Introduction
Major depressive disorder (MDD) is one of the most common psychiatric disorders and a leading cause of disability worldwide (Hasin et al., Reference Hasin, Sarvet, Meyers, Saha, Ruan, Stohl and Grant2018; Iancu, Wong, Rhebergen, van Balkom, & Batelaan, Reference Iancu, Wong, Rhebergen, van Balkom and Batelaan2020; Lu et al., Reference Lu, Xu, Huang, Li, Ma, Xu and Zhang2021). Neuroimaging studies using resting-state functional magnetic resonance imaging (rs-fMRI) have documented that MDD is associated with altered intrinsic functional connectivity (FC) in large-scale brain networks. For instance, abnormal FC patterns within high-order neural networks such as the default-mode network in MDD patients have been frequently reported (Kaiser, Andrews-Hanna, Wager, & Pizzagalli, Reference Kaiser, Andrews-Hanna, Wager and Pizzagalli2015; Luo et al., Reference Luo, Wu, Xu, Chen, Wu, Wang and Wang2021; Yan et al., Reference Yan, Chen, Li, Castellanos, Bai, Bo and Zang2019). In other studies, it was found that MDD is related to decreased FCs within the sensorimotor, visual, and auditory networks (Javaheripour et al., Reference Javaheripour, Li, Chand, Krug, Kircher, Dannlowski and Wagner2021; Lu et al., Reference Lu, Cui, Huang, Li, Duan, Chen and Chen2020; Luo et al., Reference Luo, Wu, Xu, Chen, Wu, Wang and Wang2021). These findings have significantly advanced our knowledge about neural mechanisms underlying MDD.
The clinical and biological heterogeneity in MDD has received significant attention in recent years (Nguyen et al., Reference Nguyen, Harder, Xiong, Kowalec, Hägg, Cai and Lu2022; Pine, Reference Pine2019). Age is one of the most notable contributing factors to such heterogeneity, and there have been ample studies reporting that MDD patients in different age groups differ in depressive symptomatology, risk factors, and responses to treatment (Rep et al., Reference Rep, Peyre, Sánchez-Rico, Blanco, Dosquet, Schuster and Hoertel2022; Schaakxs et al., Reference Schaakxs, Comijs, Lamers, Kok, Beekman and Penninx2018; Wagner et al., Reference Wagner, Wollschläger, Dreimüller, Engelmann, Herzog, Roll and Lieb2020). For example, it was found that vegetative symptoms such as appetite change and insomnia are more common in adolescent MDD than adult MDD (Rice et al., Reference Rice, Riglin, Lomax, Souter, Potter, Smith and Thapar2019). Beside that, MDD in late adulthood is considered to be more characterized by somatic symptoms and cognitive impairments (Hegeman, Kok, van der Mast, & Giltay, Reference Hegeman, Kok, van der Mast and Giltay2012; Thomas et al., Reference Thomas, Gallagher, Robinson, Porter, Young, Ferrier and O'Brien2009). Potential differences in MDDs between different age stages have been also indicated by past neuroimaging studies from the perspectives of brain development and aging. To be specific, FC between the sensorimotor and subcortical regions as well as volumes of several subcortical structures such as putamen have been found to be reduced with increasing age during adolescence (Sanders et al., Reference Sanders, Harms, Kandala, Marek, Somerville, Bookheimer and Barch2023; Supekar, Musen, & Menon, Reference Supekar, Musen and Menon2009; Whittle et al., Reference Whittle, Lichter, Dennison, Vijayakumar, Schwartz, Byrne and Allen2014). These age-related brain changes were found to be attenuated in adolescents with MDD, which were thought to be reflective of delayed brain maturation (Jiao et al., Reference Jiao, Ding, Lu, Su, Zhang, Wang and Liu2011; Straub et al., Reference Straub, Brown, Malejko, Bonenberger, Grön, Plener and Abler2019; Whittle et al., Reference Whittle, Lichter, Dennison, Vijayakumar, Schwartz, Byrne and Allen2014). In contrast to adolescent MDD, most studies conducted in older patients suggested an accelerated brain aging process in patients with MDD (Ballester et al., Reference Ballester, Suh, Nogovitsyn, Hassel, Strother, Arnott and Frey2021; Han et al., Reference Han, Dinga, Hahn, Ching, Eyler, Aftanas and Schmaal2021; Jha, Chin Fatt, Minhajuddin, Mayes, & Trivedi, Reference Jha, Chin Fatt, Minhajuddin, Mayes and Trivedi2023). Together, these findings support the possibility that mechanisms of MDD are associated with age, which might be instructive for personalizing the treatment strategies for MDD patients (Wagner et al., Reference Wagner, Wollschläger, Dreimüller, Engelmann, Herzog, Roll and Lieb2020).
To test the hypothesis that MDD-related brain dysfunctions are age-associated, several studies have been performed to compare the abnormal brain FC patterns between different age groups of MDD patients. Especially, it was suggested that unique abnormal FC patterns may present in pre-adult and elderly patients with MDD when compared to the early-and-middle adult patients (Hu et al., Reference Hu, Xiao, Ai, Wang, Chen, Tan and Kuang2019; Saberi, Mohammadi, Zarei, Eickhoff, & Tahmasian, Reference Saberi, Mohammadi, Zarei, Eickhoff and Tahmasian2022; Tang et al., Reference Tang, Lu, Zhang, Hu, Bu, Li and Huang2018). As an example, abnormal FC patterns involving the insula, the amygdala, and the default-mode cortices have been found to differ between adolescents and adults with MDD (Hu et al., Reference Hu, Xiao, Ai, Wang, Chen, Tan and Kuang2019; Tang et al., Reference Tang, Lu, Zhang, Hu, Bu, Li and Huang2018). Furthermore, it was suggested that unique profiles of FC abnormalities may exist in patients with ‘late-life depression’, which can be defined as MDD occurring over the age of 50 or 55 no matter whether the illness started in early or later life (Saberi et al., Reference Saberi, Mohammadi, Zarei, Eickhoff and Tahmasian2022; Tan et al., Reference Tan, Ouyang, Huang, Wu, Liu, He and Long2023; Yang et al., Reference Yang, Chen, Zhong, Zhou, Mai, Zhang and Ning2022). Nevertheless, these studies are limited in several ways. First, many studies have compared FC patterns between adolescents and adults (Tang et al., Reference Tang, Lu, Zhang, Hu, Bu, Li and Huang2018), or between young adults and older adults (Ye et al., Reference Ye, Shen, Xu, Yang, Chen, Liu and Cheng2017). However, little has been conducted to simultaneously compare MDD patients among adolescents, adults, and elderly patients. Second, some of the existing studies have reported heterogeneous and even conflicting results. For example, both decreased (Wu et al., Reference Wu, Andreescu, Butters, Tamburo, Reynolds and Aizenstein2011) and increased (Eyre et al., Reference Eyre, Yang, Leaver, van Dyk, Siddarth, Cyr and Lavretsky2016) FCs between the posterior cingulate cortex and the anterior cingulate cortex have been reported in elderly patients with MDD than healthy controls (HCs). One possible reason for such inconsistency may be due to relatively small sample sizes (<60 per group) and potentially differing experimental settings in these published studies (Chen, Lu, & Yan, Reference Chen, Lu and Yan2018).
To address the above limitations, the current study aimed to investigate the common and distinct features of functional brain network abnormalities across adolescents, adults, and elderly patients with MDD from a large-sample (n > 1200) analysis. All imaging data were preprocessed using the same standardized protocol, to minimize the potential biases and obtain as reliable results as possible. Using one of the largest and most well-powered rs-fMRI samples of MDD patients to our knowledge, we hope to gain insights into the functional disruptions among different age groups of MDD patients, facilitating future studies on the age-related heterogeneity in MDD.
Materials and methods
Subjects
The final analyzed sample in the current study consisted of 1238 participants (617 MDD patients and 621 HCs, 12–82 years old) of 350 men and 888 women recruited from 15 study sites across China. Such a sample was drawn from two sources: a publicly available multisite dataset from the REST-meta-MDD consortium (http://rfmri.org/REST-meta-MDD) (Deng et al., Reference Deng, Yue, Xu, Ma, Chen, Li and Cheng2022; Ding et al., Reference Ding, Yang, Yan, Chen, Bai, Bo and Guo2021; Liu et al., Reference Liu, Li, Zhang, Sun, Li, Chen and Zhang2021; Long et al., Reference Long, Cao, Yan, Chen, Li, Castellanos and Liu2020; Yan et al., Reference Yan, Chen, Li, Castellanos, Bai, Bo and Zang2019; Yang et al., Reference Yang, Chen, Chen, Li, Li, Castellanos and Yan2021) and a local dataset from the First Affiliated Hospital of Chongqing Medical University in China. The sample size for each site can be found in online Supplementary Table S1. All patients were diagnosed with MDD (without comorbidity of other psychiatric disorders) based on the Structured Clinical Interview for DSM-5 (SCID) and had a total score ⩾8 on the 17-item Hamilton Depression Rating Scale (HAMD-17). Following some previous studies (Long et al., Reference Long, Cao, Yan, Chen, Li, Castellanos and Liu2020; Yan et al., Reference Yan, Chen, Li, Castellanos, Bai, Bo and Zang2019; Yang et al., Reference Yang, Chen, Chen, Li, Li, Castellanos and Yan2021), exclusion criteria include incomplete demographic information, poor imaging quality, and sites with less than 10 participants. In addition, a part of the participants was randomly excluded to ensure that demographics and MDD patients’ depressive severity were matched between different age groups (see SI Methods for details). The respective local ethics committees have approved all contributing studies, and written informed consent has been obtained from all participants or their guardians at the local institutions.
All participants were categorized into three age groups: adolescents (108 MDD patients and 60 HCs,12–17 years old), early-middle adults (411 MDD patients and 449 HCs, 18–54 years old), and late adults (98 MDD patients and 112 HCs, > = 55 years old), following the criterion in some recent studies (Cuijpers et al., Reference Cuijpers, Karyotaki, Eckshtain, Ng, Corteselli, Noma and Weisz2020; Findling et al., Reference Findling, DelBello, Zuddas, Emslie, Ettrup, Petersen and Rosen2022). The average age, sex ratio, and head motion were matched between the MDD patients and HCs in each age group; besides, the sex ratio, head motion, and depressive severity (measured by the HAMD total score) were matched between different age groups of MDD patients. More details about the demographic characteristics of each group of participants can be found in Table 1.
Table 1. Demographic and clinical characteristics in the three age groups of participants
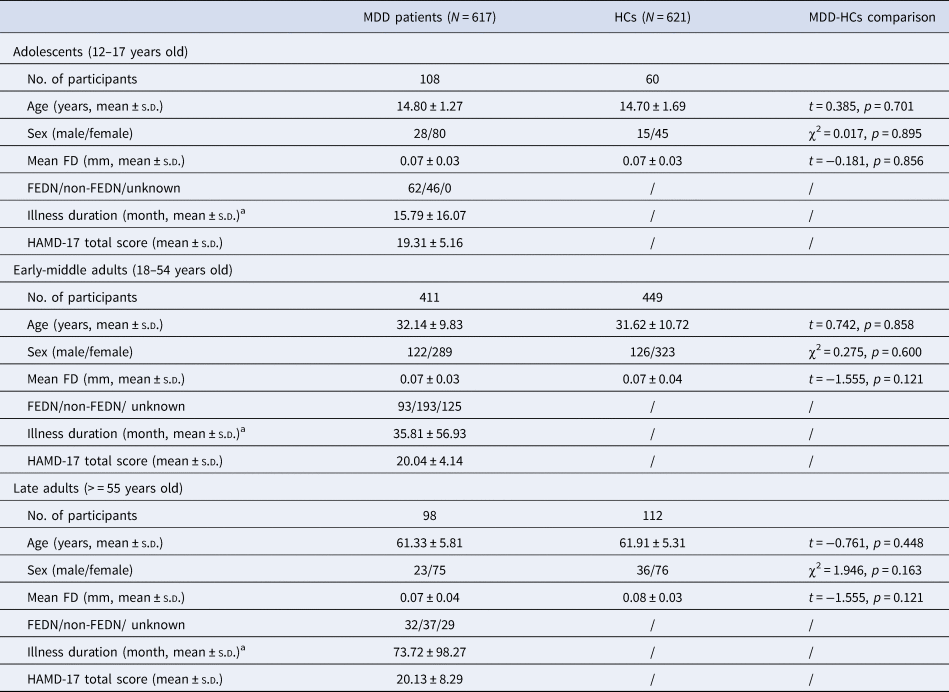
a Data on illness duration was only available for 107 adolescent patients, 371 early-middle adult patients, and 66 late adult patients, respectively. FD, framewise-displacement; FEDN, first-episode, drug-naïve; HAMD, Hamilton Depression Rating Scale; HC, healthy controls; SD, standard deviation.
Data preprocessing and quality control
All participants underwent rs-fMRI and T1-weighted structural scans at each site. The scanning parameters for each site can be found in online Supplementary Table S1. Data were preprocessed using the DPARSF software (Yan, Wang, Zuo, & Zang, Reference Yan, Wang, Zuo and Zang2016) with the standardized protocol provided by the REST-meta-MDD project, as described in several previously published studies (Long et al., Reference Long, Cao, Yan, Chen, Li, Castellanos and Liu2020; Yan et al., Reference Yan, Chen, Li, Castellanos, Bai, Bo and Zang2019; Yang et al., Reference Yang, Chen, Chen, Li, Li, Castellanos and Yan2021; Zhang et al., Reference Zhang, Palaniyappan, Deng, Zhang, Pan, Fan and Pu2021). A Chinese adolescent brain template (https://github.com/zuoxinian/CCS/tree/master/H3/GrowthCharts/Templates/IPCAS/BrainTemplate) was used for image normalization for participants who were under 18 years of age (Dong et al., Reference Dong, Castellanos, Yang, Zhang, Zhou, He and Zuo2020; Zhang et al., Reference Zhang, Palaniyappan, Deng, Zhang, Pan, Fan and Pu2021). Subjects with poor image quality or excessive head motion as determined by mean framewise-displacement (FD) >0.2 mm were excluded from the analyses. See SI Methods for more details.
Brain parcellation, edge-based FC and network-level FC
Nodes and edges between them (connections) make up a topological network. In the current study, the preprocessed images were parcellated into 264 nodes across the brain based on the 264 regions of interest (ROIs) in a validated and widely-used Power functional atlas (Liang et al., Reference Liang, Deng, Li, Greenshaw, Wang, Li and Li2020; Long et al., Reference Long, Ouyang, Yan, Wu, Huang, Pu and Palaniyappan2023; Power et al., Reference Power, Cohen, Nelson, Wig, Barnes, Church and Petersen2011). Edge-based FCs between brain nodes were defined by the z-transformed Pearson correlation coefficients between average time courses of each possible pair of nodes (264*263/2 = 34 716 edges in total) (Yan et al., Reference Yan, Chen, Li, Castellanos, Bai, Bo and Zang2019; Yang et al., Reference Yang, Chen, Chen, Li, Li, Castellanos and Yan2021). To investigate FCs at the level of large-scale brain networks, all nodes were further assigned into 10 networks including the default-mode, frontoparietal, sensorimotor, visual, subcortical, cingulo-opercular, salience, ventral attention, dorsal attention, and auditory networks referring to previous work (see online Supplementary Figure S1) (Cole et al., Reference Cole, Reynolds, Power, Repovs, Anticevic and Braver2013; Feng et al., Reference Feng, Chen, Cai, Ye, Feng, Liu and Xue2020; Mohr et al., Reference Mohr, Wolfensteller, Betzel, Mišić, Sporns, Richiardi and Ruge2016; Niu et al., Reference Niu, Sun, Wang, Yang, Wen and Xiang2022). The network-level FC strengths were calculated by averaging the z-transformed FC values across all involved edges within a specific network or between a specific pair of networks (Javaheripour et al., Reference Javaheripour, Li, Chand, Krug, Kircher, Dannlowski and Wagner2021; Li et al., Reference Li, Su, Wu, Castellanos, Li, Li and Yan2021). Since 10 networks were defined, this resulted in a total of 10 within-network and 45 between-network averaged FC values.
Separate comparisons in each age group
The edge-based and network-level FCs were firstly compared between the MDD patients and HCs in each age group separately (e.g. adolescent patients v. adolescent controls) (Fig. 1). Edge-based FCs were compared by the network-based statistics (NBS) approach (Zalesky, Fornito, & Bullmore, Reference Zalesky, Fornito and Bullmore2010). Here, NBS analyses with t tests were conducted, with sex, age, head motion as measured by mean FD, and site added as covariates. Note here, the sites were added as covariates (as dummy variables) to control possible influences of different data scanning parameters across different sites. Permutation with 5000 iterations was employed and the results were considered significant at edge p < 0.001 and cluster p < 0.05 after NBS correction for multiple comparisons (Chopra et al., Reference Chopra, Francey, O'Donoghue, Sabaroedin, Arnatkeviciute, Cropley and Fornito2021; Li et al., Reference Li, Su, Wu, Castellanos, Li, Li and Yan2021; Tsurugizawa & Yoshimaru, Reference Tsurugizawa and Yoshimaru2021). The network-level FC values were compared by analysis of covariance (ANCOVA) covarying for sex, age, head motion as well as site, and the results were considered significant at p < 0.05 after false discovery rate (FDR) correction (Li et al., Reference Li, Su, Wu, Castellanos, Li, Li and Yan2021; Sun et al., Reference Sun, Zhao, He, Chang, Wang, Wei and Yang2022). The results were visualized partly using the Brainnet Viewer toolbox (Xia, Wang, & He, Reference Xia, Wang and He2013).
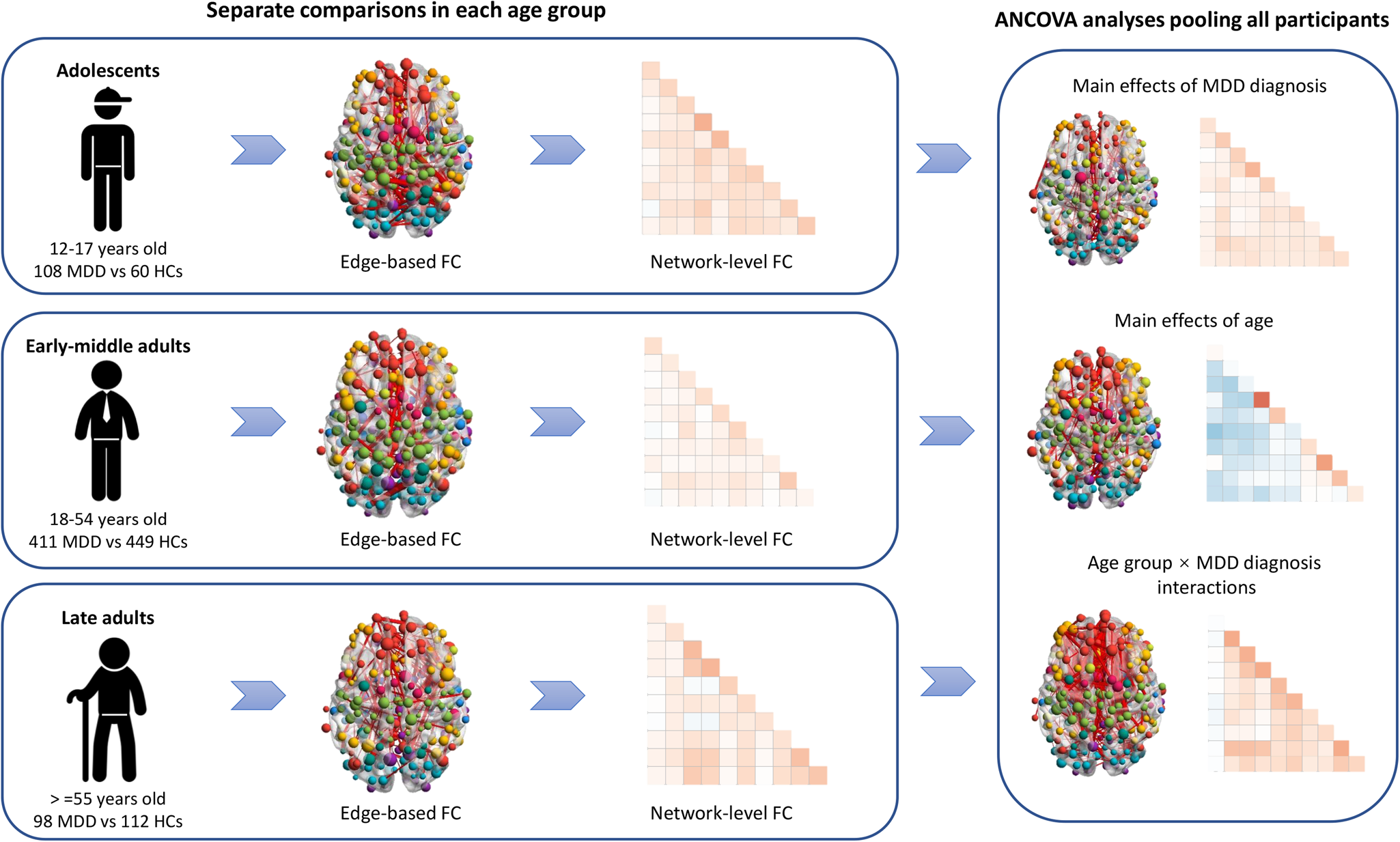
Figure 1. A summary of the main analyzing steps in the present study. ANCOVA, analysis of covariance; FC, functional connectivity; HCs, healthy controls; MDD, major depressive disorder.
Analyses using the whole pooled sample
Data of all participants were then pooled and analyzed for direct investigations on the effects of age groups and MDD diagnosis, as well as their interactions (Fig. 1). A two-by-three ANCOVA statistical model was used to analyze both the edge-based and network-level FCs, with MDD diagnosis (MDD v. HCs), age group (adolescents v. early-middle adults v. late adults) and MDD diagnosis × age group interaction as variables of interest, as well as sex, head motion and site as covariates. For edge-based FCs, such analyses were performed based on the NBS approach (5000 iterations), and the results were considered significant at edge p < 0.001 and cluster p < 0.05 after NBS corrections. For network-level FCs, the results were considered significant when p < 0.05 after FDR corrections across the multiple network-level FC values. When significant main effects of age group were found, the Bonferroni post-hoc pairwise comparisons were further adopted between different age groups and the differences were considered significant at Bonferroni p < 0.05.
Associations with clinical variables
Several post-hoc analyses were performed to explore possible associations between the observed significant brain network alterations (edge-level and network-level) and clinical characteristics in MDD patients. Firstly, the brain network alterations were correlated with HAMD-17 total score/illness duration using partial correlations adjusted for sex, age, head motion, and site effects. Secondly, the brain network alterations were compared between the first-episode, drug-naïve (FEDN) and non-FEDN patients by ANCOVA adjusted for the same above covariates. These investigations were performed both in each age group separately and in all MDD patients. For edge-level FCs, average FC values were firstly calculated across all the edges with significant alterations before being fed into the analyses. The results were considered significant at FDR-corrected p < 0.05. See SI Methods for more details.
Results
Separate comparisons in each age group
In all three age groups, the MDD patients showed significant alterations in both edge-based and network-level FCs when compared to age-matched HCs. The significant alterations in edge-based FCs (corrected p < 0.05) were shown in Fig. 2. For better visualization, we counted and presented both the raw number and normalized proportion (obtained by dividing the raw number by the maximum possible number) of edges falling into each of the within-network and between-network classes. The significant alterations in network-level FCs (corrected p < 0.05) were shown in Fig. 3.
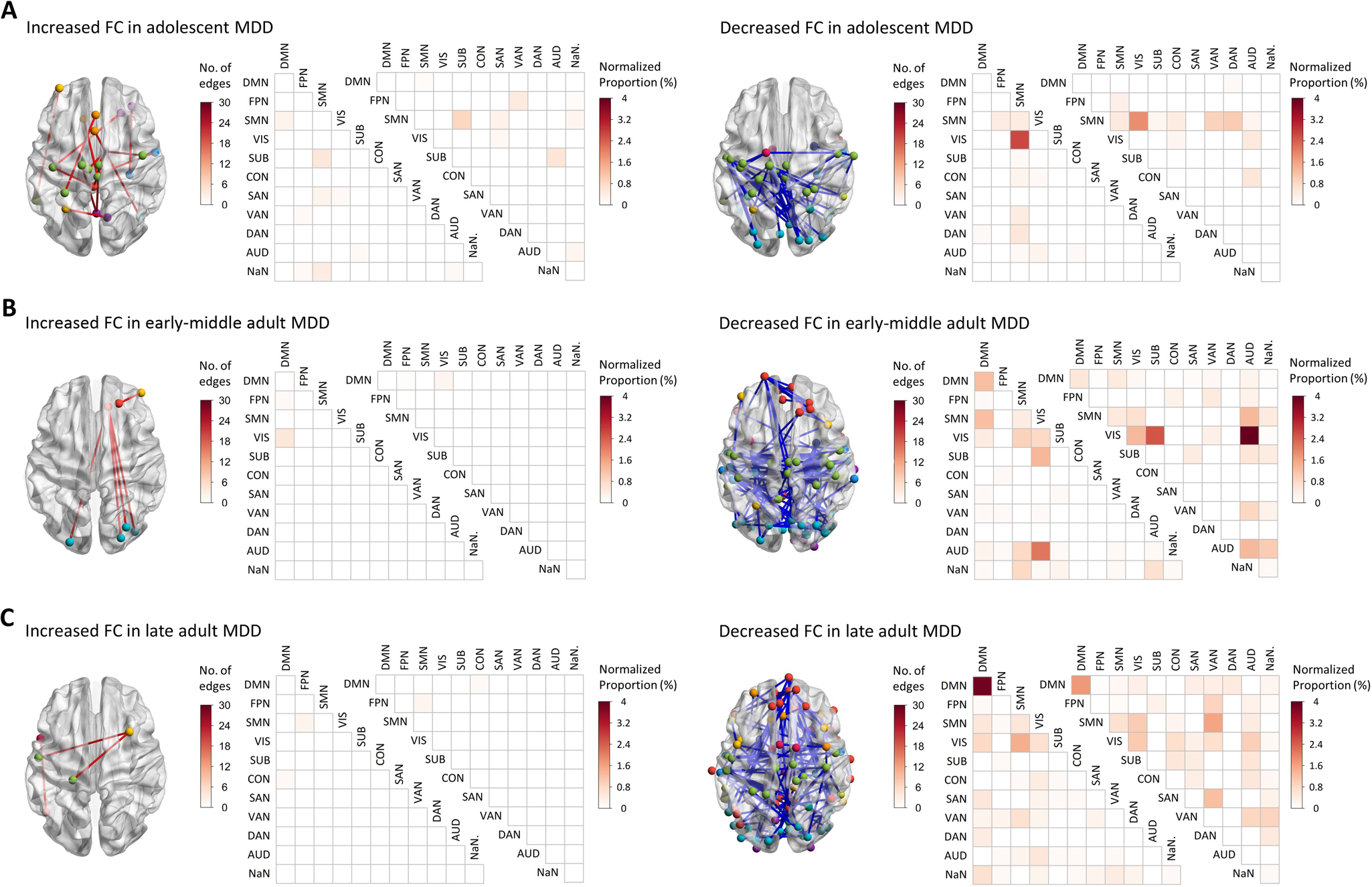
Figure 2. Results of the separate comparisons on edge-based FCs in each age group. (A) Results in the adolescents. (B) Results in the early-middle adults. (C) Results in the late adults. The edges with significantly increased or decreased FCs in MDD patients (corrected p < 0.05) were presented separately. AUD, auditory network; CON, cinguloopercular network; DAN, dorsal network; DMN, default-mode network; FC, functional connectivity; FPN, frontoparietal network; MDD, major depressive disorder; SAN, salience network; SMN, sensorimotor network; SUB, subcortical network; VAN, ventral attention network; VIS, visual network.
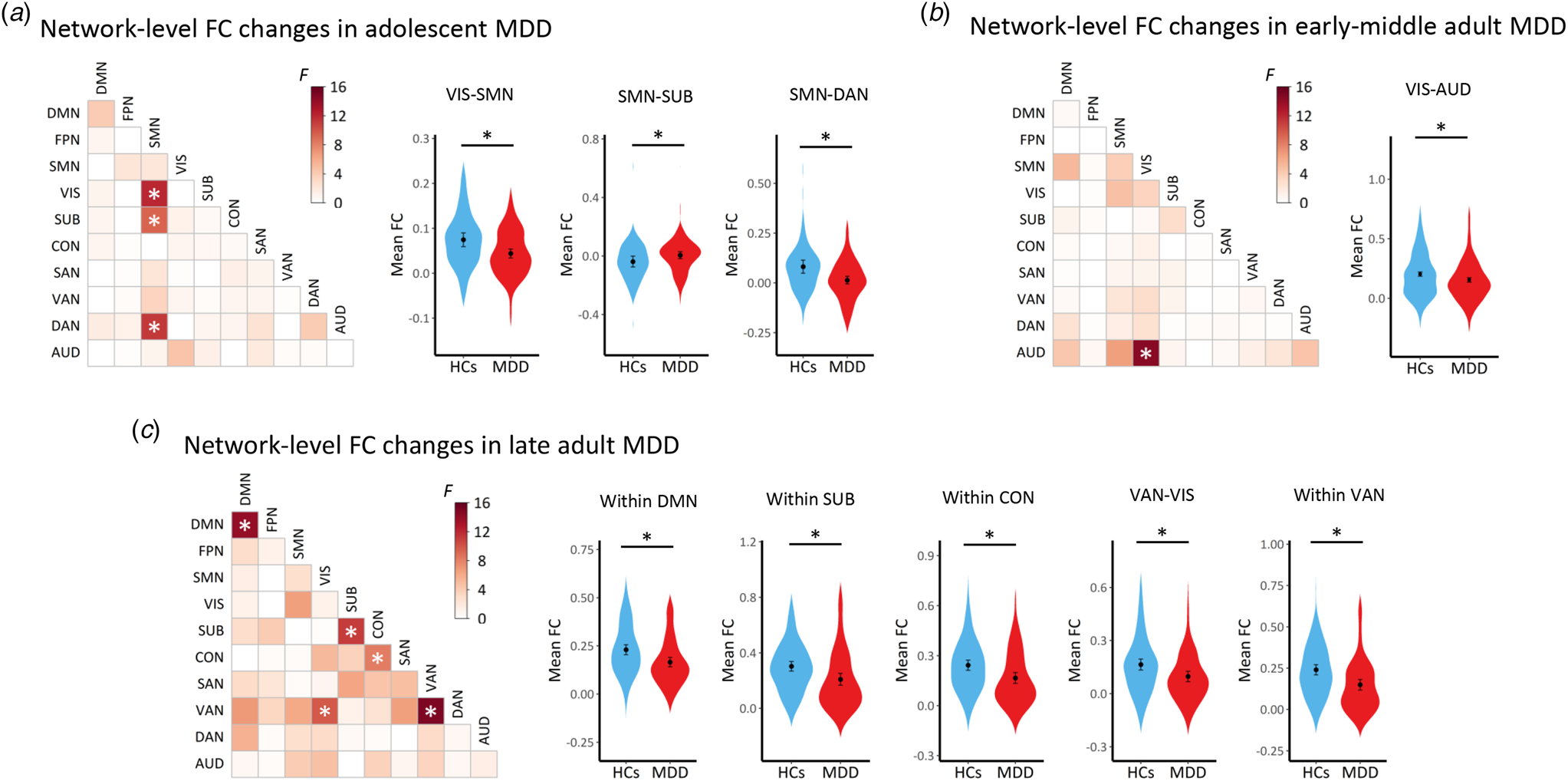
Figure 3. Results of the separate comparisons on network-level FCs in each age group. (A) Results in the adolescents. (B) Results in the early-middle adults. (C) Results in the late adults. The ‘*’ indicates a significant difference (corrected p < 0.05) between the patients and HCs. Mean values of the network-level FCs showing significant differences were also presented on the right sides. AUD, auditory network; CON, cinguloopercular network; DAN, dorsal attention network; DMN, default-mode network; FC, functional connectivity; FPN, frontoparietal network; MDD, major depressive disorder; SAN, salience network; SMN, sensorimotor network; SUB, subcortical network; VAN, ventral attention network; VIS, visual network.
Results on the edge-based FCs and network-level FCs were generally in agreement with each other, consistently suggesting significantly altered FC patterns (most of which are hypo-connected edges) in all age groups of MDD patients. Specifically, the results suggested similar widespread decreases in FCs, which involved the sensorimotor, visual, and auditory networks, in all age groups of MDD patients. Several age group-specific alterations were also observed, including increased sensorimotor-subcortical FC in adolescent patients, decreased visual-subcortical FC in early-middle adult patients, as well as decreased subcortical-, cinguloopercular-, default-mode-, and attention-involved FCs in late adult patients (Figs 2, 3).
Pooled analyses on network-level FCs
Results of the ANCOVA model on network-level FCs when pooling the data of all participants were shown in Fig. 4. Firstly, the ANCOVA model revealed significant main effects of age group (corrected p < 0.05) in a considerable proportion of within-network and between-network FC values (Fig. 4A). The post-hoc comparisons indicated that such effects were mainly driven by significant reductions of FCs in the late adult patients with MDD when compared to the adolescent and early-middle adult patients (Fig. 4B). Such results, therefore, may indicate an aging-related decline in brain-wide FCs which involved multiple brain systems.
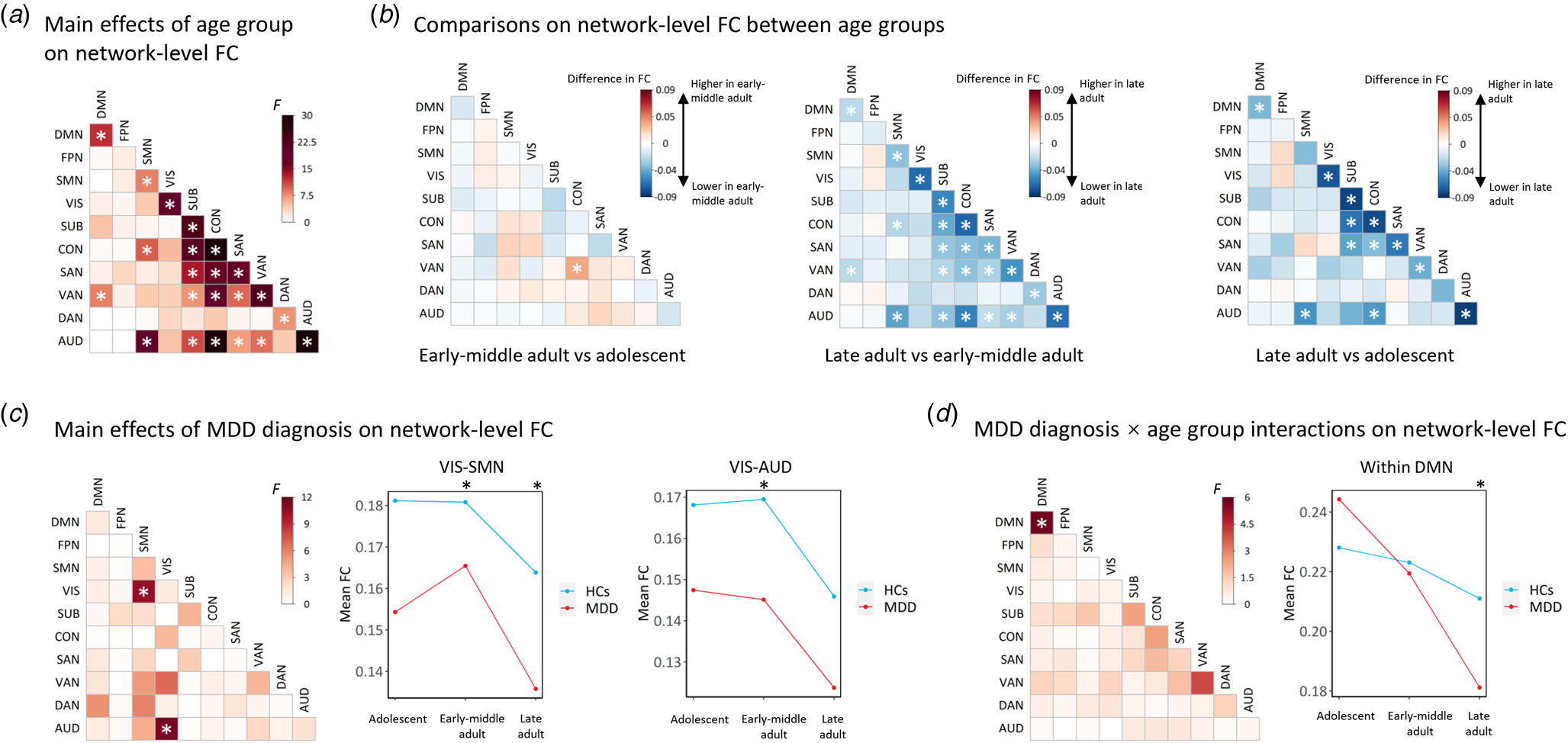
Figure 4. Results of the ANCOVA models pooling all participants on network-level FCs. (A) Results on main effects of age group. (B) Results on post-hoc comparisons between different age groups. (C) Results on main effects of MDD diagnosis. (D) Results on age group × MDD diagnosis interactions. The ‘*’ indicates significant effects with corrected p < 0.05. For network-level FCs showing significant main effects of MDD diagnosis, or significant age group × MDD diagnosis interactions, the interaction plots were also presented on the right sides. AUD, auditory network; CON, cinguloopercular network; DAN, dorsal attention network; DMN, default-mode network; FPN, frontoparietal network; HCs, healthy controls; MDD, major depressive disorder; SAN, salience network; SMN, sensorimotor network; SUB, subcortical network; VAN, ventral attention network; VIS, visual network.
The ANCOVA model also revealed significant main effects of MDD diagnosis (corrected p < 0.05) in FCs between the visual and sensorimotor networks, as well as between the visual and auditory networks (Fig. 4C). From the interaction plots, it can be seen that these FC values were consistently lower in MDD patients than HCs in all age groups (Fig. 4C). Thus, it might be concluded that the visual-sensorimotor and visual-auditory FCs are decreased in MDD patients independent of the age group.
Additionally, a significant age group × MDD diagnosis interaction was found on FC within the default-mode network (corrected p < 0.05); the interaction plots showed that the within-default-mode FCs were increased in adolescent patients, but decreased in early-middle adult and late adult patients with MDD in trends when compared to the HCs in each age groups (Fig. 4D).
Pooled analyses on edge-level FCs
Similar to the result on network-level FCs, the ANCOVA model indicated significant main effects of age group in a considerable proportion of the edges across the whole brain (corrected p < 0.05, see online Supplementary Figure S2). However, no significant main effects of MDD diagnosis or significant age group × MDD diagnosis interactions were found on the edge-level FCs (corrected p > 0.05).
Associations with clinical measures
No significant correlations were found between the brain network alterations and HAMD-17 total score/illness duration in MDD patients (all corrected p > 0.05). No significant differences in the brain network alterations were found between the FEDN and non-FEDN MDD patients, either (all corrected p > 0.05).
Discussion
In the present study, we investigated the functional brain network abnormalities in MDD patients across three major age groups using a large, multi-site sample. Overall, we found that there are both shared and distinct abnormal FC patterns across different age groups of patients. These findings might provide valuable information for improving our insight into the age-related heterogeneity in MDD from a perspective of brain dysfunctions.
The first important finding of this study is that all three age groups of MDD patients had similar decreased FCs involving the sensorimotor, visual, and auditory regions (Figs 2, 3), suggesting that MDD-related dysfunctions in these brain systems are common regardless of age stages. This was further supported by significant main effects of MDD diagnosis on the visual-sensorimotor and visual-auditory FCs in the ANCOVA analyses when pooling all participants (Fig. 4C). Multiple previous studies had observed decreased FCs within or between the sensory and motor networks in MDD (Chen et al., Reference Chen, Liu, Zuo, Xi, Long, Li and Yang2021; Lu et al., Reference Lu, Cui, Huang, Li, Duan, Chen and Chen2020; Zhuo et al., Reference Zhuo, Zhu, Wang, Qu, Ma and Qin2017). In a recent mega-analysis including 606 MDD patients aged 18 to 65 years, it was also found that hypoconnectivities (relatively lower FCs than HCs) within the somatosensory motor network, as well as between the somatosensory motor and visual networks were two of the significant findings in such a sample (Javaheripour et al., Reference Javaheripour, Li, Chand, Krug, Kircher, Dannlowski and Wagner2021). These alterations may be reflective of the impaired integration of sensory and motor processing that has been widely reported in MDD (Lu et al., Reference Lu, Cui, Huang, Li, Duan, Chen and Chen2020; Ray, Bezmaternykh, Mel'nikov, Friston, & Das, Reference Ray, Bezmaternykh, Mel'nikov, Friston and Das2021). Despite these findings, there is little knowledge about possible age effects on such MDD-related dysfunctions. To our knowledge, the present study might provide one of the first evidence that MDD-related reductions in sensory and motor FCs are shared across the lifespan from adolescence to late adulthood. Interestingly, in a recent meta-analysis on MDD-related brain structural changes, it was also shown that differences in the visual network are shared by both younger and older adults with MDD (Zhukovsky et al., Reference Zhukovsky, Anderson, Coughlan, Mulsant, Cipriani and Voineskos2021), which may partly support our hypothesis from a brain structural perspective.
Beyond the commonly shared features, some unique abnormalities in brain FC patterns were observed in each age group of MDD (Figs 2, 3). In particular, an increased sensorimotor-subcortical FC was found in the adolescent patients. Past studies suggested that brain development during adolescence is characterized by age-related increases in FCs between the subcortical and higher-order cognitive networks, but declines in FCs between the subcortical and sensory/motor regions (Sanders et al., Reference Sanders, Harms, Kandala, Marek, Somerville, Bookheimer and Barch2023). Thus, one possible explanation for our results is that age-related decrease in sensory/motor-subcortical FCs is postponed in adolescent patients, which support the delayed brain maturation hypothesis for adolescent MDD (Straub et al., Reference Straub, Brown, Malejko, Bonenberger, Grön, Plener and Abler2019; Whittle et al., Reference Whittle, Lichter, Dennison, Vijayakumar, Schwartz, Byrne and Allen2014). From the structural perspective, past studies have also found delayed subcortical volumetric changes in adolescent MDD and they were associated with symptoms of insomnia (Blank et al., Reference Blank, Meyer, Wieser, Rabl, Schögl and Pezawas2022; Whittle et al., Reference Whittle, Lichter, Dennison, Vijayakumar, Schwartz, Byrne and Allen2014). Therefore, these unique changes may partly account for the unique symptom profiles in adolescent MDD such as more common insomnia and other vegetative symptoms (Rice et al., Reference Rice, Riglin, Lomax, Souter, Potter, Smith and Thapar2019). Notably, the group of early-middle adult patients showed no similar increases in FCs between the sensory/motor and subcortical networks, but even a lower visual-subcortical FC than HCs. We therefore propose that MDD-related changes in some particular neural circuits, at least in the sensory/motor-subcortical connections, should be different between adolescent and early-middle adult stages.
As for late adult patients with MDD, it was observed that they uniquely exhibited wide FC reductions within multiple networks, especially within higher-order systems such as the default-mode, cingulo-opercular, and attention networks (Figs 2, 3). Several published reports have suggested that normal aging in later adulthood is accompanied by decreases in FCs within most brain networks (Park et al., Reference Park, Jung, Ryu, Oh, Kim, Choi and Shim2017; Staffaroni et al., Reference Staffaroni, Brown, Casaletto, Elahi, Deng, Neuhaus and Kramer2018; Zonneveld et al., Reference Zonneveld, Pruim, Bos, Vrooman, Muetzel, Hofman and Vernooij2019), which was also confirmed in our ANCOVA models (Figs 4A and 4B). Therefore, one possible explanation for our findings is that FCs within these networks may decrease more rapidly in the elderly MDD patient than in normal controls, which thus supports the hypothesis of an accelerated aging process in late adult MDD (Ballester et al., Reference Ballester, Romano, Azevedo Cardoso, Hassel, Strother, Kennedy and Frey2022; Jha et al., Reference Jha, Chin Fatt, Minhajuddin, Mayes and Trivedi2023; Tang et al., Reference Tang, Wu, Cao, Chen, Wu, Tan and Liu2022). Since the higher-order brain networks are known to be associated with advanced cognitive processes and cognitive changes in aging (Geerligs, Renken, Saliasi, Maurits, & Lorist, Reference Geerligs, Renken, Saliasi, Maurits and Lorist2015; Hardcastle et al., Reference Hardcastle, Hausman, Kraft, Albizu, Evangelista, Boutzoukas and Woods2022; Hausman et al., Reference Hausman, O'Shea, Kraft, Boutzoukas, Evangelista, Van Etten and Woods2020), such changes can also partly explain why MDD in late adulthood is more characterized by cognitive declines (Thomas et al., Reference Thomas, Gallagher, Robinson, Porter, Young, Ferrier and O'Brien2009). However, the observed larger-scale brain dysfunctions in older patients with MDD may be also partly due to potential influences of the longer average illness duration and longer exposure to antidepressant medication (Li et al., Reference Li, Su, Wu, Castellanos, Li, Li and Yan2021; Yan et al., Reference Yan, Chen, Li, Castellanos, Bai, Bo and Zang2019). Nevertheless, we failed to detect any significant relationships between the illness duration and brain network changes, or any significant differences between the FEDN and non-FEDN patients to support this possibility.
In the pooled ANCOVA analyses, a significant age group × MDD diagnosis interaction was found on FC within the default-mode network (Fig. 4D), a high-level brain system known to involve self-directed thoughts (Gandelman et al., Reference Gandelman, Albert, Boyd, Park, Riddle, Woodward and Taylor2019; Guan et al., Reference Guan, Amdanee, Liao, Zhou, Wu, Zhang and Zhang2022). In past studies, both decreased (Chen, Wang, Zhu, Tan, & Zhong, Reference Chen, Wang, Zhu, Tan and Zhong2015; Jacob et al., Reference Jacob, Morris, Huang, Schneider, Rutter, Verma and Balchandani2020; Yan et al., Reference Yan, Chen, Li, Castellanos, Bai, Bo and Zang2019) and increased (Alexopoulos et al., Reference Alexopoulos, Hoptman, Kanellopoulos, Murphy, Lim and Gunning2012; Scalabrini et al., Reference Scalabrini, Vai, Poletti, Damiani, Mucci, Colombo and Northoff2020) FCs within the default-mode network than HCs were ever reported in MDD. From the interaction plots (Fig. 4D), it can be seen that firstly, the FC within the default-mode network were decreased with increasing age in both MDD patients and HCs, but seemed to decrease more rapidly in the elderly patient than in elderly controls. Once again, this may support the hypothesis of an accelerated aging process in late adult MDD as mentioned earlier. Secondly, different age groups of MDD patients showed different trends of changes when compared to age-matched HCs: it was higher in adolescent patients (although not significant) but lower in early-middle-adult and late-adult patients. Thus, we propose that such heterogeneity may partly account for the inconsistencies in many previous studies, where MDD patients across different age stages were often estimated as a whole (Goldman et al., Reference Goldman, Sankar, Rich, Kim, Pittman, Constable and Blumberg2022; Hu et al., Reference Hu, Xiao, Ai, Wang, Chen, Tan and Kuang2019). This may again highlight the importance of considering age-related heterogeneity in rs-fMRI studies on MDD.
Our study has several limitations to note. First, due to the variations in data management practices across different study centers in the REST-meta-MDD consortium, information on illness duration and medication status for each MDD patient was not available in a part of the patients; furthermore, the exact number of prior depression episodes and medication dosage were not available in most non-FEDN participants, which limited our power to further explore the effects of antidepressant medication in non-FEDN patients. Second, the current study was carried out based on cross-sectional datasets, while future studies using longitudinal data may provide more valuable information about the effects of aging on MDD-related brain dysfunctions. Third, the rs-fMRI data from different study sites were scanned with different machines and different scan parameters; although we have controlled the site effects in all analyses, the results may still be partly influenced by them. Fourth, the depressive severity was assessed in all patients using the HAMD, which may be not optimal for the adolescent patients. It might be more accurate to use those scales specifically developed for adolescents, such as the Children's Depression Inventory or Children's Depression Rating Scale-Revised (Stallwood et al., Reference Stallwood, Monsour, Rodrigues, Monga, Terwee, Offringa and Butcher2021) in the adolescent group. Finally, while conventional analyses of static FCs were performed in the present study, some other brain network measures such as the small-world metrics (Yang et al., Reference Yang, Chen, Chen, Li, Li, Castellanos and Yan2021) and dynamic FCs (Long et al., Reference Long, Cao, Yan, Chen, Li, Castellanos and Liu2020) may provide further important information and can be investigated in future research.
In conclusion, using a large multi-center dataset, this study explored the similar and different features of functional brain network abnormalities in MDD across different age groups. Our results suggested that decreased FCs among the sensory and motor networks may be common biomarkers for MDD shared across all adolescent, early-middle adult, and late adult patients. Some unique abnormalities in brain FC patterns were also observed in each age group of MDD, e.g. increased sensorimotor-subcortical FC in adolescent patients, decreased visual-subcortical FC in early-middle adult patients, as well as wide FC reductions within the subcortical, default-mode, cingulo-opercular, and attention networks in late adult patients. These findings may provide valuable information to expand our understanding of the age-related heterogeneity in MDD.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291723002234.
Acknowledgements
This work was supported by the STI2030-Major Projects (2022ZD0212900 to XZ), the National Natural Science Foundation of China (82271565 to XZ, 82201692 to YL, 82071506 to ZL), and the Natural Science Foundation of Hunan Province, China (2021JJ40851 to YL). The data of this study were partly provided by the members of the REST-meta-MDD Consortium.
Author's contributions
Y. L., X. L., X. O. and X. Z. contributed to the conception and design of the study. Y. L., X. L., Z. L., X. O., Y. H., M. L. and X. Z. contributed to the data acquisition. Y. L., X. L., H. C., M. Z., B. L., X. O. and X. Z. contributed to the analysis and interpretation of data. Y. L. and X. L. drafted the manuscript. H. C., B. L., M. X., J. S., X. O. and X. Z. revised the manuscript. All authors read and approved the final manuscript.
Competing interest
None.








