Introduction
Unnatural causes of death due to traffic accidents (TA) or suicide attempt (SA) constitute a major burden on global health. According to the World Health Organisation (WHO) about 1.4 million people die each year as a result of road injuries, rendering it among the 10 leading causes of death globally for years (Naghavi et al., Reference Naghavi, Abajobir and Abbafati2017). Between 20 and 50 million more people suffer from non-fatal road accidents, with many incurring a disability as a result of their injury. According to the WHO, TA are among the most important problems with regard to social, economic and human health issues (Naghavi et al., Reference Naghavi, Abajobir and Abbafati2017). Likewise, suicide also has a huge impact on society, with 0.8 million people dying annually due to self-harm (Naghavi et al., Reference Naghavi, Abajobir and Abbafati2017). For every fatal SA, 10–20 persons are estimated to attempt suicide, with approximately 10 million people attempting suicide worldwide annually (Turecki and Brent, Reference Turecki and Brent2016).
Both these causes of unnatural death are considered to be multifactorial, with psychological, social and biological factors involved (Turecki and Brent, Reference Turecki and Brent2016; Lutz et al., Reference Lutz, Mechawar and Turecki2017). Resources are directed at addressing modifiable factors in policymaking (Perron et al., Reference Perron, Burrows, Fournier, Perron and Ouellet2013; Goniewicz et al., Reference Goniewicz, Goniewicz, Pawlowski and Fiedor2016; Herbert et al., Reference Herbert, Gilbert, Cottrell and Li2017) (i.e. making more difficult to jump of bridges, increasing road safety, preventing drug abuse) and healthcare (Goniewicz et al., Reference Goniewicz, Goniewicz, Pawlowski and Fiedor2016; Turecki and Brent, Reference Turecki and Brent2016; Gilissen et al., Reference Gilissen, De Beurs, Mokkenstorm, Merelle, Donker, Terpstra, Derijck and Franx2017) (improved immediate care for people involved in a car crash, lowering barriers for people with suicidal ideation to receive help) in order to diminish health burden. These interventions showed regional successes although without affecting global mortality rates in the last decade (Naghavi et al., Reference Naghavi, Abajobir and Abbafati2017). Recently, a hitherto unsuspected, but potentially modifiable biological factor has received increasing attention with its association to both SA and TA: the latent infection with Toxoplasma gondii (T. gondii) (Flegr et al., Reference Flegr, Klose, Novotna, Berenreitterova and Havlicek2009; Pedersen et al., Reference Pedersen, Mortensen, Norgaard-Pedersen and Postolache2012).
T. gondii is an intracellular and neurotropic parasite affecting most of the warm-blooded animals including humans. In humans, infection is acquired by ingestion of contaminated water or food that contains tissue cysts. It is estimated that approximately 30% of the global population is infected with the parasite, with large regional variances (Montoya and Liesenfeld, Reference Montoya and Liesenfeld2004). The parasite has a complex life cycle, whereby it needs to end up in the intestine of felines (cats) in order to complete its lifecycle (Montoya and Liesenfeld, Reference Montoya and Liesenfeld2004). Interestingly, non-feline mammals show cognitive and behavioural changes that increase their risk of being caught by felines (Berdoy et al., Reference Berdoy, Webster and Macdonald2000; Webster, Reference Webster2007), which are considered evolutionary adaptations of the parasite facilitating its survival. There is evidence that the parasite accomplishes this behavioural change by influencing neurotransmitters in the brain (Flegr, Reference Flegr2013; Parlog et al., Reference Parlog, Schluter and Dunay2015). Studies have shown that the parasite is able to increase dopamine release in infected neurons (Prandovszky et al., Reference Prandovszky, Gaskell, Martin, Dubey, Webster and McConkey2011) and potentially influences the kynurenine pathway, which could influence glutamate signalling (Notarangelo et al., Reference Notarangelo, Wilson, Horning, Thomas, Harris, Fang, Hunter and Schwarcz2014).
Since these findings emerged, research focussed increasingly on possible cognitive and behavioural changes by a latent T. gondii infection in humans. Indeed, neurocognitive changes have been reported in people with a latent T. gondii infection, whereby increased impulsiveness has been reported in people with T. gondii infection, although results so far are heterogeneous (Dickerson et al., Reference Dickerson, Stallings, Origoni, Katsafanas, Schweinfurth, Savage, Khushalani and Yolken2014; Gale et al., Reference Gale, Brown, Erickson, Berrett and Hedges2015; Sugden et al., Reference Sugden, Moffitt, Pinto, Poulton, Williams and Caspi2016; Peng et al., Reference Peng, Brenner, Mathai, Cook, Fuchs, Postolache, Groer, Pandey, Mohyuddin, Giegling, Wadhawan, Hartmann, Konte, Brundin, Friedl, Stiller, Lowry, Rujescu and Postolache2018). Nonetheless, meta-analyses did show overall a significant association of exposure to T. gondii with several psychiatric disorders, especially schizophrenia, suggesting that the infection could impact human behaviour as well (Sutterland et al., Reference Sutterland, Fond, Kuin, Koeter, Lutter, van Gool, Yolken, Szoke, Leboyer and de Haan2015).
The objective of the study was to determine if T. gondii infection is indeed associated with SA and/or TA by conducting a systematic review and meta-analysis.
Methods
We followed the systematic review guidelines provided by the PRISMA statement (Moher et al., Reference Moher, Liberati, Tetzlaff and Altman2009). We conducted a systematic search (see online Supplementary Appendix 1) throughout Medline, PsychInfo and EMBASE until 11 February 2019 (Prospero #CRD42018090206).
Inclusion criteria were: (i) original research papers with comparable quantitative data; (ii) any language; (iii) analysis of latent T. gondii infection by measuring IgG antibodies using one of the following diagnostic assays: Sabin–Feldman dye test, complement fixation, immune haemagglutination, immune fluorescence or enzyme-linked immunosorbent assay (ELISA); (iv) case–control or cohort studies with human subjects; (v) data on SA and/or TA. Exclusion criteria were: (i) studies without a control group, (ii) case reports or case series and (iii) studies with immunocompromised patients. Additional studies were sought in references to all reviews on this topic. Screening of search results by manuscript titles and abstracts were performed by three researchers (AS, BK, AK). Final screening by reading the whole manuscript was performed to validate inclusion (AS, AK). The corresponding authors were asked to provide additional data if not included in the original publications and for unpublished results.
All selected articles were screened on study quality independently by two researchers (AS, GF) following Cochrane criteria of quality on case–control or cohort studies (Higgins et al., Reference Higgins, Altman and Sterne2011). If there was a difference in quality score, the difference was discussed. If consensus could not be reached, a third opinion was asked (LdH) for a final decision.
Some studies included only subjects which had committed SA as cases (e.g. cases presented at the emergency department due to a SA) and collected healthy controls as a control population in order to compare antibody levels against T. gondii. In contrast, other studies included subjects with certain psychiatric disorder(s) cross-sectionally and defined caseness as having a history of SA(s) and control population as subjects not having this history. In order to evaluate whether this difference in sampling method mattered, we stratified studies based on the type of control population used.
Meta-analysis of eligible studies
For all studies an odds ratio (OR) was calculated. ORs adjusted for confounders were used if available. The random-effects model was applied for all analyses. Heterogeneity was assessed by eye-balling and calculating I 2. Meta-analytical calculations were carried out with Comprehensive Meta-analysis Software 3.0 (Borenstein et al., Reference Borenstein, Hedges, Higgins and Rothstein2013).
Effect of potential moderators on heterogeneity was assessed if possible (study quality, mean age, sex, seroprevalence of the control population, fatality, type of control population, diagnosis of cases and timing of outcome measurement: prospective or retrospective). Whether studies had a prospective design was determined based on the fulfilment of one of the following two criteria: (a) the study was designed as a longitudinal cohort study in which the assessment of seropositivity to T. gondii preceded the outcome (TA or SA) or (b) in a cross-sectional study the outcome (TA or SA) had occurred at or shortly before the measurement of IgG antibodies against T. gondii (since these antibodies are indicative of a latent infection and emerge months after primary infection). Considering the diagnosis of cases, specifically for SA, studies were grouped in studies that had included only cases with schizophrenia (schizophrenia only) compared with studies that had included other or any psychiatric disorder (various psychiatric disorders).
Where applicable meta-regression analysis (for continuous data) or subgroup analysis (for categorical data) were performed with the methods of moments and the mixed-effects model, respectively (Borenstein et al., Reference Borenstein, Hedges, Higgins and Rothstein2009).
To assess by which proportion moderators influenced the variance of the true effect, regression analyses were performed investigating the moderator as a covariate. The amount of variance was expressed by R 2. Due to the number of included studies, moderator effects were analysed individually (Borenstein et al., Reference Borenstein, Hedges, Higgins and Rothstein2009).
For seropositivity definition, cut-off scores used for the assays in the respective studies were used. If multiple assays were used in a study, we utilized the results of the assay that showed the smallest effect size.
Higher average antibody titres (compared with controls) have been called serointensity, whereby preferably numbers on high v. low, but still positive, antibody titres were used to see if this influenced the magnitude of the OR. If these were not available, average titres in groups were used and converted into an OR.
The potential for publication bias was assessed by examination of funnel plots and by the Egger's test (which was considered significant if the one-sided p value was <0.10). If applicable, Duval and Tweedie's trim and fill method was used for a better estimation of the true OR.
Since the global incidence of TA and SA are both low (0.3–0.7% per year and 0.14% per year, respectively), OR becomes identical to the risk ratio (RR) (Zhang and Yu, Reference Zhang and Yu1998). As the current meta-analysis provides an estimation of ORs and the global prevalence (Pglob) of a latent T. gondii is estimated to be 30% (Montoya and Liesenfeld, Reference Montoya and Liesenfeld2004), the population attributable fraction (PAF) can be calculated. The formula PAF = [Pglob × (RR − 1)]/[Pglob × (RR − 1) + 1] was applied (Levin, Reference Levin1953).
Results
The systematic search rendered in a total of 715 studies. After duplicate removal, 636 studies remained. After title and abstract screening for inclusion criteria, 51 studies were selected for full-text screening. Ten studies were added after reference screening of reviews. After full-text screening, 24 studies were finally selected for quantitative synthesis, with a total of 4229 cases and 12 234 controls in TA and 2259 cases and 9400 controls in SA (see Fig. 1 for flowchart and Table 1 for study characteristics).

Fig. 1. Flowchart of the study selection process.
Table 1a. (a) Study characteristics of studies reporting T. gondii infection and traffic accidents
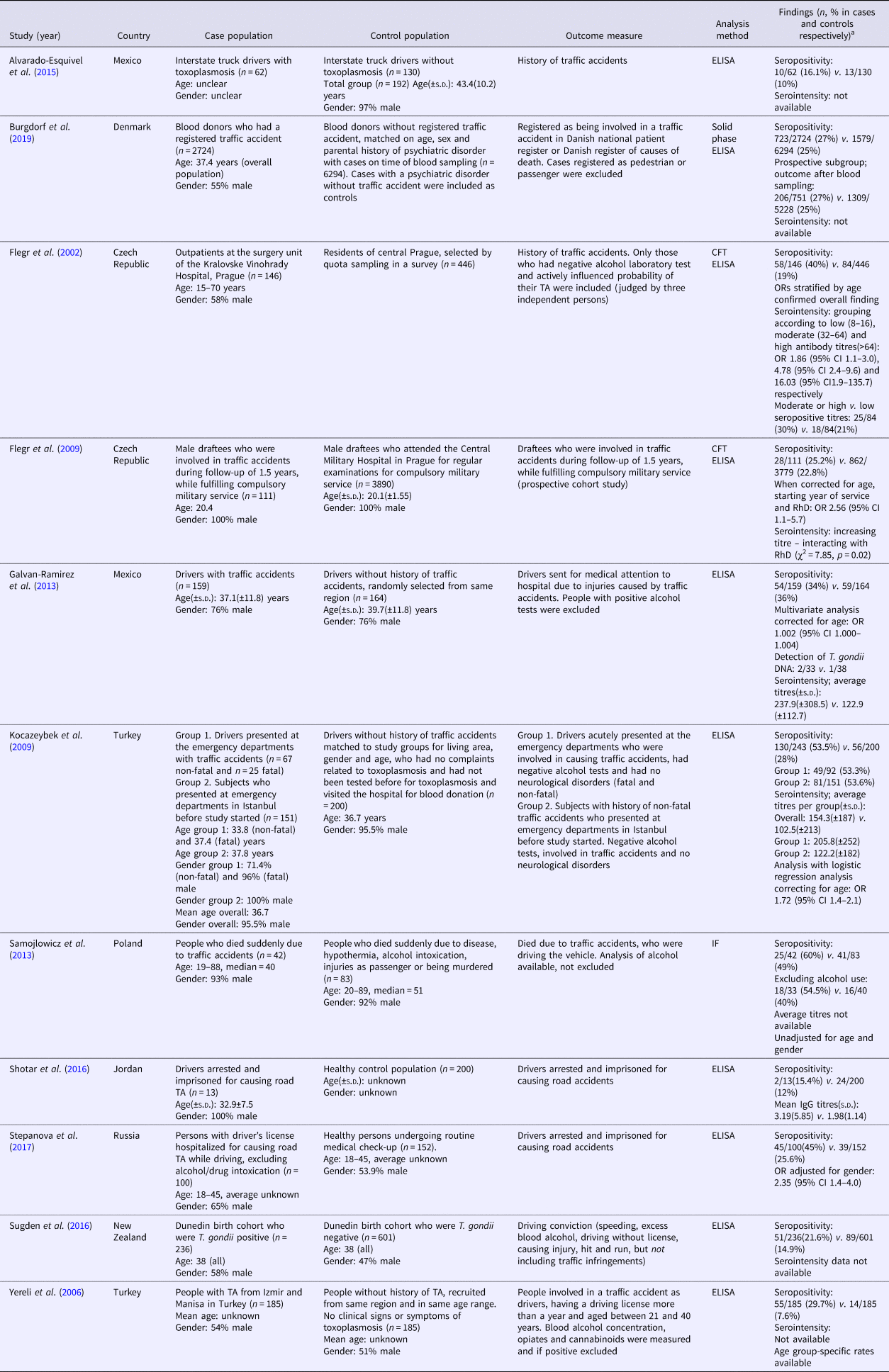
ELISA, enzyme linked immunoassays; CFT, complement fixation test; IF, indirect fluorescence test.
a Data of cases are presented first, control population second.
Table 1b. (b) Study characteristics of studies reporting T. gondii infection and suicide attempts
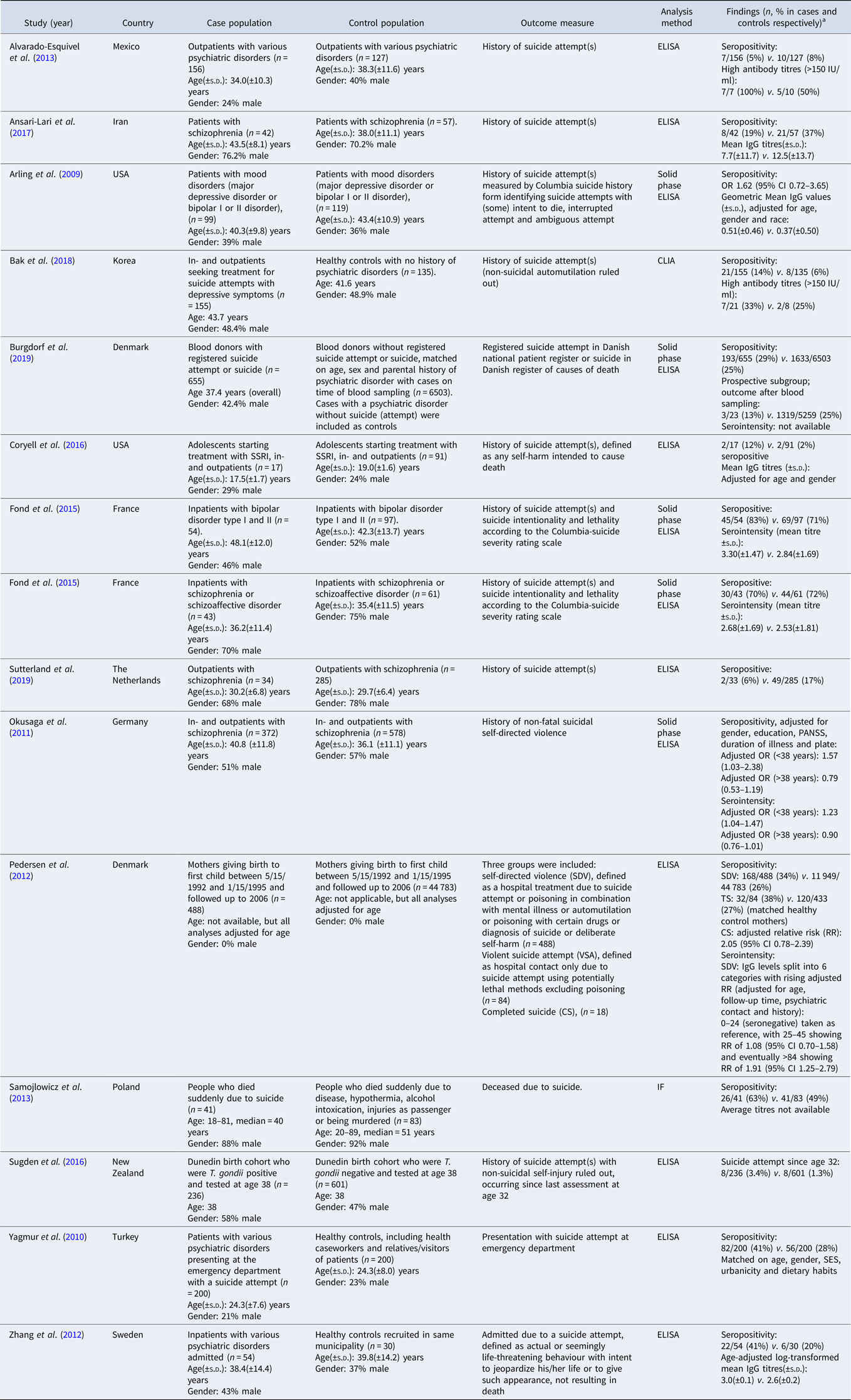
ELISA, enzyme linked immunoassays; CFT, complement fixation test; IF, indirect fluorescence test; CLIA, chemiluminescent immunoassay.
a Data of cases are presented first, control population second.
Traffic accidents
In total, 11 studies comparing seropositivity to T. gondii with the risk of being involved in a TA were identified (Flegr et al., Reference Flegr, Havlicek, Kodym, Maly and Smahel2002; Yereli et al., Reference Yereli, Balcioglu and Ozbilgin2006; Flegr et al., Reference Flegr, Klose, Novotna, Berenreitterova and Havlicek2009; Kocazeybek et al., Reference Kocazeybek, Oner, Turksoy, Babur, Cakan, Sahip, Unal, Ozaslan, Kilic, Saribas, Aslan, Taylan, Koc, Dirican, Uner, Oz, Ertekin, Kucukbasmaci and Torun2009; Galvan-Ramirez et al., Reference Galvan-Ramirez, Sanchez-Orozco, Rodriguez, Rodriguez, Roig-Melo, Troyo Sanroman, Chiquete and Armendariz-Borunda2013; Samojlowicz et al., Reference Samojlowicz, Borowska-Solonynko and Golab2013; Alvarado-Esquivel et al., Reference Alvarado-Esquivel, Pacheco-Vega, Hernandez-Tinoco, Salcedo-Jaquez, Sanchez-Anguiano, Berumen-Segovia, Rabago-Sanchez and Liesenfeld2015; Shotar et al., Reference Shotar, Alzyoud and Alkhatib2016; Sugden et al., Reference Sugden, Moffitt, Pinto, Poulton, Williams and Caspi2016; Stepanova et al., Reference Stepanova, Kondrashin, Sergiev, Morozova, Turbabina, Maksimova, Brazhnikov, Shevchenko and Morozov2017; Burgdorf et al., Reference Burgdorf, Trabjerg, Pedersen, Nissen, Banasik, Pedersen, Sorensen, Nielsen, Larsen, Erikstrup, Bruun-Rasmussen, Westergaard, Thorner, Hjalgrim, Paarup, Brunak, Pedersen, Torrey, Werge, Mortensen, Yolken and Ullum2019), showing an overall OR of 1.69 (95% CI 1.20–2.38, p = 0.003), see Fig. 2. The Egger's test did indicate the presence of publication bias (p = 0.06), however the Duval and Tweedie's trim and fill rendered the same OR using the random-effects model if missing studies were searched left from the mean. Also, the funnel plot using the random-effects model did not indicate publication bias (online Supplementary Appendix 2). Heterogeneity was considerable and significant (I 2 = 86%, p < 0.0001, τ 2 = 0.247).
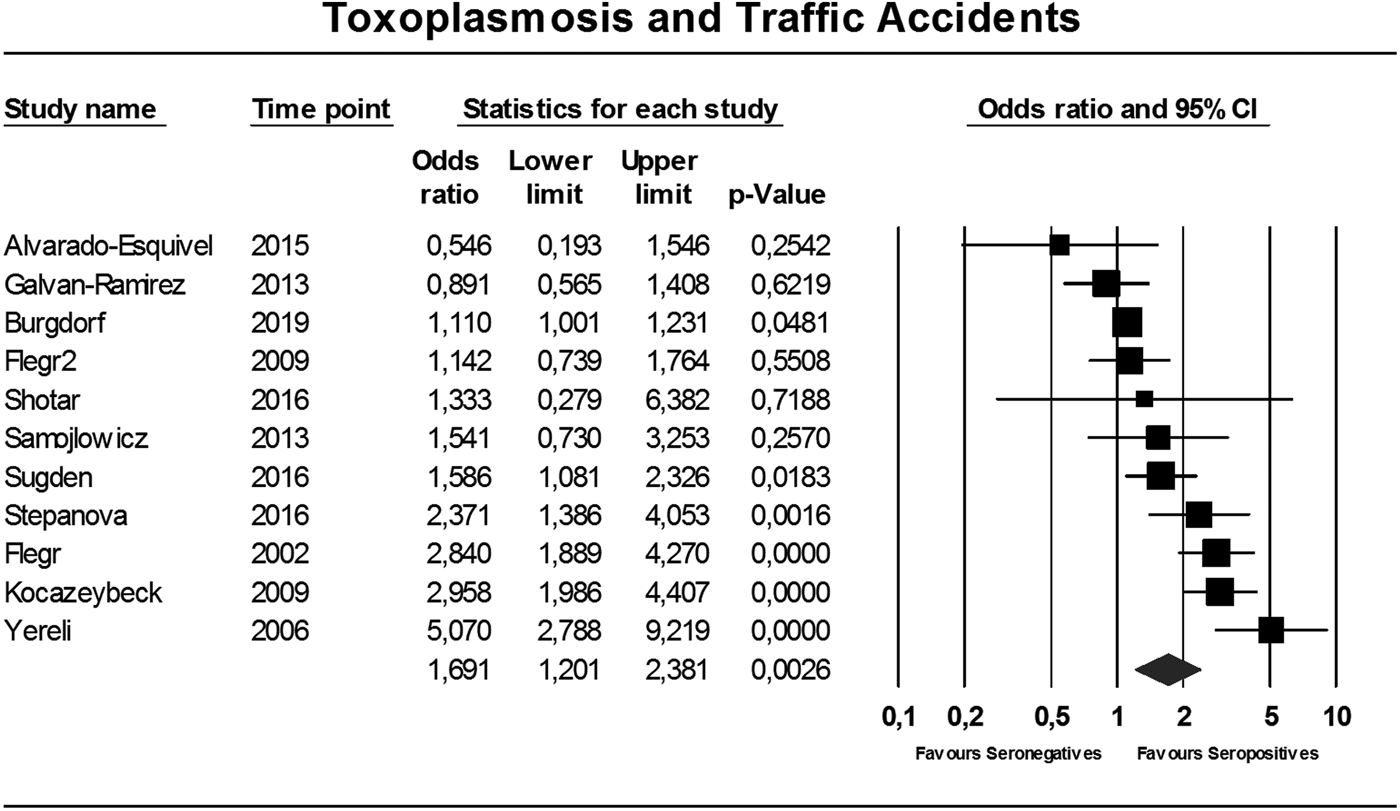
Fig. 2. Forest plot toxoplasmosis and traffic, accidents using the random-effects model.
To explore heterogeneity, moderators were assessed for their impact on the between-study variance. No effect of overall study quality on OR was found. Noteworthy, when quality of the definition of cases in TA was considered separately, the OR was significant in studies using a more concise description of TA (excluding alcohol use, making sure the driver was responsible for the accident), as opposed to those with broader inclusion criteria. Importantly, studies selectively assessing fatal TA as well as studies measuring antibodies against T. gondii occurring directly after or before the occurrence of TA (representing prospective study designs, as IgG antibodies against T. gondii represents a latent infection) both showed significant associations (Table 2a).
Table 2a. (a) Moderator assessment in association traffic accidents with toxoplasmosis
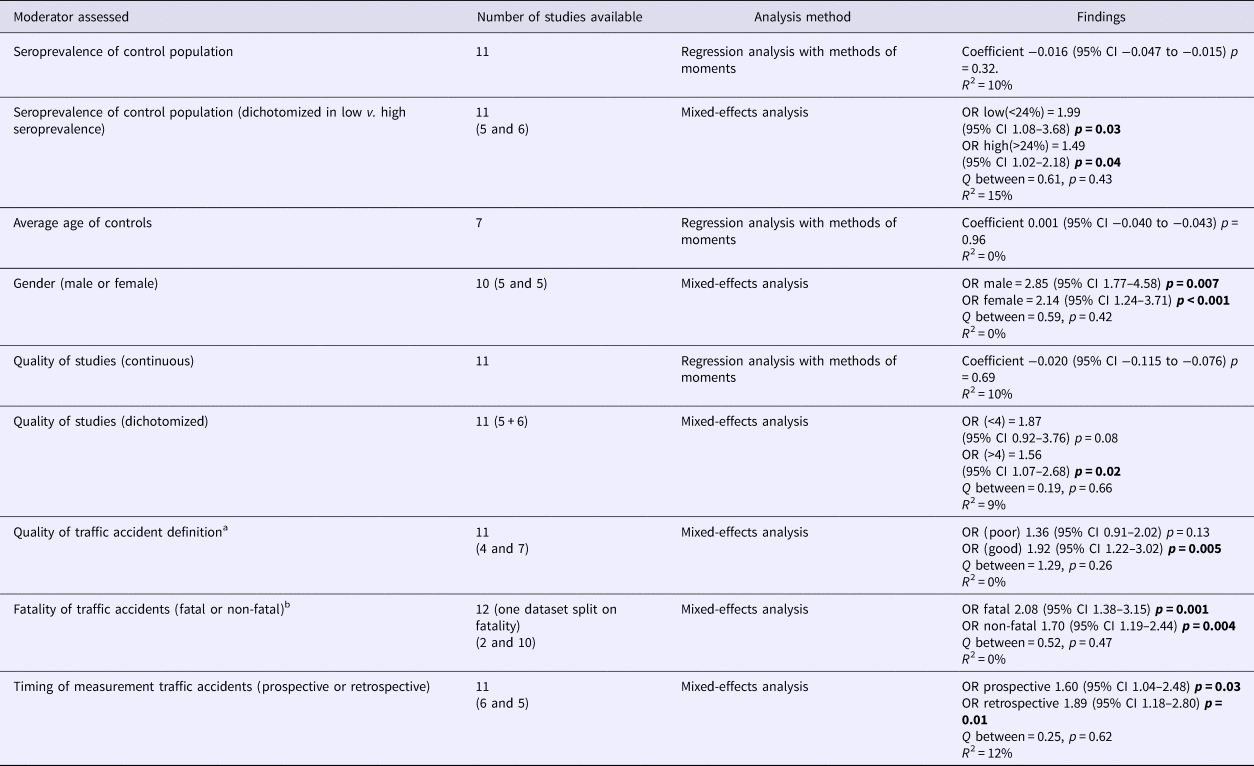
p-values < 0.05 are highlighted in bold.
a Good definition of being a case suffering a traffic accident in a study is assuring the case is the driver, which has (most probably) caused the traffic accident, excluding people who had drunk alcohol and/or used drugs.
b Studies reporting both fatal and non-fatal were excluded when separate data were not available.
b For this moderator prospective data only were analysed for the study of Burgdorf et al.
Table 2b. (b) Moderator assessment in association suicide attempts with toxoplasmosis
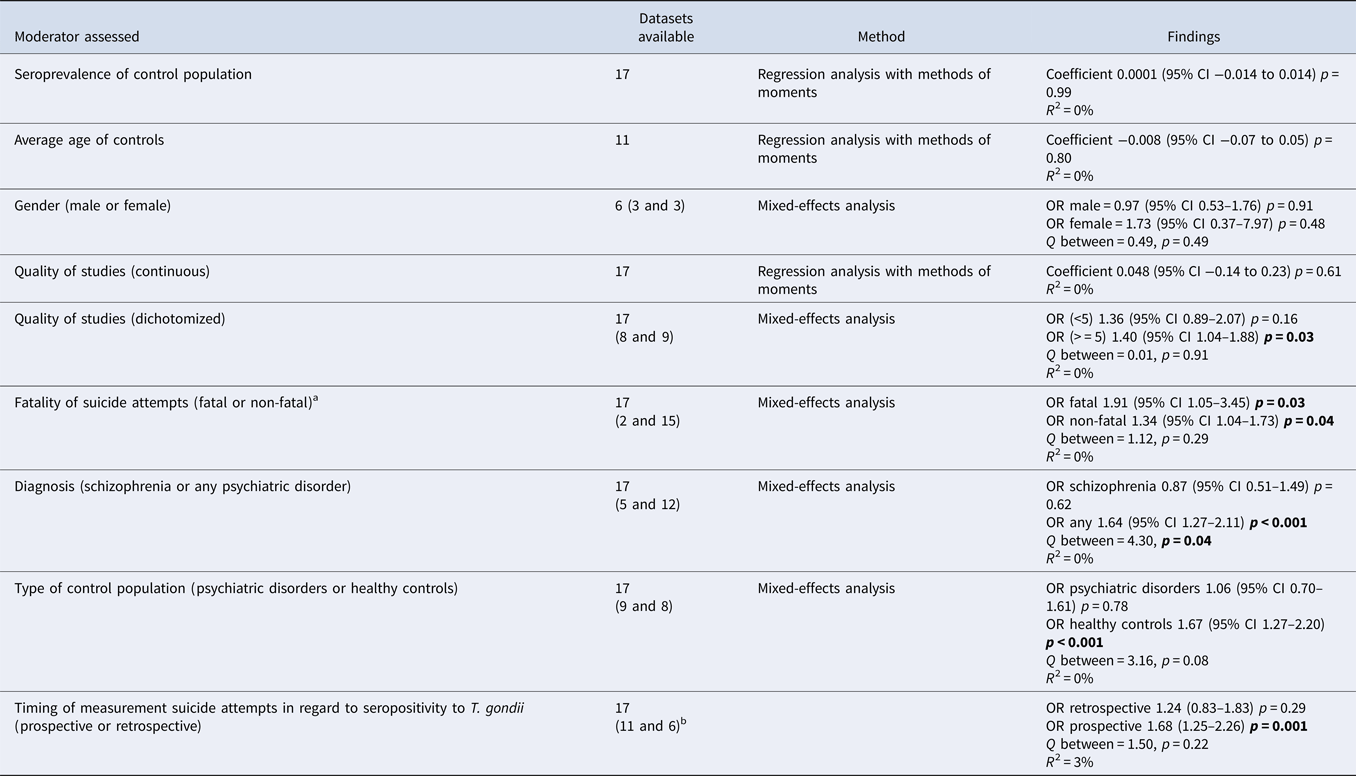
p-values < 0.05 are highlighted in bold.
a Studies reporting both fatal and non-fatal suicide attempts were excluded when separate data were not available. To measure the OR for fatal suicide attempts in the study of Pedersen et al., the adjusted relative risk had to be used.
b For this moderator prospective data only were analysed for the study of Burgdorf et al.
None of the moderators had a significant impact on the variance of the overall effect.
Serointensity and TA
In total, five studies reported serointensity (Flegr et al., Reference Flegr, Havlicek, Kodym, Maly and Smahel2002; Flegr et al., Reference Flegr, Klose, Novotna, Berenreitterova and Havlicek2009; Kocazeybek et al., Reference Kocazeybek, Oner, Turksoy, Babur, Cakan, Sahip, Unal, Ozaslan, Kilic, Saribas, Aslan, Taylan, Koc, Dirican, Uner, Oz, Ertekin, Kucukbasmaci and Torun2009; Galvan-Ramirez et al., Reference Galvan-Ramirez, Sanchez-Orozco, Rodriguez, Rodriguez, Roig-Melo, Troyo Sanroman, Chiquete and Armendariz-Borunda2013; Shotar et al., Reference Shotar, Alzyoud and Alkhatib2016). Overall, the odds for TA were increased in cases with high titres compared with low titres; OR 1.75 (95% CI 1.28–2.38; p < 0.001). Significant heterogeneity was present (I 2 = 65%, τ 0.299), for which no explanation was found with available moderators (seroprevalence, age, quality, study design).
SA or suicide
Thirteen studies studying the association between SA and latent T. gondii infection and one unpublished study were identified (Arling et al., Reference Arling, Yolken, Lapidus, Langenberg, Dickerson, Zimmerman, Balis, Cabassa, Scrandis, Tonelli and Postolache2009; Yagmur et al., Reference Yagmur, Yazar, Temel and Cavusoglu2010; Okusaga et al., Reference Okusaga, Langenberg, Sleemi, Vaswani, Giegling, Hartmann, Konte, Friedl, Groer, Yolken, Rujescu and Postolache2011; Pedersen et al., Reference Pedersen, Mortensen, Norgaard-Pedersen and Postolache2012; Zhang et al., Reference Zhang, Traskman-Bendz, Janelidze, Langenberg, Saleh, Constantine, Okusaga, Bay-Richter, Brundin and Postolache2012; Alvarado-Esquivel et al., Reference Alvarado-Esquivel, Sanchez-Anguiano, Arnaud-Gil, Lopez-Longoria, Molina-Espinoza, Estrada-Martinez, Liesenfeld, Hernandez-Tinoco, Sifuentes-Alvarez and Salas-Martinez2013; Samojlowicz et al., Reference Samojlowicz, Borowska-Solonynko and Golab2013; Fond et al., Reference Fond, Boyer, Gaman, Laouamri, Attiba, Richard, Delavest, Houenou, Le Corvoisier, Charron, Krishnamoorthy, Oliveira, Tamouza, Yolken, Dickerson, Leboyer and Hamdani2015; Coryell et al., Reference Coryell, Yolken, Butcher, Burns, Dindo, Schlechte and Calarge2016; Sugden et al., Reference Sugden, Moffitt, Pinto, Poulton, Williams and Caspi2016; Ansari-Lari et al., Reference Ansari-Lari, Farashbandi and Mohammadi2017; Bak et al., Reference Bak, Shim, Kwon, Lee, Kim, Yoon and Lee2018; Burgdorf et al., Reference Burgdorf, Trabjerg, Pedersen, Nissen, Banasik, Pedersen, Sorensen, Nielsen, Larsen, Erikstrup, Bruun-Rasmussen, Westergaard, Thorner, Hjalgrim, Paarup, Brunak, Pedersen, Torrey, Werge, Mortensen, Yolken and Ullum2019; Sutterland et al., Reference Sutterland, Kuiper, Ribbens, Mounir, Van Gool and de Haan2019), with an overall OR of 1.39 (95% CI 1.10–1.76, p = 0006), see Fig. 3. One of the studies provided data on suicide deaths, violent SA as well as self-directed violence (Pedersen et al., Reference Pedersen, Mortensen, Norgaard-Pedersen and Postolache2012). Subjects with self-directed violence were excluded from the analysis. The Egger's test did not indicate the presence of publication bias (p = 0.15) and Duval and Tweedie's trim and fill analysis using the random-effects model rendered a similar and significant OR. There was evidence of heterogeneity (I 2 = 55%, p = 0.003, τ 2 = 0.103).

Fig. 3. Forest plot toxoplasmosis and suicide attempts using the random effects model.
To explore heterogeneity, moderators were assessed for their impact on the between-study variance. Two moderators had a significant impact on the between-study variance: the proportion of patients with schizophrenia (schizophrenia only v. various psychiatric disorders) [ORschizophrenia = 0.87 (95% CI 0.51–1.49), p = 0.62 v. ORvarious = 1.8 (95% CI 1.44–2.24), p < 0.001] and whether the control population consisted of healthy controls or patients with psychiatric disorders [ORhealthy controls = 1.9 (95% CI 1.48–2.44), p < 0.001 v. ORpsychiatric controls = 1.06 (95% CI 0.70–1.61), p = 0.78] (Table 2b). In order to determine whether the effect of one moderator could drive the other, the effect of diagnosis within studies that used controls with psychiatric disorders was assessed post-hoc in studies with various psychiatric disorders as control subjects (n = 4) compared with only schizophrenia as control subjects (n = 5). A non-significant larger association was found [ORvarious = 1.49 (95% CI 0.71–3.15), p = 0.29 v. ORschizophrenia = 0.87 (95% CI 0.51–1.49), p = 0.62; Q-between 1.32, p = 0.25, R 2 = 0%]. It was not possible to investigate the reverse, since all studies investigating SA in subjects with schizophrenia did not use healthy controls as a comparison.
Importantly, studies that selectively assessed SA within a prospective design as well as fatal SA showed significant associations with T. gondii infection (Table 2b).
Even though the type of diagnosis of case population or type of control population explained a (trend-)significant amount of between-study variance, the estimated proportion of between-study variance was 0% for both moderators as expressed by R 2.
Serointensity and SA
Eight studies reported serointensity (Arling et al., Reference Arling, Yolken, Lapidus, Langenberg, Dickerson, Zimmerman, Balis, Cabassa, Scrandis, Tonelli and Postolache2009; Okusaga et al., Reference Okusaga, Langenberg, Sleemi, Vaswani, Giegling, Hartmann, Konte, Friedl, Groer, Yolken, Rujescu and Postolache2011; Pedersen et al., Reference Pedersen, Mortensen, Norgaard-Pedersen and Postolache2012; Zhang et al., Reference Zhang, Traskman-Bendz, Janelidze, Langenberg, Saleh, Constantine, Okusaga, Bay-Richter, Brundin and Postolache2012; Alvarado-Esquivel et al., Reference Alvarado-Esquivel, Sanchez-Anguiano, Arnaud-Gil, Lopez-Longoria, Molina-Espinoza, Estrada-Martinez, Liesenfeld, Hernandez-Tinoco, Sifuentes-Alvarez and Salas-Martinez2013; Fond et al., Reference Fond, Boyer, Gaman, Laouamri, Attiba, Richard, Delavest, Houenou, Le Corvoisier, Charron, Krishnamoorthy, Oliveira, Tamouza, Yolken, Dickerson, Leboyer and Hamdani2015; Ansari-Lari et al., Reference Ansari-Lari, Farashbandi and Mohammadi2017; Bak et al., Reference Bak, Shim, Kwon, Lee, Kim, Yoon and Lee2018), rendering an overall OR of 1.22 (95% CI 0.96–1.55, p = 0,11). There was no indication of publication bias (Egger's test p = 0.17). The heterogeneity of the observed effects between studies was high (I 2 = 62%, p = 0.004, τ = 0.252). When exploring possible reasons for the heterogeneity, there was a significant effect of studies focussing on subjects with schizophrenia (n = 5) v. various other psychiatric disorders (n = 5): OR 0.99 (95% CI 0.77–1.29) v. OR 1.66 (95% CI 1.29–2.12, p < 0.001), R 2 = 61%, p = 0.004.
Population attributable fraction
Assuming an average infection rate of T. gondii of 30% in humans globally, the calculated PAF, [0.3 × (OR − 1)]/[0.3 × (OR − 1) + 1], showed that if T. gondii infection would theoretically be completely prevented, the casualties due to TA would decrease with approximately 17% (95% CI 6–29%) and due to SA with 10% (95% CI 3–19%).
Discussion
Overall we found significant associations between a T. gondii infection and both SA and TA. Importantly, the associations did not seem to be influenced by publication bias. If the associations would be explained by the causal hypothesis, i.e. that the T. gondii infection influences the risk of committing a SA or suffering a TA, this would imply that a latent T. gondii infection would lead to much more morbidity and mortality than hitherto assumed. Taking into account the calculated PAFs, T. gondii infection would emerge as a major public health concern, comparable with global meningitis mortality, with estimated 318 000 annual deaths potentially due to the behavioural effects of this infection (Naghavi et al., Reference Naghavi, Abajobir and Abbafati2017). Therefore, it is paramount to evaluate whether the results of the current meta-analysis and the available literature to date indeed provide enough support for the plausibility of a causal relationship and whether alternative explanations for these associations could be given.
When assessing whether a factor (here a T. gondii infection) could be causal to a certain condition, Koch's postulates of causality can be used as a theoretical framework (Antonelli and Cutler, Reference Antonelli and Cutler2016). The postulates that need to be addressed in order for a factor to be considered causal to a certain condition are: consistency of the relationship, a temporal relationship (the factor preceding the emergence of the condition), a gradient in the relationship (more exposure to the factor leads to a higher risk of getting the condition), experimental proof of the relationship and a plausible biological mechanism.
The consistency of the relationship is addressed with the current meta-analysis. Overall the associations were significant, although the amount of studies was modest and heterogeneity high. In TA the Egger's test indicated the presence of publication bias. Nevertheless, statistical adjustment for possible bias by the Duval and Tweedie's trim and fill analysis did not change the overall finding. This is probably due to the different underlying mathematical method these tests use. The Egger's test relies on the fixed-effects model to see whether the sample size influences overall effect, whereas the Duval and Tweedie's trim and fill analysis can be applied to both the fixed- and random-effects model (Borenstein et al., Reference Borenstein, Hedges, Higgins and Rothstein2009). This implies that the Egger's test relies more heavily on sample size, whereby the large Danish cohort study that found a small but significant association of T. gondii with TA has a very large weight (>70%) relative to the other studies (all 5% or less) (Burgdorf et al., Reference Burgdorf, Trabjerg, Pedersen, Nissen, Banasik, Pedersen, Sorensen, Nielsen, Larsen, Erikstrup, Bruun-Rasmussen, Westergaard, Thorner, Hjalgrim, Paarup, Brunak, Pedersen, Torrey, Werge, Mortensen, Yolken and Ullum2019). When funnel plots were examined using both the fixed- and random-effects model, it became clear that with the random-effects model (which gives relatively less weight to large studies than the fixed-effects model) there does not seem to be a publication bias (see online Supplementary Appendix 2). Additionally, even when the fixed-effects model was used for statistical correction, the OR remained significantly increased albeit smaller (data available upon request). In our view, despite the significant Egger's test, the data still indicate a relationship between T. gondii and TA, although the magnitude is less certain. When exploring heterogeneity in TA, assessable moderators explained a negligible and non-significant amount of the observed variance. In favour of the consistency of the relationship with TA was that the OR seemed to be stronger when studies were grouped which had carefully selected subjects with a higher chance to have influenced their own risk of becoming involved in TA (not intoxicated, having caused the accident as a driver).
In SA there was no indication of publication bias, but (trend-)significant effects on heterogeneity were found among studies that included subjects with schizophrenia only and those that used healthy subjects as control population. A secondary analysis seemed to indicate that selection of schizophrenia cases is particularly important, whereby the association of T. gondii infection with SA was absent in cases with schizophrenia. This might be due to the fact that T. gondii infection is a risk factor for schizophrenia in itself (Sutterland et al., Reference Sutterland, Fond, Kuin, Koeter, Lutter, van Gool, Yolken, Szoke, Leboyer and de Haan2015) or that the infection has little effect on suicidality in schizophrenia specifically, where other factors may overshadow such a relationship. For example, Toxoplasma infection has been associated with dopamine modulation in brain neurons (Prandovszky et al., Reference Prandovszky, Gaskell, Martin, Dubey, Webster and McConkey2011), while dopamine disturbances are generally recognized abnormalities described in the brain of most subjects with schizophrenia whether or not they are infected with T. gondii (Howes et al., Reference Howes, McCutcheon, Owen and Murray2017). Nonetheless, the association of T. gondii infection with SA being partly explained by using healthy subjects as controls remains a concern, suggesting the higher prevalence of antibodies against T. gondii in several psychiatric disorders might drive the association. On one hand this possible confounding seems less likely as the large cohort study of Pedersen et al. was able to control for psychiatric disorders in the population and still showed a significant association between T. gondii infection and suicidal behaviour (Pedersen et al., Reference Pedersen, Mortensen, Norgaard-Pedersen and Postolache2012), but on the other hand the study of Burgdorf et al. found a small but non-significant effect, whereby they used a different method to control for psychiatric disorders in the population (Burgdorf et al., Reference Burgdorf, Trabjerg, Pedersen, Nissen, Banasik, Pedersen, Sorensen, Nielsen, Larsen, Erikstrup, Bruun-Rasmussen, Westergaard, Thorner, Hjalgrim, Paarup, Brunak, Pedersen, Torrey, Werge, Mortensen, Yolken and Ullum2019). Future studies should keep trying to disentangle these confounding factors and assess other potential factors influencing heterogeneity.
One of those candidates is Rhesus factor positivity, which has been suggested as relevant (Flegr et al., Reference Flegr, Klose, Novotna, Berenreitterova and Havlicek2009; Flegr et al., Reference Flegr, Preiss and Klose2013). For example, cognitive dysfunction was only found in subjects with a latent T. gondii infection which were Rhesus negative. Why the Rhesus factor could protect humans against deletorial effects of T. gondii is unclear. Another candidate to explore is the strain hypothesis, which states that strains of T. gondii differ in virulence and in ability to influence human behaviour (Xiao et al., Reference Xiao, Buka, Cannon, Suzuki, Viscidi, Torrey and Yolken2009). This strain hypothesis is supported by the finding of Xiao et al., that the association of T. gondii infection with affective psychosis was only found in a subgroup who were infected with strain type I and not in those infected with type II or other types (Xiao et al., Reference Xiao, Buka, Cannon, Suzuki, Viscidi, Torrey and Yolken2009). Since then, one other study has found a similar effect with strain type I in humans (Groer et al., Reference Groer, Yolken, Xiao, Beckstead, Fuchs, Mohapatra, Seyfang and Postolache2011).
The postulate of a temporal relationship seemed plausible as studies with a prospective design rendered similar findings indicating the infection preceded the outcome. Additionally, the serointensity analyses showed that significant increases in the ORs were found with higher antibody titres for TA and for SA, the latter within studies that did not include schizophrenia cases only. This could imply that the severity of the infection plays a role, indicating a gradient in the relationship. Nevertheless, it is much less clear if Toxoplasma prevalence influences the numbers of SA and TA. One study has suggested that a relationship between national T. gondii prevalence and suicide incidence in European countries exists (Lester, Reference Lester2010). On the other hand, suicide numbers have varied considerably the last decades, while it has been suggested that T. gondii prevalence steadily decreased in the same time period (Jones et al., Reference Jones, Kruszon-Moran, Rivera, Price and Wilkins2014; Gargate et al., Reference Gargate, Ferreira, Vilares, Martins, Cardoso, Silva, Nunes and Gomes2016; Dyvesether et al., Reference Dyvesether, Nordentoft, Forman and Erlangsen2018; Huikari et al., Reference Huikari, Miettunen and Korhonen2019). This could be explained by other factors that have a larger impact on the occurrence of suicide than Toxoplasmosis (which has an estimated PAF of 10%), such as, for instance, the unemployment rates (Huikari et al., Reference Huikari, Miettunen and Korhonen2019). Concerning TA, no study to date has examined if regional T. gondii prevalence is related to TA numbers.
The experimental proof for a relationship is challenging with the studied outcomes. Animal models have been examined, which could be applicable to these outcomes. Several behavioural changes after infection with T. gondii in mice and rats have been documented, including increased reaction time, slower neural processing speed, decreased attention span and increased risk-taking behaviour (Webster, Reference Webster2007; Daniels et al., Reference Daniels, Sestito and Rouse2015; Tan et al., Reference Tan, Soh, Lim, Daniel, Zhang and Vyas2015). These factors can increase the risk of both TA and SA. However, the methodology underlying these findings has been questioned (Worth et al., Reference Worth, Andrew Thompson and Lymbery2014). Human studies have indicated cognitive abnormalities concurring with T. gondii infection, increasing the risk of suffering from TA (Guenter et al., Reference Guenter, Bielinski, Deptula, Zalas-Wiecek, Piskunowicz, Szwed, Bucinski, Gospodarek and Borkowska2012; Pearce et al., Reference Pearce, Hubbard, Rivera, Wilkins, Fisch, Hopkins, Hasenkamp, Gross, Bliwise, Jones and Duncan2013; Pearce et al., Reference Pearce, Kruszon-Moran and Jones2014). However, these findings were not always replicated (Gale et al., Reference Gale, Brown, Erickson, Berrett and Hedges2015; Sugden et al., Reference Sugden, Moffitt, Pinto, Poulton, Williams and Caspi2016).
Finally, a plausible biological mechanism should be available to explain how a latent T. gondii infection could lead to an increased risk of causing TA and SA. Several biological mechanisms have been postulated. First of all, as tryptophan is essential for T. gondii replication, increased tryptophan breakdown by activating the kynurenine pathway is a major line of defence for the host against T. gondii infection, leading to increased kynurenine (KYN) and quinolinic acid (QUIN) levels (Miller et al., Reference Miller, Boulter, Ikin and Smith2009). Increased KYN as well as QUIN levels, including in CSF and post-mortem, have been associated with suicidal behaviour (Sublette et al., Reference Sublette, Galfalvy, Fuchs, Lapidus, Grunebaum, Oquendo, Mann and Postolache2011; Steiner et al., Reference Steiner, Bogerts, Sarnyai, Walter, Gos, Bernstein and Myint2012; Erhardt et al., Reference Erhardt, Lim, Linderholm, Janelidze, Lindqvist, Samuelsson, Lundberg, Postolache, Traskman-Bendz, Guillemin and Brundin2013). Okusaga et al. found that the risk of SA was increased in cases with both seropositivity to T. gondii and high KYN levels (Okusaga et al., Reference Okusaga, Duncan, Langenberg, Brundin, Fuchs, Groer, Giegling, Stearns-Yoder, Hartmann, Konte, Friedl, Brenner, Lowry, Rujescu and Postolache2016). QUIN is an N-methyl-D-aspartate (NMDA) receptor agonist and considered to be neurotoxic, whereby recent evidence indicates that ketamine, an NMDA receptor antagonist can acutely reduce suicidality (DiazGranados et al., Reference DiazGranados, Ibrahim, Brutsche, Ameli, Henter, Luckenbaugh, Machado-Vieira and Zarate2010). Secondly, it has been demonstrated that a T. gondii infection can increase dopamine levels both in vitro and in mice (Skallova et al., Reference Skallova, Kodym, Frynta and Flegr2006; Prandovszky et al., Reference Prandovszky, Gaskell, Martin, Dubey, Webster and McConkey2011). The genome of T. gondii is able to express the enzyme tyrosine hydroxylase in infected cells, which is the rate-limiting enzyme in dopamine synthesis (Prandovszky et al., Reference Prandovszky, Gaskell, Martin, Dubey, Webster and McConkey2011). Hyperdopaminergic states, together with NMDA agonism, can lead to increased arousal, neurotoxicity and impulsivity (Barake et al., Reference Barake, Evins, Stoeckel, Pachas, Nachtigall, Miller, Biller, Tritos and Klibanski2014).
Although the suggested biological mechanisms may be plausible, studies confirming the role of one of these mechanisms in humans are challenging and remain scarce (Okusaga et al., Reference Okusaga, Duncan, Langenberg, Brundin, Fuchs, Groer, Giegling, Stearns-Yoder, Hartmann, Konte, Friedl, Brenner, Lowry, Rujescu and Postolache2016).
Alternative explanations for an association between T. gondii infection and both SA and TA should be explored as well. As it is known that felines are the definite host of the parasite, households with cats are more prone to get infected by T. gondii. While we are unaware of studies showing that families who prefer cats as pets are also more prone to psychiatric illness, SA or TA, this reverse causality cannot be ruled out. Conversely, evidence that households with cats have an increased risk of developing schizophrenia has been published (Torrey et al., Reference Torrey, Simmons and Yolken2015), although these findings have been challenged (Solmi et al., Reference Solmi, Hayes, Lewis and Kirkbride2017).
When elaborating further on factors that could give rise to a so-called spurious association (i.e. toxoplasmosis and TA or SA are not directly related to each other but both rise or fall due to another factor), it should be noted that households with low incomes are known to be at a higher risk of being involved in a TA or committing a SA (Qin et al., Reference Qin, Agerbo and Mortensen2003; Ghaffar et al., Reference Ghaffar, Hyder, Govender and Bishai2004; Hawton and van Heeringen, Reference Hawton and van Heeringen2009). There is an indication that this could also be applicable to the risk of contracting a latent T. gondii infection (Mareze et al., Reference Mareze, Benitez, Brandao, Pinto-Ferreira, Miura, Martins, Caldart, Biondo, Freire, Mitsuka-Bregano and Navarro2019). Studies included in our meta-analysis did not adjust for socio-economic status, which would be important to examine in future studies.
Furthermore, it is possible that the biological susceptibility of humans to contract a latent T. gondii infection, for example, through differences in the immune defence system or reactivity of the kynurenine pathway, also influences the risk of TA and SA. In SA, many studies have shown changes in cytokine profiles in cases compared with controls (Gananca et al., Reference Gananca, Oquendo, Tyrka, Cisneros-Trujillo, Mann and Sublette2016), but in TA this has not been examined as far as we are aware. More studies with a prospective design focussing on biological susceptibility are needed to shed further light on this matter.
Strengths and limitations
One of the strengths of the meta-analysis is the extensive sensitivity analysis addressing heterogeneity, after retrieval of additional data from several authors. This showed variation in the association of SA with T. gondii infection, due to several factors, which can guide the field in further research into this topic. It was also able to demonstrate that the severity of T. gondii infection further increased the association with SA and TA and that the infection precedes these adverse outcomes. One of the limitations, however, is that the amount of studies was still relatively modest, leaving some uncertainty about the robustness of the finding and limiting options for sensitivity analyses with multiple moderators at once. Another limitation was that it was not possible to use individual patient data.
Conclusion
Overall, the findings of this meta-analysis in conjunction with the available literature provide substantial support for the hypothesis that a latent T. gondii infection may play an important role in the risk of TA and SA. However, although there is some support for the interpretation that the association is causal, causality is not proven. Nevertheless, the additional impact that toxoplasmosis potentially has on health [besides the well-known consequences in immunocompromised patients and de novo infection during pregnancies and the possible mental health consequences (Montoya and Liesenfeld, Reference Montoya and Liesenfeld2004)] validates the question what the gain could be for devoting more public health resources into this issue. Further research concerning the prevention of primary infections on a larger scale should be considered. It is presumed that transmission occurs most often through ingestion of intact oocysts containing the parasite, which can be contracted by eating raw or undercooked meat, changing cat litter, by not washing hands after gardening, eating unwashed vegetables or fruit or by drinking contaminated water (Montoya and Liesenfeld, Reference Montoya and Liesenfeld2004). It would be good to increase the knowledge about the most common route of transmission in humans and try to prevent this. Alternatively, resources could be directed into developing a vaccine for humans or cats. Secondly, little is known how to diminish or prevent deleterious consequences by an acquired latent infection. Targeting specific high-risk populations, such as traffic offenders or people who have committed a SA could be an option to investigate these questions. Finally, possible candidates that could be responsible for the heterogeneous findings (rhesus factor positivity, differences in strain virulence, type of case and control population) should be explored further.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291719000813
Author ORCIDs
Arjen L. Sutterland, 0000-0001-9206-2492
Acknowledgements
We would like to thank Joost Daams, clinical librarian, for help on developing the search strategy. We would also like to thank the authors who provided additional data on their studies for this meta-analysis: Cosme Alvarado-Esquivel, Karen Sugden, Bouke Kuiper, Marianne Pedersen and Guillaume Fond.
Financial support
Not applicable.









