The main source of vitamin D is from ultraviolet-B radiation on the skin. It is also obtained from foods such as fish, eggs, dairy products and fortified margarine or cereals. Sunlight deprivation is more important than inadequate nutrition as a cause of vitamin D deficiency and therefore failure to spend time outdoors, covering up with clothes or the use of sunscreens results in decreased skin synthesis of vitamin D.
Other secondary causes of vitamin D deficiency are malabsorption, the use of anticonvulsants, such as carbamazepine and phenytoin, and hepatic and renal disorders, which affect vitamin D metabolism. Risk factors for vitamin D deficiency also include obesity, although the reason is unclear, and pigmented skin, which filters out ultraviolet-B radiation involved in the production of vitamin D.
Vitamin D deficiency has been well documented in older people, especially among those living in institutions. In a population survey that sampled the vitamin D levels of 1766 people over the age of 65, the prevalence of vitamin D deficiency in those living in institutions was over 30% compared with 9.6% of men and 15% of women living in private households (Reference Hirani and PrimatestaHirani & Primatesta, 2005). More recently, vitamin D deficiency has been reported in psychiatric patients. A programme developed in a residential community in Australia including complete health assessments for all residents found that out of 30 residents tested, 3 had vitamin D levels in the normal range, 20 in the deficient range and 7 in the severely depleted range (Reference Howard, Waygood and DesmondHoward et al, 2006).
Method
25-hydroxycholecalciferol (25OHD) is the best indicator of vitamin D status. However, there is no generally accepted criterion for vitamin D deficiency. Some specialists suggest that 25OHD has to be above 50 nmol/l for people to have true vitamin D sufficiency and for all the parameters of calcium metabolism to be normal. For the psychiatric in-patients in this study, the normal range of 25OHD was 25–120 nmol/l. Concentrations below 25 nmol/l are consistent with vitamin D deficiency, which may be associated with osteomalacia (Anonymous, 2006a , 2006b ). For the purpose of this study, 25OHD levels in the range 25–50 nmol/l were taken to indicate borderline deficiency.
Study setting and patient population
The patient population is based in a low secure psychiatric service where individuals are referred for rehabilitation from acute adult wards, psychiatric intensive care units and prisons in West London. For security reasons, some in-patients have limited freedom to leave the unit without a nurse escorting them, and constraints on nursing numbers mean this is not always feasible. The average length of admission is in excess of 1 year.
Initially, vitamin D status was queried in two in-patients: one had gait disturbance and a history of peritoneal tuberculosis, the other was taking the anti-obesity drug orlistat which can affect absorption of fat-soluble vitamins. To quantitatively determine 25OHD we used 25-Hydroxy Vitamin D kit (Immunodiagnostic Systems, Boldon, Tyne and Wear, UK; www.idsltd.com/), which is a conventional liquid phase radioimmunoassay. These two individuals were both found to have 25OHD levels in the deficient range (<25 nmol/l). As low vitamin D levels can be associated with activation of latent tuberculosis, and there had been three cases of tuberculosis on the ward in the previous 18 months, other in-patients with a history of active or latent tuberculosis were then investigated. After finding consistently low levels of 25OHD in these in-patients, all the individuals coming into the service were offered vitamin D tests as part of their routine blood monitoring.
The study population consisted of males aged 24–53 years old. At the start of the study, out of 18 in-patients, 17 agreed to have blood samples taken as part of their routine care. Calcium and ergocalciferol (calcium and vitamin D) tablets containing 10 μg(400 units) ergocalciferol and calcium 97 mg are available for use as a daily supplement. A higher (‘pharmacological’) strength ergocalciferol formulation containing 250 μg (10 000 units) ergocalciferol is available but necessitates regular monitoring for hypercalcaemia. A decision to use the lower strength preparation was made on the basis that this would be safer, given the potential for cardiac arrhythmias was already raised in this patient population owing to the use of antipsychotic drugs and that frequent checking for hypercalcaemia would be impractical.
In this study, the lower strength daily supplement (10 μg ergocalciferol) at a dose of one tablet daily was offered to in-patients with 25OHD levels lower than 25 nmol/l; 25OHD test levels were repeated after approximately 7 months (mean=19, s.d.=82).
Results
Of the 17 in-patients who had a blood test performed, none had vitamin D sufficiency using the strictest criteria. Two individuals had 25OHD levels in the range 25–50 nmol/l indicating borderline deficiency. For the other 15 individuals, vitamin D levels were below 25 nmol/l, including 7 people whose 25OHD level was below 17.5 nmol/l (i.e. below the level for which the test can provide an accurate quantitative result).
Of the 17 in-patients in the study, 10 were African or African-Caribbean, 2 were of mixed ethnicity (African-Caribbean/White European), 1 was Vietnamese and 4 were White European. For analysis by ethnicity the ethnic groups were collapsed into White or Black and minority ethnic groups. All the Black and minority ethnic group in-patients were found to have 25OHD levels in the deficient range (below 25 nmol/l); of the 4 White European in-patients, 2 had deficiency and 2 had borderline deficiency. The difference between the White and Black and minority ethnic groups was significant using two-tailed Fisher's exact test (P=0.044).
In 12 in-patients who received treatment and in whom repeat measures were performed, an improvement in the 25OHD level was noted (P<0.01, paired t-test) in all but 1 patient (Fig. 1); 8 patients moved into the borderline deficiency range (25OHD 25–50 nmol/l) but no one moved into the sufficient range (25OHD>50 nmol/l). Patients reported no adverse side-effects during treatment with calcium and ergocalciferol.
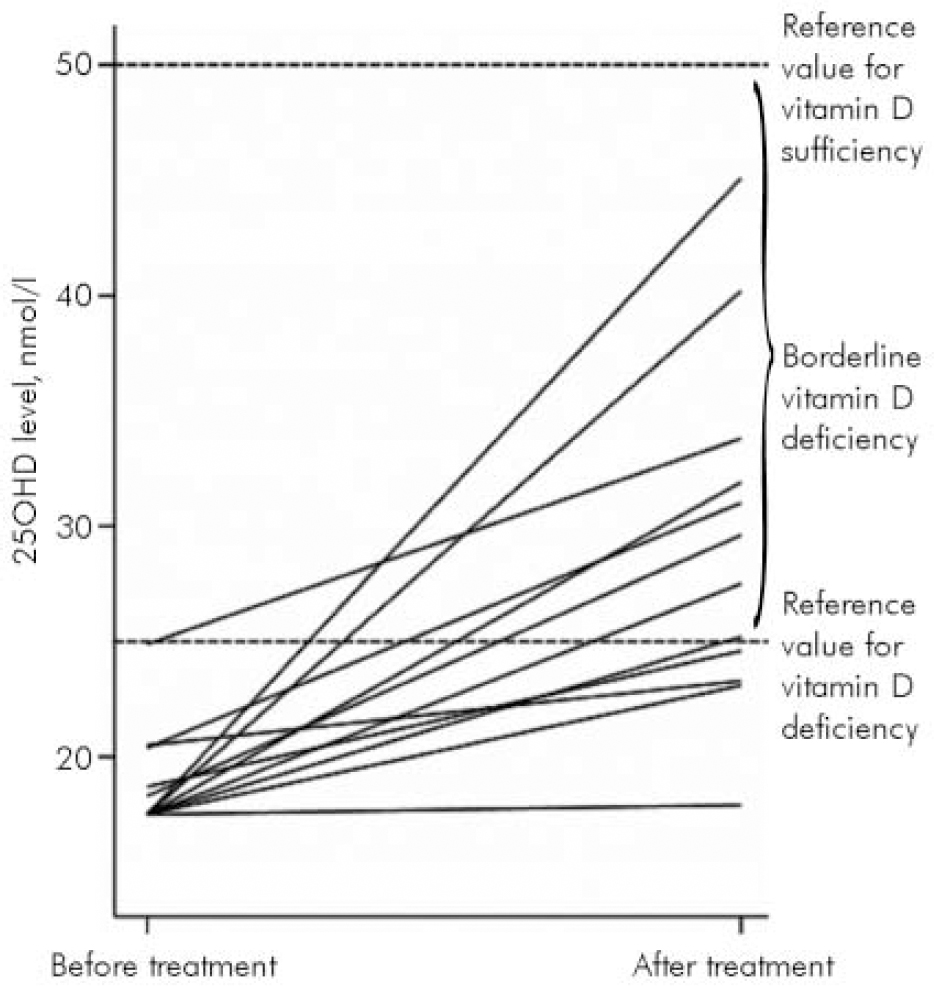
Fig. 1. Vitamin D (25OHD) levels before and after treatment.
There was no significant difference in repeat 25OHD levels in four patients who did not receive treatment and who were tested again after an average interval of 105 days. This group included two recently admitted patients, whose 25OHD level dropped during the study, and one patient who went on leave to a hostel, whose 25OHD level improved.
Discussion
The principal finding in the present case series was that 15 of the 17 long-stay psychiatric in-patients had evidence of vitamin D deficiency using the most demanding criterion (25OHD<25 nmol/l) and none met the strictest criteria for vitamin D sufficiency (25OHD>50 nmol/l).
Effects of vitamin D deficiency
Vitamin D regulates calcium and phosphate absorption and metabolism. Monitoring of serum calcium, phosphate and alkaline phosphatase may be helpful in diagnosing vitamin D deficiency, but although levels of alkaline phosphatase may increase early in vitamin D deficiency, calcium and phosphate may only fall in long-standing, symptomatic vitamin D deficiency (Anonymous, 2006b ).
Vitamin D deficiency has long been associated with bone diseases such as rickets in children and osteomalacia and osteoporosis in adults as the result of inadequate mineralisation of the bone matrix. It is also one of the causes of hypocalcaemia.
More recently, vitamin D has been noted to have more widespread effects on health. It appears to play an important role in muscle function – most individuals with vitamin D deficiency who present clinically do so because of muscle weakness or muscle pain (Anonymous, 2006b ). Studies have also shown that deficiency of vitamin D is associated with a reduction in grip strength and that its supplementation in older people reduced the incidence of fractures by improving neuromuscular function (Reference GrantGrant, 2006). Vitamin D has also been associated with reduction in the risk of specific cancers – the evidence that it reduces the risk of cancer is strongest for colon cancer, based on ecologic case – control cohort and laboratory studies (Reference GrantGrant, 2006). The evidence is also strong for lung cancer and there is reasonable evidence for other cancers including breast, ovarian, prostate, rectal and non-Hodgkin lymphoma (Reference GrantGrant, 2006). There is increasing evidence that vitamin D reduces both the risk of development and severity of symptoms of multiple sclerosis and that it reduces the risk of type 1 diabetes.
There is weaker evidence that vitamin D deficiency is associated with rheumatoid arthritis, osteoarthritis, type 2 diabetes, hypertension and cardiovascular disease (Reference GrantGrant, 2006). Vitamin D deficiency also commonly associates with tuberculosis. In a study in West London, vitamin D deficiency was associated with active tuberculosis and undetectable levels of 25OHD carried a higher risk of tuberculosis among Gujarati Asians (Reference Wilkinson, Llewelyn and ToossiWilkinson et al, 2000). Another study in London showed that vitamin D deficiency commonly associates with tuberculosis among all ethnic groups apart from White Europeans and Chinese or South East Asians (Reference Ustianowski, Shaffer and CollinUstianowski et al, 2005).
Vitamin D and mental health
Psychiatric in-patients may be particularly vulnerable to vitamin D deficiency because of lack of exposure to sunlight, poor dietary habits, the use of anticonvulsants and overrepresentation of ethnic groups known to be at greater risk. Individuals with psychiatric problems often have poor physical health and many of the problems which are associated with vitamin D deficiency compound existing medical morbidity in this group.
Similar epidemiology of vitamin D deficiency and schizophrenia has led some authors to suggest that low vitamin D levels can themselves predispose people to mental disorders. A study looking at a birth cohort from Northern Finland found use of vitamin D supplements in the first year of life was associated with a reduced risk of developing schizophrenia in later life in males but not females (Reference McGrath, Saari and HakkoMcGrath et al, 2004). However, initial studies looking for association between polymorphisms of the vitamin D receptor and risk of schizophrenia have been negative (Reference Handoko, Nancarrow and MowryHandoko et al, 2006).
Limitations of the study
Our study was prompted by the chance finding of vitamin D deficiency in our patients. There is no control group and it is difficult to know whether the results could be generalised to in-patients in other settings. The sample size is too small to indicate whether the fall in vitamin D levels correlates with length of admission to hospital and is therefore something specific to the hospital milieu (e.g. lack of sunlight, poor nutrition or medication). Tests on two recently admitted patients show normal levels that drop within the first 2 months but this needs further investigation. Further investigation is also required to find the optimum dosing regime to return 25OHD levels to normal and to find out how long such treatment needs to continue.
Implications
Vitamin D should be routinely monitored in psychiatric in-patients as it appears they are a group at increased risk of deficiency. Replacement therapy can be commenced with calcium and ergocalciferol tablets (containing 10 μg of ergocalciferol), which produce a significant rise to at least borderline deficiency in most patients. Further study is required to clarify optimal dosing and whether this should be continued until patients are in the sufficient range.
All psychiatric in-patients should have adequate exposure to sunlight and this should be taken into consideration when reviewing leave policies and access to outdoor facilities such as secure gardens or balconies. Already there has been much consideration given to the development of metabolic syndrome in psychiatric patients and regulation of hospital food and dietary advice. Close attention to diet is essential to ensure that psychiatric in-patients also receive their recommended daily allowance of all vitamins and minerals, including vitamin D. The widespread vitamin D deficiency among psychiatric in-patients in this study highlights the need for comprehensive primary healthcare to be provided for this patient group.
Declaration of interest
None.
Acknowledgements
We thank Gary Baxter, Professor of Pharmacology at the University of Cardiff, Richard Ball, Dietician at the West London Mental Health NHS Trust, and Mandy Donaldson, Principal Biochemist at the Hammersmith Hospital, London.




eLetters
No eLetters have been published for this article.