Introduction
Catatonia is a neuropsychiatric syndrome characterised by disturbance of volition, speech and movement with increased autonomic nervous system activity (Walther, Stegmayer, Wilson, & Heckers, Reference Walther, Stegmayer, Wilson and Heckers2019). It has been described as a feature of a range of other psychiatric diagnoses such as depression, mania, schizophrenia and autism (Rasmussen, Mazurek, & Rosebush, Reference Rasmussen, Mazurek and Rosebush2016; Taylor & Fink, Reference Taylor and Fink2003; Wing & Shah, Reference Wing and Shah2000), as well as in various neurological and medical conditions such as autoimmune disorders, metabolic disturbances or viral infections (Oldham, Reference Oldham2018). However, despite 150 years elapsing since its first description, catatonia has been poorly studied with research findings generally based on small case series and surveys in highly heterogeneous clinical samples. In a recent meta-analysis, most studies of catatonia prevalence were small with a mean sample size of 125 (Solmi et al., Reference Solmi, Pigato, Roiter, Guaglianone, Martini, Fornaro and Correll2018).
The population prevalence of catatonia has only been estimated indirectly (Taylor & Fink, Reference Taylor and Fink2003) and there has been a suggestion in the literature that catatonia has been observed to ‘virtually disappear’ (Mahendra, Reference Mahendra1981). The longitudinal course of catatonia has been poorly characterised and it is not clear to what extent catatonia represents a temporary state as opposed to an underlying predisposition that manifests with periodic relapses (Walther & Strik, Reference Walther and Strik2016). Gjessing described a periodic catatonia in the mid-20th century, but the most comprehensive study of catatonia relapse to date has been a case series of only 30 patients, finding that the number of episodes varied between 2 and 12 (Lin, Hung, Tsai, & Huang, Reference Lin, Hung, Tsai and Huang2016). One recent study found a considerably raised mortality in schizophrenia with catatonic stupor compared to other patients with schizophrenia, but this has not been generalised to catatonia as a whole (Funayama, Takata, Koreki, Ogino, & Mimura, Reference Funayama, Takata, Koreki, Ogino and Mimura2018).
Previous smaller studies have suggested that rates of catatonia vary across different countries (World Health Organization, 1973) (although this was not confirmed by a recent meta-analysis) (Solmi et al., Reference Solmi, Pigato, Roiter, Guaglianone, Martini, Fornaro and Correll2018) and between ethnic groups within the same country (Chandrasena, Reference Chandrasena1986). Several studies have been suggestive of higher rates among patients of Black ethnicity, but these have been limited to specific patient groups (Hutchinson, Takei, Sham, Harvey, & Murray, Reference Hutchinson, Takei, Sham, Harvey and Murray1999; Lee, Schwartz, & Hallmayer, Reference Lee, Schwartz and Hallmayer2000), have lacked a control group (Dealberto, Reference Dealberto2008) or have not been statistically significant (Mustafa, Bassim, Abdel Meguid, Sultan, & Al Dardiry, Reference Mustafa, Bassim, Abdel Meguid, Sultan and Al Dardiry2012); all have been comparatively small.
Moreover, novel research is posing new questions about the condition. For example, catatonia has been reported in encephalitis due to infections of the central nervous system (CNS) and antibodies to certain neuronal antigens (Rogers, Pollak, Blackman, & David, Reference Rogers, Pollak, Blackman and David2019; Samra, Rogers, Mahdi-Rogers, & Stanton, Reference Samra, Rogers, Mahdi-Rogers and Stanton2020). Notably, up to 88% of patients with N-methyl-d-aspartate (NMDA) receptor encephalitis exhibit catatonia at some point in their illness (Dalmau et al., Reference Dalmau, Gleichman, Hughes, Rossi, Peng, Lai and Lynch2008), although it is unclear whether NMDA receptor autoantibodies are present at higher rates in patients with catatonia generally. Movement disorders in NMDA receptor encephalitis are an early feature and tend to be prolonged, but it is possible that some catatonic features may be observed without the syndrome of catatonia being identified (Varley et al., Reference Varley, Webb, Balint, Fung, Sethi, Tijssen and Irani2019).
Several small studies have investigated serum iron, which initially appeared to be reduced in patients with catatonia (Carrol & Goforth, Reference Carrol and Goforth1995; Lee, Reference Lee1998), but small case–control studies have been equivocal (Haouzir et al., Reference Haouzir, Lemoine, Desbordes, Follet, Meunier, Baarir and Petit2009; Lakshmana, Khanna, & Christopher, Reference Lakshmana, Khanna and Christopher2009; Peralta et al., Reference Peralta, Cuesta, Mata, Serrano, Perez-Nievas and Natividad1999). Low serum iron has also been found to be predictive of neuroleptic malignant syndrome and fever after antipsychotic administration in patients with catatonia (Carrol & Goforth, Reference Carrol and Goforth1995; Conca et al., Reference Conca, Bertsch, Küng, Waschgler, Hrubos, König and Hansen2003; Lee, Reference Lee1998). Iron is a negative acute phase marker that is present at lower levels in acute inflammatory states and numerous autoimmune disorders have been reported with catatonia, so we have previously proposed that catatonia may also be associated with central and peripheral inflammation (Rogers et al., Reference Rogers, Pollak, Blackman and David2019).
This study aimed to examine the occurrence, correlates and outcomes of catatonia. Our first objective was to establish the incidence of catatonia and its relapse rate. The second objective was to investigate evidence for peripheral inflammation by comparing inpatients with catatonia to psychiatric inpatients without catatonia in terms of C-reactive protein (CRP), white cell count, creatine kinase (CK) and NMDA receptor autoantibodies. The third objective was to conduct a longitudinal comparison of patients with catatonia to psychiatric patients without catatonia in terms of their duration of hospitalisation and mortality.
Method
Setting
The study used the Clinical Records Interactive Search (CRIS) system, which is a large repository of anonymised electronic healthcare records from patients receiving care from the South London and Maudsley NHS Foundation Trust, UK. This Trust is the largest unit provider of secondary mental health services in the UK, serving four London boroughs with a combined 2016 population of 1 317 000, as well as providing some specialist services to the UK population nationally. Patients are seen in settings as diverse as community teams, outpatient departments, psychiatric wards and allied general hospitals. Unified electronic records were introduced between 2005 and 2006, importing previous electronic records dating back to 1999. CRIS was developed in 2008 and incorporates these previous records as well as adding current records up to the present day (Stewart et al., Reference Stewart, Soremekun, Perera, Broadbent, Callard, Denis and Lovestone2009). It currently contains records for over 500 000 individuals. The initial data extraction did not specify a time period in order to obtain the most expansive chronological data.
We used existing electronic healthcare records to identify patients with catatonia. Data were initially extracted on 17/12/2018 with subsequent data extraction occurring on 24/01/2019, 04/02/2019, 17/12/2019 and 3/09/2021. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The CRIS system has approval from the Oxfordshire C Research Ethics Committee (ref: 18/SC/0372) and this study was approved by the CRIS Oversight Committee (ref: 17-102).
Identifying patients with catatonia
To identify catatonia, we first applied a bespoke natural language processing algorithm for mentions of catatonic symptoms/syndrome, which had been developed against manually extracted gold standard annotations to a performance level of 0.86 precision (positive predictive value) and 0.87 recall (sensitivity) (Jackson et al., Reference Jackson, Patel, Jayatilleke, Kolliakou, Ball, Gorrell and Stewart2017). One of the three investigators (JPR, NB and AG) examined each positive record retrieved by the algorithm to ensure that: a diagnosis of catatonia was made by a clinician, there was a date given for the diagnosis and there was clear evidence in the case record of at least two features of catatonia as defined by the Bush–Francis Catatonia Screening Instrument (BFCSI), a tool that has a high degree of interrater reliability, construct validity, sensitivity and specificity in the identification of catatonia (Bush, Fink, Petrides, Dowling, & Francis, Reference Bush, Fink, Petrides, Dowling and Francis1996a, Reference Bush, Fink, Petrides, Dowling and Francis1996b; Subramaniyam, Muliyala, Suchandra, & Reddi, Reference Subramaniyam, Muliyala, Suchandra and Reddi2020). Where a patient had multiple episodes of catatonia, only one episode was required to list the catatonic features present.
To assess interrater reliability, 30 of the first patients' case notes were examined by more than one rater (10 by all three raters, 10 by JPR and NB and 10 by JPR and AG). Cohen's kappa coefficient for caseness on the BFCSI was 0.68, which is considered ‘substantial’ agreement (Landis & Koch, Reference Landis and Koch1977). In order to assist with comparability with other studies, we also calculated the number of individuals who met DSM-5 criteria for catatonia (American Psychiatric Association, 2013).
The derivation of demographic, clinical and laboratory characteristics is described in online Supplementary eTable 1.
Descriptive analysis
To maximise generalisability in terms of treatment setting, disease spectrum and time, all catatonia patients meeting the inclusion criteria above were included in the descriptive analyses. Index year and number of cases were assessed with Pearson's correlation. Further descriptive analyses divided annual frequency by the size of the catchment population, as estimated by the UK Office for National Statistics (Office for National Statistics, 2011). Descriptive statistics were used to investigate relapse and treatment settings.
Case–control study
Our comparison group was drawn from the structured fields of electronic healthcare records and was composed of all individuals admitted to psychiatric wards within the same mental health trust between 2007 and 2016, covering patients with a variety of diagnoses and ages, including services treating children, adults and older people. To ensure comparability of the two groups, patients with catatonia were included only if they were inpatients on psychiatric wards admitted between these dates.
Diagnoses (other than identification of catatonia) were made according to the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) and we reported primary diagnoses (World Health Organization, 1992). We grouped these as organic disorders (ICD-10 codes F00–F09 and non-F codes); neurodevelopmental disorders (F70–89, F90 and F95); schizophrenia and related disorders (F20–F29); mood disorders (F30–F39); neurotic disorders (F40–59); personality and behavioural disorders (F50–69, F91–F94, F98) and substance use disorders (F10–F19).
Analysis of laboratory markers was conducted in Viapath Laboratory at King's College Hospital, apart from neuronal autoantibody analyses, which were conducted in the Oxford NHS Diagnostic Neuroimmunology Service. Serum NMDA receptor antibody level results (using the Oxford live cell-based assay prior to July 2015 and the Euroimmun fixed cell-based assay thereafter) (Oxford Diagnostic Immunology Service, 2015) were officially reported by the laboratory as negative, weakly positive or positive. Due to the evidence that weakly positive peripheral antibodies can be associated with autoimmune encephalitis and high titres in the CSF (Cai et al., Reference Cai, Zhou, Xie, Yu, Wang and Ren2018; Qin et al., Reference Qin, Wu, Huang, Xu, Zhang, Zheng and Zheng2017), we grouped the weakly positive with the positive results to create two categories: negative and positive. Antibodies against the voltage-gated potassium channel, as measured by radioimmunoassay were reported, as data were collected prior to the reporting of antibodies to the LGi1 and CASPR2 antigens by the laboratory.
Age, sex, ethnicity, diagnostic group and laboratory markers were compared between patients with and without catatonia using logistic regression. Odds ratios (ORs) for laboratory results were calculated unadjusted and adjusted for age, sex and Black ethnicity, as these demographic factors are known to affect the results of numerous laboratory tests. Where a high degree of positive skew was present in the laboratory results, natural logarithmic transformations were used; where zero values were present, a logn(x + 1) transformation was used. Where the ORs for laboratory results had very narrow confidence intervals (CIs), the results were divided by their standard deviations prior to transformation.
Due to missing data in the laboratory results, the number of cases for each individual result is reported. Associations with missing data were analysed. However, multiple imputation was not conducted because computational problems can develop in data with high proportions of missing data, resulting in misleading estimates (Sterne et al., Reference Sterne, White, Carlin, Spratt, Royston, Kenward and Carpenter2009).
Cohort study
As in the case–control study, the comparison group was composed of all individuals admitted to psychiatric wards between 2007 and 2016. Patients with catatonia were included only if they were inpatients on psychiatric wards admitted between these dates. When analysing the duration of admission, patients with catatonia were included only where catatonia occurred within 7 days of admission, to avoid a bias where catatonia becomes more likely due to patients spending longer in hospital. When analysing mortality, patients with catatonia were included only where catatonia was recorded within 3 days of its onset, to avoid a survival bias in which only surviving patients would have the opportunity to have catatonia retrospectively recorded in their notes. Data were ascertained in the same way for patients with and without catatonia. Mortality data were obtained from linked national records. The time from index date to outcome (hospital discharge or death) was analysed using a Cox proportional baseline hazard model survival analysis, adjusting for age, sex, ethnicity and index year. After interaction with peer reviewers, an additional analysis adding diagnostic group as a covariate to the model was conducted. The proportionality assumption was checked using visual inspection of the Kaplan–Meier plot.
Statistical analysis
Statistical analysis was conducted using Stata MP (version 15) with a threshold for statistical significance set to p < 0.05. The manuscript was written according to STROBE recommendations (checklist is given in online Supplementary eTable 2) (von Elm et al., Reference von Elm, Altman, Egger, Pocock, Gøtzsche and Vandenbroucke2007).
Results
Occurrence
The sample consisted of 2130 episodes of catatonia in 1456 subjects, as shown in Fig. 1. Overall, in the 10 year period from 2007 to 2016, among patients who were resident in the healthcare provider's catchment area, there were 1316 episodes of catatonia (852 unique patients) for the provider's catchment population of 1 242 055, giving an incidence of 10.6 (95% CI 10.0–11.1) episodes per 100 000 person-years. Where the more stringent DSM-5 criteria were used, there were 901 episodes of catatonia in 586 individuals. Among adults, there were 1214 episodes of catatonia in a mean population of 968 064, giving an incidence of 12.5 (95% CI 11.8–13.3) episodes per 100 000 person-years. Among children, there were 102 episodes of catatonia in a mean population of 273 990, giving an incidence of 3.7 (95% CI 3.0–4.5) episodes per 100 000 person-years. Examining only those episodes that were contemporaneously reported between 2007 and 2016, there was a positive correlation between index year and number of episodes (r = 0.70, p = 0.02), as shown in Fig. 2. This remained after adjusting for the mean age of the population each year (r = 0.71, p = 0.03).
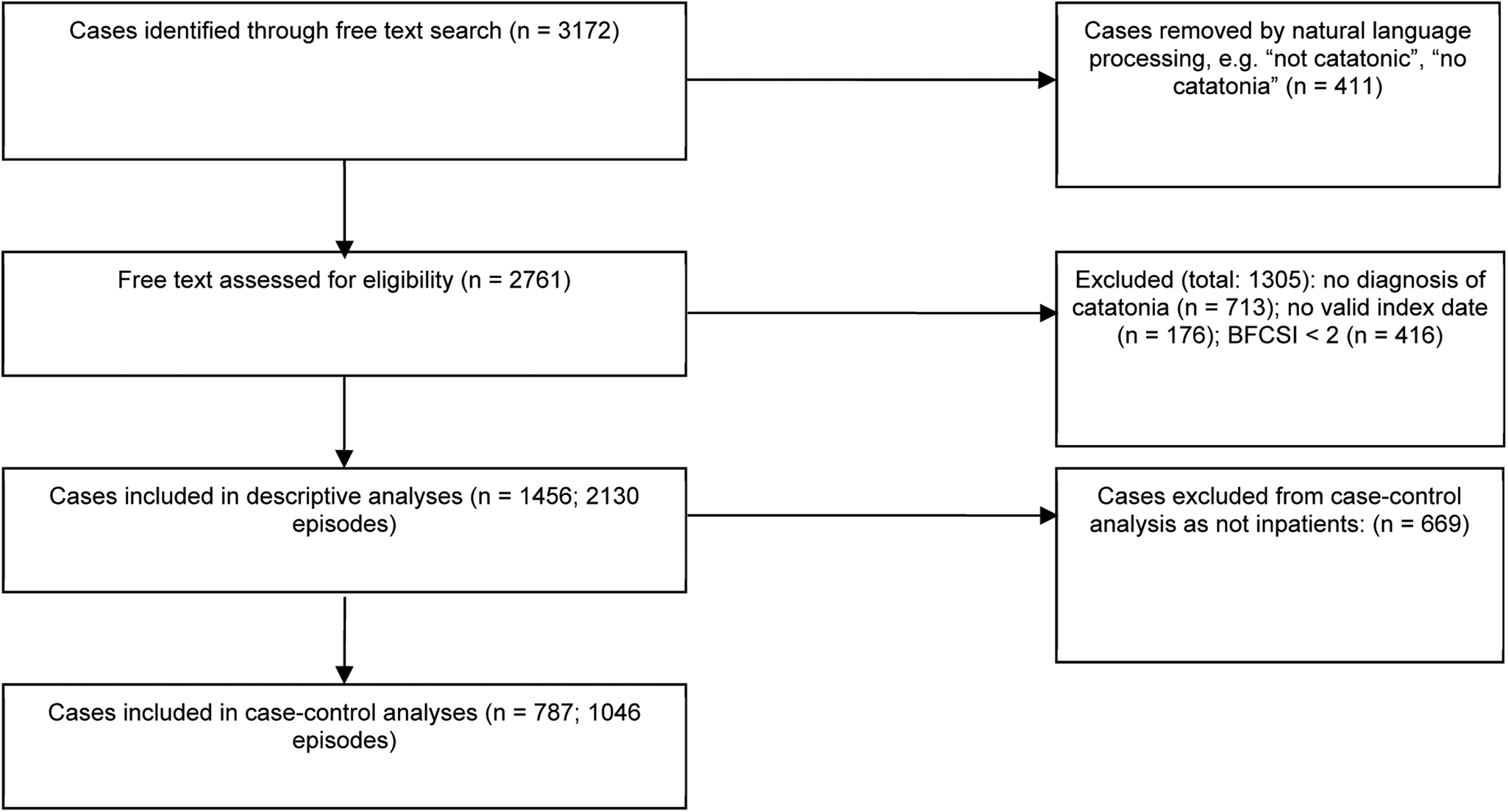
Fig. 1. Identification and screening of cases.
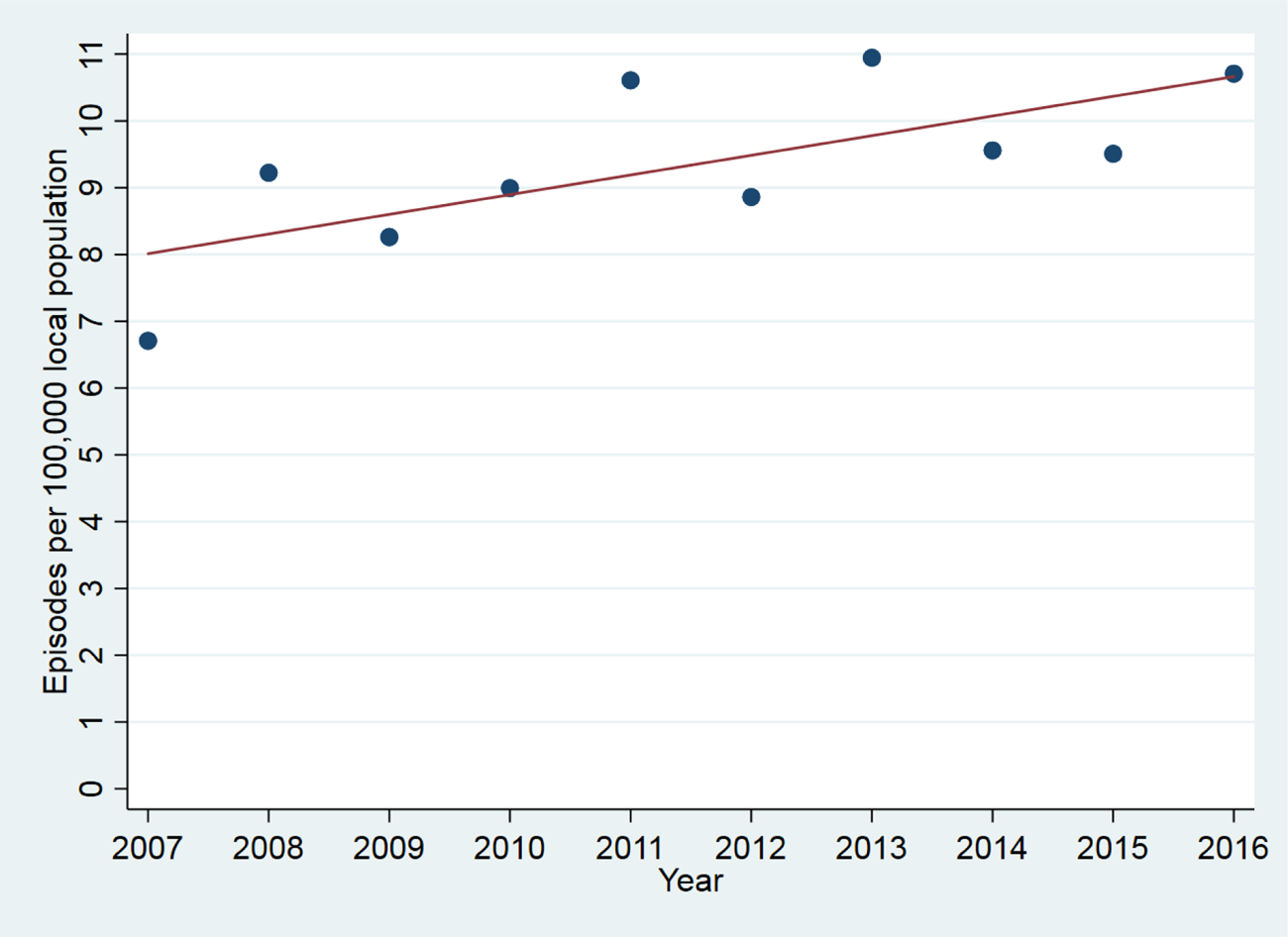
Fig. 2. Catatonic episode per 100 000 local population by year.
Number of episodes per patient ranged between 1 and 27 (mean 1.5, s.d. 1.2, median 1, IQR 1–2) over a mean follow-up time of 7.0 years (s.d. 5.1 years). After the first episode, subsequent episodes occurred at a rate of 0.035 episodes (s.d. 0.086) per year with 25.1% experiencing at least two episodes within the follow-up period. However, after five episodes, further episodes occurred in 55.9%.
The age range for the patients at the time of first recorded diagnosis of catatonia was between 5 and 91 years (mean 35.4, median 32, s.d. 16.2, IQR 23–45 years). In terms of treatment setting at the time of diagnosis of catatonia, 1046 episodes (49.1%) were diagnosed when the individual was an inpatient in a psychiatric ward, 462 (21.7%) were in a community mental health team, 217 (10.2%) were in a general hospital, 54 (2.5%) were in a crisis resolution and home treatment team, 28 (1.3%) were in a health-based place of safety and in 323 (15.2%) the treatment setting was not specified. A total of 1022 (48.0%) were detained under the Mental Health Act for compulsory treatment within 2 weeks of the index date. The mean number of features of the BFCSI present was 3.6 (s.d. 1.7). Patients with adult and paediatric first presentation of catatonia are compared in online Supplementary eTable 3.
Case–control study
The comparison group was drawn from all inpatients admitted to the Trust between 2007 and 2016 and represented 24 956 patients with 37 456 inpatient episodes. Demographic comparisons are made in Table 1. Patients with catatonia were similar to the control group in sex ratio but younger and more likely to be from an ethnic minority background. There were significant differences in the underlying diagnoses of the two groups.
Table 1. Characteristics of groups: OR for catatonia according to age, sex, ethnicity and diagnosis
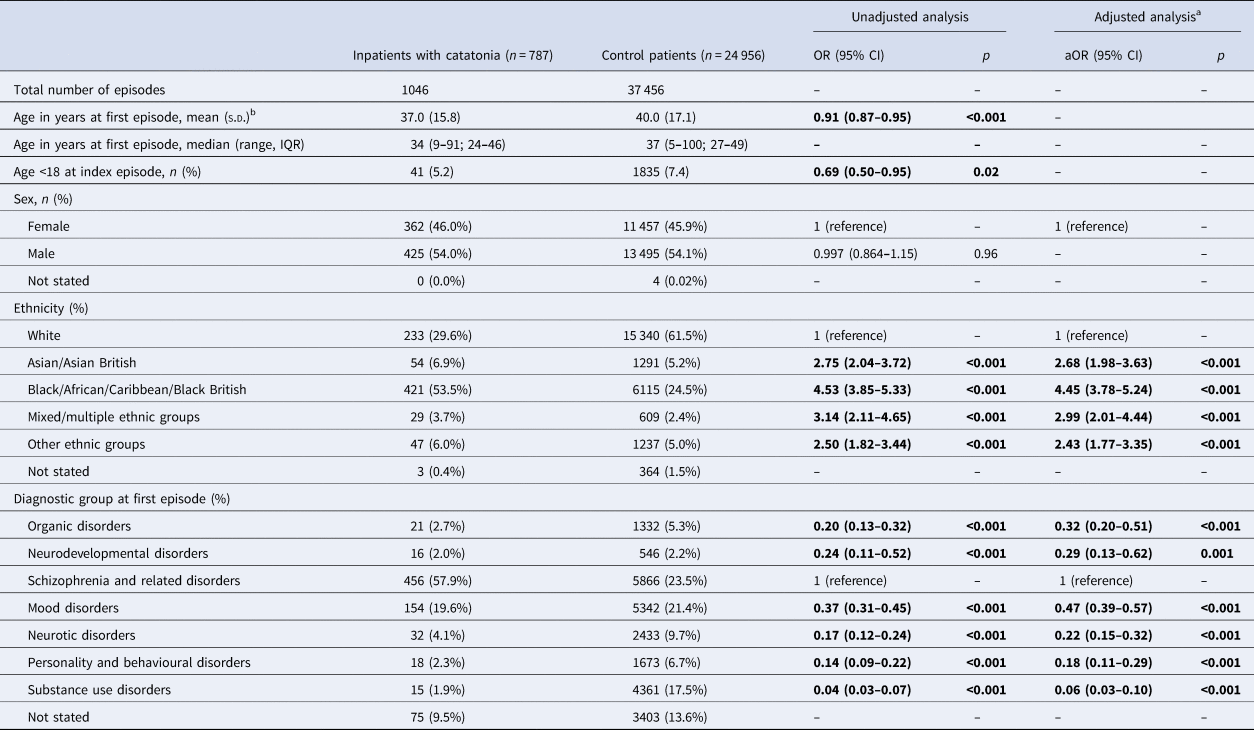
a Ethnicity adjusted for age and sex. Diagnostic group adjusted for age, sex and Black ethnicity.
b ORs calculated using age in decades.
The main laboratory test results are compared in Table 2 with additional exploratory outcomes presented in online Supplementary eTable 4. As an additional exploratory analysis, we investigated whether serum iron and creatinine kinase were altered at baseline, or whether there was a change that corresponded to the onset of catatonia. We included all patients with catatonia who had laboratory results both for a catatonic episode and that was at least 1 month from any catatonic episode. Paired t tests were then used to compare the result from when the patient had catatonia with the average of the non-catatonic results. No statistically significant differences were detected between CK or iron at baseline compared to during an episode of catatonia, but numbers were small (see online Supplementary eTable 5). Comparing selected laboratory test results between patients in the catatonia group who did and did not have low serum iron revealed no significant differences after adjustment (see online Supplementary eTable 6). We also explored whether the high CK within the catatonia group was related to muscular rigidity or to rhabdomyolysis due to immobility by testing the associations between CK and each of rigidity and immobility. We found no significant relationship between CK and either of these clinical features, either in a simple measure of association or in a multivariable analysis, as shown in online Supplementary eTable 7. When receiver operating characteristic analysis was conducted for CK and catatonia diagnosis, the area under the curve was 0.64, as shown in online Supplementary eFig. 1.
Table 2. Laboratory results for patients with catatonia and the comparison group
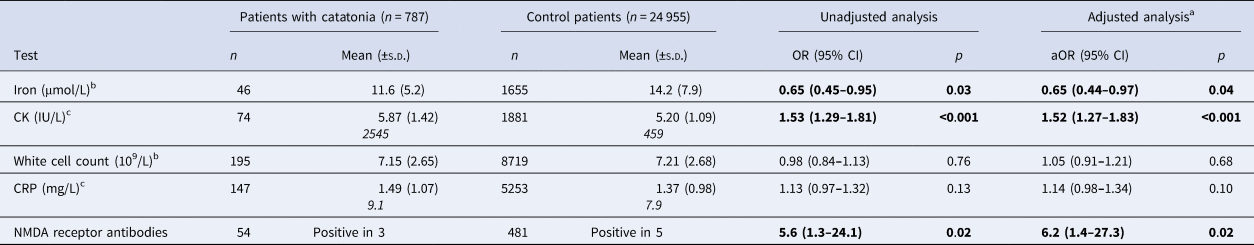
a Adjusted for age, sex and ethnicity.
b Due to very small CIs, these ORs have been calculated by dividing the laboratory result by its standard deviation.
c Due to positive skew, these results underwent a natural logarithm transformation. Logn results are in normal text with original results in italics (analyses performed using logn results).
In terms of missing data, three valid laboratory values were present for 9.1% of the inpatient episodes with catatonia and 6.3% of the inpatient control episodes. When the missing data were analysed, there were significant associations with catatonic group membership, age, sex and Black ethnicity, but the absolute differences were very small. Associations are shown in online Supplementary eTable 8.
Cohort study
When we compared the 556 episodes of catatonia (473 patients) recognised within 7 days of admission with the control group using survival analysis with hospital discharge as the outcome, we found that the baseline proportional hazards assumption was reasonable (see Kaplan–Meier plots in online Supplementary eFigs 2 and 3). The median duration of inpatient stay was 43 days (95% CI 40–49 days) among patients with catatonia, compared to 25 days (95% CI 25–26 days) in the comparison group. The unadjusted Cox proportional hazard ratio (HR) for hospital discharge was 0.77 (95% CI 0.71–0.84, p < 0.001); after adjusting for age, sex, Black ethnicity and year of admission, it was 0.78 (95% CI 0.72–0.85, p < 0.001). After the addition of diagnostic group as a covariate to the model, the HR was 0.83 (95% CI 0.76–0.90, p < 0.001). When the analysis was restricted to those subjects with a first episode in adulthood, the results were similar [unadjusted HR 0.77 (95% CI 0.70–0.83), p < 0.001; adjusted HR 0.76 (95% CI 0.69–0.83), p < 0.001].
When we compared the 646 patients with catatonia recorded within 3 days of its occurrence with the control group with mortality as the outcome, we found that the baseline proportional hazards assumption was reasonable (see Kaplan–Meier plot in online Supplementary eFig. 4). 3535 deaths (58 in the catatonia group) occurred during a mean follow-up time of 7.0 years (s.d. 3.2). While there was a lower mortality among patients with catatonia in the unadjusted analysis [HR 0.66 (95% CI 0.51–0.85), p = 0.001], after adjustment for age, sex, Black ethnicity and year of admission, there was no evidence for a difference between patients with and without catatonia [adjusted HR = 0.93 (95% CI 0.72–1.21), p = 0.60]. After the addition of diagnostic group as a covariate to the model, the HR was 1.12 (95% CI 0.86–1.45, p = 0.42). When the analysis was restricted to those subjects with a first episode in adulthood, the results were similar [unadjusted HR 0.64 (95% CI 0.49–0.83), p = 0.001; adjusted HR 0.92 (95% CI 0.71–1.20), p = 0.54].
Discussion
This study used data from electronic patient records and is, to our knowledge, the largest clinical study on catatonia published to date (Solmi et al., Reference Solmi, Pigato, Roiter, Guaglianone, Martini, Fornaro and Correll2018). Our results show that patients diagnosed with catatonia had a similar sex ratio to the general population of psychiatric inpatients, but those with catatonia were slightly younger. There was a considerable difference in ethnicity between the two groups with Black patients being substantially overrepresented among those with recorded catatonia. It has previously been proposed that this disparity is due to different interpretation of symptoms by clinicians of the dominant culture (Hutchinson et al., Reference Hutchinson, Takei, Sham, Harvey and Murray1999). Other possible explanations include cultural differences in illness expression and genetic factors. It has been reported that schizophrenia is more common among migrant populations (Saha, Chant, Welham, & McGrath, Reference Saha, Chant, Welham and McGrath2005), but we found that the overrepresentation of schizophrenia in our catatonia population did not fully explain the ethnicity differential. Non-European origin has been shown to be a risk factor for tardive dyskinesia, another movement disorder commonly seen in psychiatric practice (Tenback, van Harten, & van Os, Reference Tenback, van Harten and van Os2009).
Mahendra famously hypothesised, based on clinical experience, that catatonia was becoming less common (Mahendra, Reference Mahendra1981). In this study, we found greater annual numbers of patients with catatonia between 2007 and 2016. Apart from increased recognition, one possible reason for an increase in catatonia diagnosis would be the use of certain novel psychoactive drugs (such as synthetic cannabinoids, synthetic cathinones and phenethylamines) (Mdege, Meader, Lloyd, Parrott, & McCambridge, Reference Mdege, Meader, Lloyd, Parrott and McCambridge2017), some of which have been linked to catatonia (Khan, Pace, Truong, Gordon, & Moukaddam, Reference Khan, Pace, Truong, Gordon and Moukaddam2016; Richman et al., Reference Richman, Skoller, Fokum, Burke, Hickerson and Cotes2018), and this will be the subject of further investigation with this dataset. Our overall incidence of catatonia (10.6 episodes per 100 000 person-years) is somewhat lower than a previous US estimate of 33.0 per 100 000 person-years that estimated rates of catatonia indirectly using proportions reported in other diagnoses (Taylor & Fink, Reference Taylor and Fink2003). Our figures likely represent an underestimate of the true incidence of catatonia, given that most cases of catatonia are not recognised by clinicians and catatonic signs are poorly identified (Takács, Ungvari, Antosik-Wójcińska, & Gazdag, Reference Takács, Ungvari, Antosik-Wójcińska and Gazdag2021; van der Heijden et al., Reference van der Heijden, Tuinier, Arts, Hoogendoorn, Kahn and Verhoeven2005). This may be particularly the case in a general hospital, where catatonia has been found to be common among critically ill patients (Grover, Ghosh, & Ghormode, Reference Grover, Ghosh and Ghormode2014; Wilson et al., Reference Wilson, Carlson, Duggan, Pandharipande, Girard, Wang and Ely2017); it is possible that such patients did not come to the attention of a psychiatric team.
The diagnostic heterogeneity of catatonia in our study differed somewhat from other studies in that schizophrenia and related disorders, rather than mood disorders, were most common (Taylor & Fink, Reference Taylor and Fink2003). It is likely that our data represent overdiagnosis of schizophrenia, as a relic of the Kraepelinian concept of catatonia as existing purely as a subtype of schizophrenia (Shorter & Fink, Reference Shorter and Fink2018). According to one survey of psychiatrists in Hungary, most clinicians still view catatonia as residing within the framework of schizophrenia (Takács et al., Reference Takács, Ungvari, Antosik-Wójcińska and Gazdag2021). In addition, the diagnostic coding used is still ICD-10, which only formally recognises catatonia in the context of F20.2 – Catatonic schizophrenia and F06.1 – Organic catatonic disorder. Our data show that, although approximately half of cases of catatonia are recognised on psychiatric wards, appreciable rates of diagnosis occur in treatment settings such as in community teams and general hospitals. However, our data are limited to patients presenting to psychiatric services and it is likely that many cases present in general hospitals and are not assessed by a psychiatrist. We should emphasise that we report the treatment setting and whether patients were detained at the point at which catatonia was recognised, so it is possible that many patients were admitted to psychiatric hospitals shortly after catatonia recognition.
Our data on catatonia relapse show that for three quarters of patients with a first reported catatonic episode, during an average follow-up period of 7.0 years, there were no further episodes. However, there was evidence to suggest that in patients with multiple episodes, the probability of relapse is much higher, providing some evidence for Gjessing's description of periodic catatonia (Gjessing, Reference Gjessing1932). Relapse was more common in those with an underlying psychotic disorder, although this may reflect an understanding that individuals with relapsing catatonia must necessarily be suffering from catatonic schizophrenia.
In what we believe to be the largest study of biomarkers in catatonia, we have found that iron was low relative to psychiatric controls [adjusted OR (aOR) 0.65, 95% CI 0.44–0.97] and this result remained after adjusting for demographic variables. Our initial hypothesis was that, as iron is a negative acute phase marker, this represented a peripheral inflammatory response (Rogers et al., Reference Rogers, Pollak, Blackman and David2019), but the lack of difference in CRP, total white cell count and erythrocyte sedimentation rate does not support this. Another possibility is that low iron is a hallmark of malnutrition, which is likely to occur in catatonia (Clinebell, Azzam, Gopalan, & Haskett, Reference Clinebell, Azzam, Gopalan and Haskett2014) and could be a consequence of the prolonged hospitalisation. However, there was no evidence for several other markers of malnutrition – low albumin, vitamin B12, folate or creatinine (Keller, Reference Keller2019; Thongprayoon, Cheungpasitporn, & Kashani, Reference Thongprayoon, Cheungpasitporn and Kashani2016) – compared to the comparison group. The relationship between iron and catatonia is therefore not clear and may relate to phenotypic heterogeneity, medication use or a more subtle immune response. It is possible that low iron creates hypokinetic symptoms by causing hypodopaminergia in the basal ganglia, as several enzymes necessary for dopamine synthesis are iron-dependent (Zucca et al., Reference Zucca, Segura-Aguilar, Ferrari, Muñoz, Paris, Sulzer and Zecca2017); this could also explain the close association between catatonia and neuroleptic malignant syndrome (Rasmussen et al., Reference Rasmussen, Mazurek and Rosebush2016). Interestingly, low serum iron has also been demonstrated in a meta-analysis of Parkinson's disease, alongside raised iron in the substantia nigra, demonstrating that alterations in serum and CNS biometals in movement disorders are not necessarily in the same direction (Genoud, Senior, Hare, & Double, Reference Genoud, Senior, Hare and Double2019). Our finding that there was no significant difference between serum iron levels during and between catatonic episodes might suggest an underlying diathesis, but it is also possible that these results were contaminated by other unrecognised episodes of catatonia.
Examining the white cell differential (online Supplementary eTable 4) suggests that the total figure masks a more complex picture. Absolute counts of neutrophils are raised (on the adjusted analysis), while lymphocytes, eosinophils and basophils are reduced. Ratios of these cell counts have more recently been used as markers of disease activity in conditions as diverse as chronic obstructive pulmonary disease, solid organ tumours, stroke and acute coronary syndromes, and their use has also been suggested for psychiatric disorders (Zulfic et al., Reference Zulfic, Weickert, Weickert, Liu, Myles and Galletly2020). There is evidence that the neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR) and monocyte–lymphocyte ratio (MLR) are higher in the manic or hypomanic phase of bipolar affective disorder than in the depressed phase (Fusar-Poli et al., Reference Fusar-Poli, Natale, Amerio, Cimpoesu, Filioli, Aguglia and Aguglia2021), while these same ratios have also been found to be higher in relapse of schizophrenia than in remission (Özdin & Böke, Reference Özdin and Böke2019). In catatonia, one study has found raised NLR compared to healthy controls, although there was no evidence for difference in terms of PLR and MLR. In the present study, we found NLR, MLR and PLR to be raised in catatonia relative to a psychiatric comparison group. It is possible that a relatively low lymphocyte count represents the impact of malnutrition or certain psychotropic medications (Gergely, Reference Gergely1999; Keller, Reference Keller2019), but lymphopaenia has also been linked to autoimmunity (Schulze-Koops, Reference Schulze-Koops2004). The effect sizes are small, but the prospect that white cell count ratios may reflect disease activity in psychiatric disorders merits further study.
The most striking laboratory finding was the CK, where the mean result in patients with catatonia (2545 IU/L) was several times higher than that of the comparison group (459 IU/L). Three studies have previously investigated this with only one finding a significant difference, but these all used smaller samples than the present investigation (Haouzir et al., Reference Haouzir, Lemoine, Desbordes, Follet, Meunier, Baarir and Petit2009; Meltzer, Reference Meltzer1968; Northoff, Wenke, & Pflug, Reference Northoff, Wenke and Pflug1996). Raised CK may be due to muscle injury resulting from the immobility, posturing and rigidity that occur in catatonia, although the use of intramuscular injections may also have contributed. It is also possible that the group with catatonia was contaminated with patients who may have been developing neuroleptic malignant syndrome, although the result remained significant following exclusion of extreme values. Raised thyroxine in catatonia (online Supplementary eTable 4) is interesting given previous research on periodic catatonia suggesting an increased metabolic rate during episodes and a reduced rate in the intervals, which appeared to be responsive to treatment with thyroid hormones (Gjessing, Reference Gjessing1964; Gjessing, Reference Gjessing1975; Gunne & Gemzell, Reference Gunne and Gemzell1956), although we should note that the difference in thyroxine levels in our study was very small.
Given that almost 250 cases of catatonia have been reported co-occurring with NMDA receptor encephalitis and that catatonic features occur in up to 88% of cases of NMDA receptor encephalitis (Dalmau et al., Reference Dalmau, Gleichman, Hughes, Rossi, Peng, Lai and Lynch2008; Rogers et al., Reference Rogers, Pollak, Blackman and David2019), we hypothesised that NMDA receptor antibodies would be present at higher rates in the patients with catatonia. Although our data supported this hypothesis with a large OR [5.6 (95% CI 1.3–24.1)], the sample size for antibody tests was small and relied on only three positive results in the group with catatonia. It is consistent with the existing literature, where more severe catatonic features have been found in patients at ultra-high risk of psychosis who have NMDA receptor antibodies and a continuous measure of NMDA receptor immunofluorescence found higher positivity in patients with catatonia (Lin, Hung, Tsai, & Huang, Reference Lin, Hung, Tsai and Huang2017; Pollak et al., Reference Pollak, Iyegbe, Kempton, Irani, Vincent, Stone and McGuire2018). However, it requires replication in a larger sample.
One particularly striking finding was that patients with catatonia remained in the hospital for 134 days longer than other psychiatric inpatients, which represents an enormous degree of morbidity and a substantial economic cost. However, there was no evidence that patients with catatonia had increased mortality in multivariable analysis, in contrast to a recent Japanese study of patients with schizophrenia that found a higher mortality among those with catatonic stupor [OR 4.8 (95% CI 2.0–10.6)] (Funayama et al., Reference Funayama, Takata, Koreki, Ogino and Mimura2018). The discrepancy might be explained by the restriction of the analysis in Funayama et al.'s study to patients with schizophrenia who had been hospitalised. It is possible that the mortality in our study is not elevated precisely because the patients with catatonia are more unwell than the comparison group and are treated for longer in the hospital, where their physical healthcare may be superior to the community. Moreover, some patients may have died before catatonia was formally recorded.
Strengths and limitations
This study had several strengths, notably its large sample size, naturalistic data, psychiatric control group, rigorous standards for defining catatonia and linkage to national records to define mortality. However, using routine clinical records also brings several disadvantages, including a reliance on clinician identification of disorders and symptoms, which means that incidences are likely underestimated, perhaps especially in general hospitals, where our figures were particularly low. Moreover, it is likely that a reliance on clinician identification will preferentially exclude patients without classical features of catatonia, such as those with excited or less acute presentations. Prospective studies, which continue to take place (Espinola-Nadurille et al., Reference Espinola-Nadurille, Flores-Rivera, Rivas-Alonso, Vargas-Cañas, Fricchione, Bayliss and Ramirez-Bermudez2019; Sarkar et al., Reference Sarkar, Sakey, Mathan, Bharadwaj, Kattimani and Rajkumar2016), are able to avoid this bias, although case numbers tend to be much smaller.
In terms of measurement, although the Bush–Francis has previously been used in paediatric populations (Grover, Chauhan, Sharma, Chakrabarti, & Avasthi, Reference Grover, Chauhan, Sharma, Chakrabarti and Avasthi2017), it has not been specifically validated in this group (Benarous et al., Reference Benarous, Consoli, Raffin, Bodeau, Giannitelli, Cohen and Olliac2016).
Regarding detection of markers of autoimmunity, we were limited to serum samples, but there is evidence that titres are higher in cerebrospinal fluid, thus potentially conferring greater sensitivity (Dalmau et al., Reference Dalmau, Gleichman, Hughes, Rossi, Peng, Lai and Lynch2008).
In terms of missing data, the epidemiological estimates may also have been biased by patients who were lost to follow-up by moving out of the area; this may have preferentially affected individuals who are less unwell. The missing diagnoses may differ from the available diagnoses and this is likely, as clinical presentation may affect the probability of making or recording a diagnosis. The fact that only a minority of patients had a valid laboratory result for any given test means that selection bias is possible and exploration of the missing data did suggest small but statistically significant associations with ethnicity and membership of the catatonia group.
In terms of confounding, there was evidence of confounding by age, sex and ethnicity, but after adjusting for these factors, the major findings remained. Given the high proportion of individuals from a Black ethnic group in the catchment area for the service and our finding that Black patients were more likely to have catatonia, this may have inflated our estimates of incidence above what is nationally representative. One important unmeasured confounder in the laboratory test results is medication use, which means it is not possible to establish if differences in laboratory test results are due to intramuscular injection or the development of neuroleptic malignant syndrome.
Finally, despite the large sample size, due to the relatively low number of deaths in the catatonia group, our statistical power for detecting a difference in mortality between the two groups was limited. It is possible that some of the findings arose by chance, given that several hypotheses were tested.
Conclusions
This study demonstrates that catatonia remains an important clinical problem with significant morbidity and may even be increasing in frequency of diagnosis in certain areas. Catatonia features in a large array of psychiatric diagnoses and is associated with a very prolonged hospital admission. In terms of biomarkers, there remains evidence for low serum iron and raised CK, but prospective controlled studies are necessary for confirmation.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291721004402
Data
A data dictionary defining each variable used in the study is available in Supplementary eTable 1. Data are owned by a third party, Maudsley Biomedical Research Centre (BRC) Clinical Records Interactive Search (CRIS) tool, which provides access to anonymised data derived from South London and Maudsley NHS Foundation Trust electronic medical records. These data can only be accessed by permitted individuals from within a secure firewall (i.e. the data cannot be sent elsewhere), in the same manner as the authors. For more information please contact: cris.administrator@slam.nhs.uk.
Acknowledgements
This paper represents independent research part-funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Author contributions
JPR, TAP and ASD designed the project. MP, MB and AK conducted data extraction with advice from RS and RP. JPR, NB, AG and JK manually reviewed the patient records. JPR conducted the statistical analysis with support from BC and GL. JPR drafted the manuscript with specialist advice from TAP, AB, AA, MSZ, RS, GL and TRN. All authors reviewed and approved the final manuscript.
Financial support
JPR was supported by an NIHR Academic Clinical Fellowship (ACF-2016-17-007) and a Wellcome Trust Ph.D. Training Fellowship for Clinicians (220659/Z/20/Z). TAP is supported by an NIHR Clinical Lectureship. RP has received support from a Medical Research Council (MRC) Health Data Research UK Fellowship (MR/S003118/1) and a Starter Grant for Clinical Lecturers (SGL015/1020) supported by the Academy of Medical Sciences, The Wellcome Trust, MRC, British Heart Foundation, Arthritis Research UK, the Royal College of Physicians and Diabetes UK. MSZ, GL and ASD are supported by the NIHR University College London Hospitals Biomedical Research Centre. MB, MP and AK are part-funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at the South London and Maudsley NHS Foundation Trust and King's College London. RS is part-funded by: (i) the National Institute for Health Research (NIHR) Biomedical Research Centre at the South London and Maudsley NHS Foundation Trust and King's College London; (ii) a Medical Research Council (MRC) Mental Health Data Pathfinder Award to King's College London; (iii) an NIHR Senior Investigator Award; (iv) the National Institute for Health Research (NIHR) Applied Research Collaboration South London (NIHR ARC South London) at King's College Hospital NHS Foundation Trust. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Role of the funding source
The funders of the individuals working on this paper had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Conflict of interest
JPR has held one advisory meeting with Promentis Pharmaceuticals, Inc. in an unpaid capacity. MSZ reports receiving personal fees from UCB Pharma for lecturing, outside the submitted work. RS declares research support received in the last 36 months from Janssen, GSK and Takeda. RP has received grant funds from Janssen and consultancy fees from Induction Healthcare and Holmusk outside the present study. All other authors declare no competing interests.







