Introduction
The effects of neuromodulation on psychiatric problems have been investigated for a long time (Woods et al., Reference Woods, Antal, Bikson, Boggio, Brunoni, Celnik and Nitsche2016). For classifying the techniques of neuromodulation, various concepts can be considered: the distinct forms of energy [electrical and magnetic, with repetitive transcranial magnetic stimulation (rTMS) belonging to the latter], whether surgical implantation of the stimulator is needed [deep brain stimulation (DBS) and vagus nerve stimulation (VNS) both need an invasive operation] and whether selectivity of brain regions exists [electroconvulsive therapy (ECT) is an example of non-selective stimulation] (Lewis, Thomson, Rosenfeld, & Fitzgerald, Reference Lewis, Thomson, Rosenfeld and Fitzgerald2016). ECT is an effective option for treatment-refractory psychiatric conditions such as schizophrenia, mania and depression; DBS was investigated more for neurological disorders than psychiatric disorders; VNS was considered to be a treatment option for depression (Ali, Mathur, Malhotra, & Braga, Reference Ali, Mathur, Malhotra and Braga2019; Bottomley, LeReun, Diamantopoulos, Mitchell, & Gaynes, Reference Bottomley, LeReun, Diamantopoulos, Mitchell and Gaynes2019; Sharma, Sengupta, Chitnis, & Amara, Reference Sharma, Sengupta, Chitnis and Amara2018). rTMS was found to have a good effect on treatment-resistant depression but its cost is usually high (Dell'Osso, Priori, & Altamura, Reference Dell'Osso, Priori and Altamura2011; De Risio et al., Reference De Risio, Borgi, Pettorruso, Miuli, Ottomana, Sociali and Zoratto2020; Li, Cui, Li, Liu, & Chen, Reference Li, Cui, Li, Liu and Chen2021; Zhao et al., Reference Zhao, Tor, Khoo, Teng, Lim and Mok2018). Beyond the above options, there are still several types of non-invasive electrical stimulation; these therapies are usually less expensive and have relatively high accessibility, thus becoming the favored choices for those patients who do not want to receive pharmacotherapy (Dell'Osso et al., Reference Dell'Osso, Priori and Altamura2011; Sauvaget et al., Reference Sauvaget, Tostivint, Etcheverrigaray, Pichot, Dert, Schirr-Bonnais and Riche2019). In this article, we have named this group of therapies ‘non-invasive, non-convulsive electrical neuromodulation (NINCEN)’.
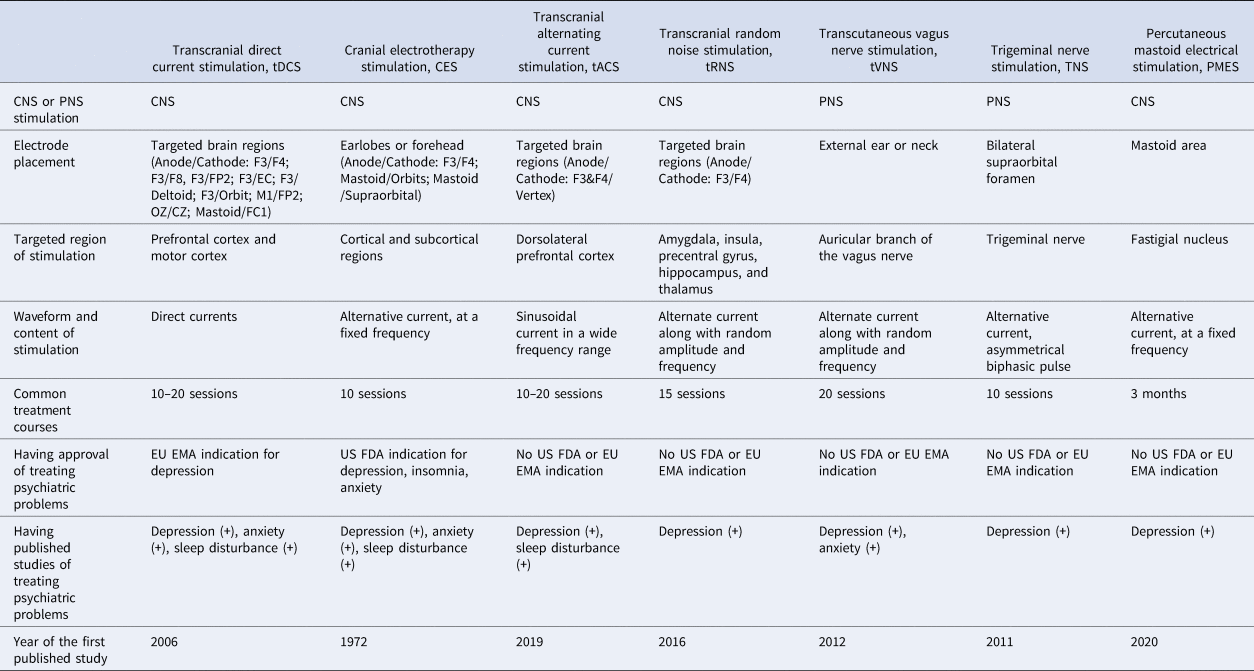
The different types of NINCEN in the literature are listed in Table 1. These therapies can be briefly classified according to the regions of stimuli and the waveforms (Guleyupoglu, Schestatsky, Edwards, Fregni, & Bikson, Reference Guleyupoglu, Schestatsky, Edwards, Fregni and Bikson2013). Among them, transcranial direct current stimulation (tDCS) and cerebral electrotherapy stimulation (CES) are the most frequently mentioned in the public and academic field. CES was developed several decades ago; its form of stimulus is an alternative current of fixed frequency and the electrodes are often placed on the earlobe or forehead (Kavirajan, Lueck, & Chuang, Reference Kavirajan, Lueck and Chuang2014). The form of stimulus for tDCS is a direct current; electrodes can be put in several regions but the dorsolateral prefrontal cortex (DLPFC) is the most common target brain region in the literature (Mehrsafar, Rosa, Zadeh, & Gazerani, Reference Mehrsafar, Rosa, Zadeh and Gazerani2020; Wolkenstein, Zeiller, Kanske, & Plewnia, Reference Wolkenstein, Zeiller, Kanske and Plewnia2014). Among other treatment options, transcranial alternating current stimulation, transcranial random noise stimulation (these two treatments are similar to tDCS in their current ranges and electrode placement but their waveforms are distinct) and percutaneous mastoid electrical stimulation are focused on the stimulation of the central nervous system with different waveforms; transcutaneous VNS and trigeminal nerve stimulation are techniques that stimulate the peripheral nervous system (the targeted areas of these two treatments are the cranial nerves) (Generoso et al., Reference Generoso, Taiar, Garrocini, Bernardon, Cordeiro, Uchida and Shiozawa2019; Guleyupoglu et al., Reference Guleyupoglu, Schestatsky, Edwards, Fregni and Bikson2013; Hein et al., Reference Hein, Nowak, Kiess, Biermann, Bayerlein, Kornhuber and Kraus2013; Lu et al., Reference Lu, He, Zhang, Wang, Zhang, Dong and Yang2020; Reed & Cohen Kadosh, Reference Reed and Cohen Kadosh2018; Shiozawa, da Silva, Netto, Taiar, & Cordeiro, Reference Shiozawa, da Silva, Netto, Taiar and Cordeiro2015). The effects of these therapies have been widely investigated on depression, anxiety and sleep disturbance in the psychiatric field (Kosari, Dadashi, Maghbouli, & Mostafavi, Reference Kosari, Dadashi, Maghbouli and Mostafavi2019; Lin et al., Reference Lin, Chang, Huang, Tzeng, Kao and Chang2021; Morriss, Xydopoulos, Craven, Price, & Fordham, Reference Morriss, Xydopoulos, Craven, Price and Fordham2019; Taremian, Nazari, Moradveisi, & Moloodi, Reference Taremian, Nazari, Moradveisi and Moloodi2019; Wagenseil, Garcia, Suvorov, Fietze, & Penzel, Reference Wagenseil, Garcia, Suvorov, Fietze and Penzel2018; Yennurajalingam et al., Reference Yennurajalingam, Kang, Hwu, Padhye, Masino, Dibaj and Bruera2018; Zanardi et al., Reference Zanardi, Poletti, Prestifilippo, Attanasio, Barbini and Colombo2020; Zhou et al., Reference Zhou, Yu, Yu, Zhang, Liu, Hu and Zhou2020). Depression and anxiety are highly comorbid psychiatric conditions; furthermore, sleep disturbance is one of the common presentations of depression/anxiety. Therefore, these conditions may have shared mechanisms and thus could benefit from similar treatment options such as NINCEN.
Table 1. Comparison of different types of electrical neuromodulation

CNS, central nervous system; PNS, peripheral nervous system; EU EMA, European Union European Medicines Agency; US FDA, United States Food and Drug Administration.
In recent decades, large and well-designed randomized controlled trials on NINCEN have emerged, the most common being those adopting tDCS, followed by CES (Blumberger, Tran, Fitzgerald, Hoy, & Daskalakis, Reference Blumberger, Tran, Fitzgerald, Hoy and Daskalakis2012; Brunoni et al., Reference Brunoni, Boggio, De Raedt, Bensenor, Lotufo, Namur and Vanderhasselt2014; Loo et al., Reference Loo, Husain, McDonald, Aaronson, O'Reardon and Alonzo2018; Padberg et al., Reference Padberg, Kumpf, Mansmann, Palm, Plewnia, Langguth and Bajbouj2017; Palm et al., Reference Palm, Schiller, Fintescu, Obermeier, Keeser, Reisinger and Padberg2012; Sharafi, Taghva, Arbabi, Dadarkhah, & Ghaderi, Reference Sharafi, Taghva, Arbabi, Dadarkhah and Ghaderi2019). Most of these studies revealed that NINCEN is safe but the efficacies in distinct studies were discrepant (Barclay & Barclay, Reference Barclay and Barclay2014; Chan et al., Reference Chan, Alonzo, Martin, Mitchell, Sachdev and Loo2013; Loo et al., Reference Loo, Sachdev, Martin, Pigot, Alonzo, Malhi and Mitchell2010; Padberg et al., Reference Padberg, Kumpf, Mansmann, Palm, Plewnia, Langguth and Bajbouj2017; Sampaio-Junior et al., Reference Sampaio-Junior, Tortella, Borrione, Moffa, Machado-Vieira, Cretaz and Brunoni2018). There have been meta-analyses with larger sample sizes to support the efficacy of CES and tDCS on depression (Price, Briley, Haltiwanger, & Hitching, Reference Price, Briley, Haltiwanger and Hitching2021; Zhang et al., Reference Zhang, Lam, Peng, Zhang, Zhang, Huang and Lee2021). These meta-analyses provided important clinical insights but we noticed several unsolved issues. First, these meta-analyses are focused on the treatment of depression; data on anxiety and sleep disturbance were not included (Price et al., Reference Price, Briley, Haltiwanger and Hitching2021; Zhang et al., Reference Zhang, Lam, Peng, Zhang, Zhang, Huang and Lee2021). Second, NINCEN studies are heterogeneous in their included subjects and treatment parameters. For example, when recruiting patients with mild depression and patients with treatment-resistant depression, the clinical meaning is quite distinct (Li et al., Reference Li, Du, Chu, Liao, Pan, Li and Hung2019b; Meron, Hedger, Garner, & Baldwin, Reference Meron, Hedger, Garner and Baldwin2015; Mutz, Edgcumbe, Brunoni, & Fu, Reference Mutz, Edgcumbe, Brunoni and Fu2018). Therefore, we consider that the influence of patients' subpopulations, measurements and treatment settings should be managed with subgroup analyses for better interpretation of the results. Furthermore, the above concepts are ‘across different techniques’, so the integration of data on different types of NINCEN becomes possible (Guleyupoglu et al., Reference Guleyupoglu, Schestatsky, Edwards, Fregni and Bikson2013; Reed & Cohen Kadosh, Reference Reed and Cohen Kadosh2018). We believe that adding the above analyses could provide more understanding of the clinical meanings of NINCEN.
The present meta-analysis was designed based on the above background information. All types of NINCEN were included in this study. Depression, anxiety and sleep disturbance are continuous problems in clinical and non-clinical populations, so the two populations were both included. There are several major aims in this analysis: to analyze the effects of tDCS and CES on depression, anxiety and sleep disturbance and to investigate the effects of all types of NINCEN on these psychiatric problems; and to estimate the influence of demographic data and treatment parameters on the analytical results using meta-regression.
Methods
Data sources and search strategy
This systematic review and meta-analysis was prepared according to the PRISMA statement guidelines (Moher, Liberati, Tetzlaff, & Altman, Reference Moher, Liberati, Tetzlaff and Altman2009). We conducted an electronic literature search of PubMed, Embase, PsycInfo, PsycArticles and CINAHL from the earliest available date of their inception to March 2021 and used the search string presented in the online Supplementary material. The literature search was conducted by three researchers independently (Y-C Cheng, M-I Su and W-L Huang). All titles meeting the inclusion criteria were retrieved and reviewed in full text. Original studies investigating the effect of NINCEN on depression, anxiety and sleep disturbance were eligible for review. Additional eligible studies were sought by searching the reference lists from primary articles and relevant reviews to identify any further studies that were not found with the electronic search. The protocol for this study was registered with PROSPERO (ID No. CRD42021227132).
Inclusion and exclusion criteria
We aimed to determine the effects of NINCEN on symptoms of depression, anxiety and sleep in all clinical and non-clinical populations. Eligible studies were those in which: (1) human randomized clinical trials used various types of NINCEN and intended to measure the mean changes of mood and sleep symptoms at baseline and at the end of the intervention; (2) active NINCEN and sham stimulation were conducted in two parallel groups; and (3) sufficient data were provided for obtaining the mean and standard deviation (s.d.).
Data extraction and quality assessment
Two investigators (Y-C Cheng and M-I Su) independently extracted relevant information from the included studies and evaluated the methodological quality of eligible trials using the Cochrane Collaboration assessment tool to assess the risk of bias. Any discrepancies were resolved by consensus with a third investigator (W-L Huang).
The following data on studies were obtained: the last name of the first author, publication year, study population, type of NINCEN device, concomitant psychotropic agent (including antidepressants, benzodiazepines, anticonvulsants) use or not, number of participants receiving active and sham stimulation, age, number of females, electrode position, electrode size, stimulation parameter and outcome measurement; some data were further analyzed using subgroup analyses or meta-regressions.
Efficacy outcomes
Our primary outcome was the change in depressive symptoms before and after active and sham treatment using any clinically validated rating scale. The means and standard deviations of changes from baseline were extracted. For depressive symptoms measured by more than one standardized rating scale, we used a predefined hierarchy. When the measurement was reported at multiple time points, we only extracted the data from the baseline and at the longest time point. When different stimulation parameters of the same NINCEN were used within a trial, a weighted average of the change and a pooled estimate of the variance were used to summarize the data. Because the efficacy of NINCEN on clinical and non-clinical populations may be different, we separated the subjects' severity of depression into several groups: mild depression, moderate depression (both based on cutoffs of the questionnaires) and major depressive disorder (based on diagnostic criteria) (Apaydin et al., Reference Apaydin, Maher, Shanman, Booth, Miles, Sorbero and Hempel2016). This issue was then managed in the subgroup analyses.
Secondary outcomes included anxiety (measured by the mean change of anxiety scale), sleep (measured by the mean change of sleep measurement), a response rate of depressive symptoms (estimated as the proportion of patients who achieved a reduction of 50% or more in the depression rating score) and depression remission rate (measured by the proportion of patients who had a depression score under the remission cut-off). For the studies in which relevant data were missing, the study authors were contacted to request the necessary information. The analyses regarding depression, anxiety and sleep disturbance were performed separately.
Statistical analysis
We estimated the relative treatment effects of the competing interventions by using standardized mean differences (Hedges' g) for continuous outcomes and the odds ratio (OR) for dichotomous outcomes, along with 95% confidence intervals. Hedges' g was also calculated for the post-intervention score change between the active and sham groups. A positive effect size indicated superior effects of the intervention v. the sham groups. Heterogeneity was performed using the I 2 test (Higgins & Thompson, Reference Higgins and Thompson2002). A random-effects model was used to show that the true effect size could vary among studies and thus offer more generalizable results. Publication bias was examined using a funnel plot and also Egger's regression test (Egger, Davey Smith, Schneider, & Minder, Reference Egger, Davey Smith, Schneider and Minder1997). Subgroup analysis was stratified based on the severity of depression, specific depression measurements and stimulation parameters (current, electrode placement, number of treatment sessions). Leave-one-out sensitivity analysis was performed by the sequential exclusion of one trial at a time to examine whether the pooled effects remained robust. We also performed meta-regressions across the study to estimate the effects of some continuous variables on the results. The purposes of sensitivity analysis and meta-regressions were different: the heterogeneity from one specific study was managed from the sensitivity analysis whereas the continuous variables across the studies were analyzed using meta-regression. All meta-analytic computations were performed with R software (using meta package version 3.5.2).
Results
Baseline characteristics of included studies
Figure 1 summarizes the review flowchart in accordance with the PRISMA statement. Of the 1616 references screened, 65 studies met the inclusion criteria for systematic review and 58 studies were entered for quantitative analysis. In quantitative analysis, a total of 2686 participants were included. The mean age of the participants was 43.23 years (range 12–71.94 years) and the median female proportion was 52.53% (range 0–100%). The sample size ranged from 16 to 256. The mean number of treatment sessions with NINCEN was 17.4 (range 5–180). The characteristics of the included participants are summarized in online Supplementary Table S1. The results of the quality assessment on the included trials in our meta-analysis using the Cochrane Collaboration tool are presented in online Supplementary Figs S1 and S2.

Fig. 1. PRISMA flowchart of included studies.
Pooled effects of tDCS on depression and anxiety
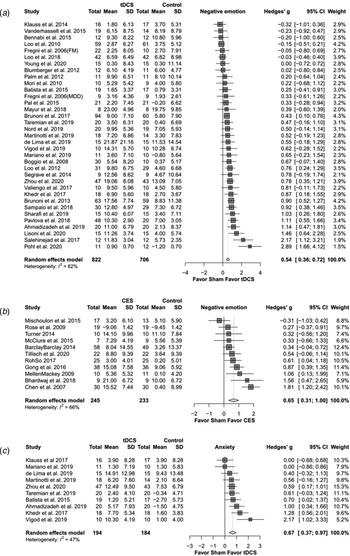
Meta-analysis examining the active tDCS group showed a significant effect on depressive symptoms (Hedges' g = 0.544, p < 0.0001) (Fig. 2a). Regarding secondary study outcomes, the active tDCS group showed a significant effect on anxiety (Hedges' g = 0.667, p < 0.0001) (Fig. 2c). The active tDCS group also showed a significant effect on the response rate of depressive symptoms (OR = 1.959, p = 0.013) but not on the remission rate (OR = 1.500, p = 0.076) (Table 2a).
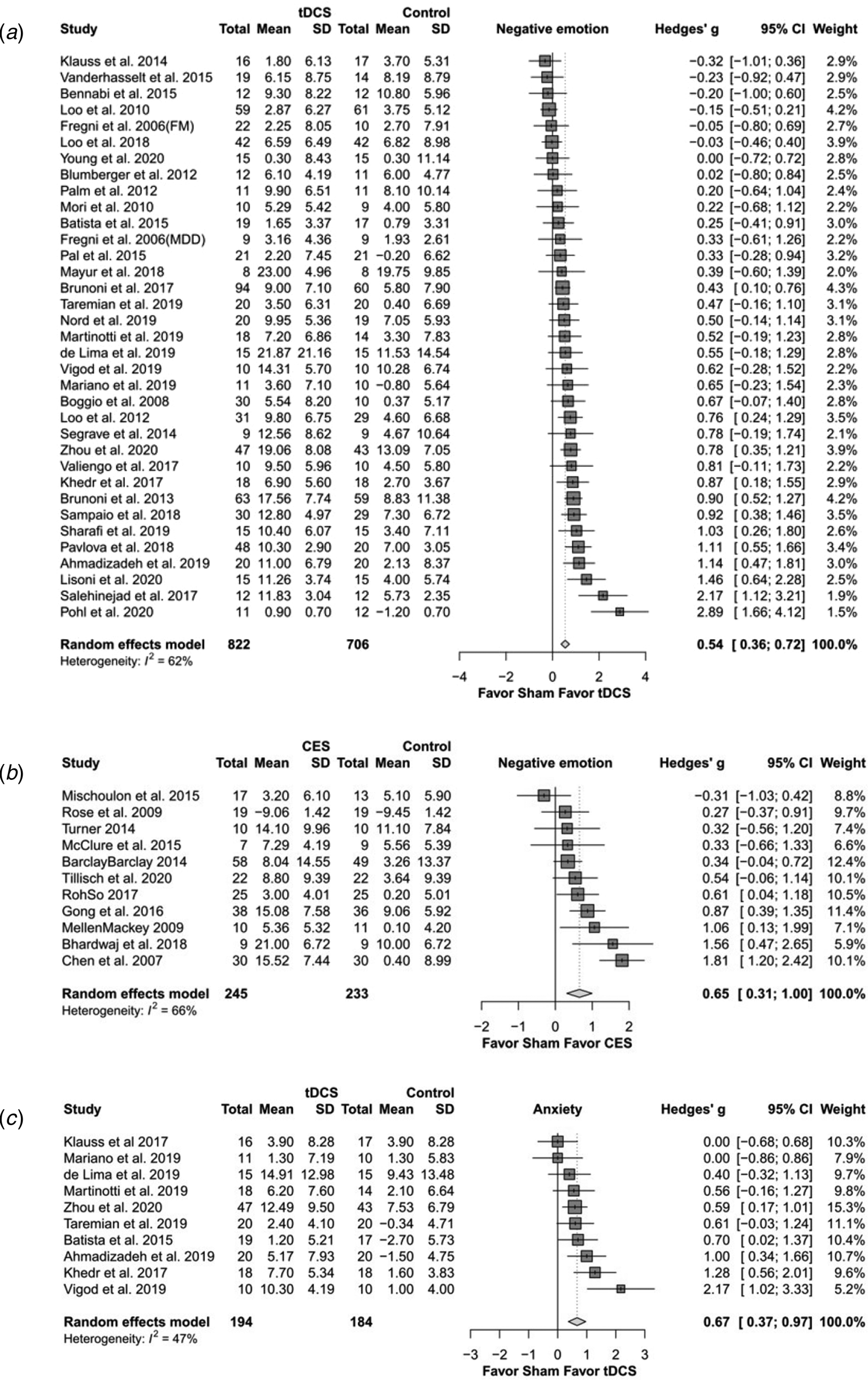
Fig. 2. The effects of transcranial direct current stimulation (tDCS) and cranial electrotherapy stimulation (CES) on depression and anxiety: forest plots. (a) tDCS on depression; (b) CES on depression; (c) tDCS on anxiety.
Table 2. The effects of electrical neuromodulation on depression, anxiety and sleep disturbance: different conditions. (a) only tDCS; (b) only CES; (c) all types of neuromodulation
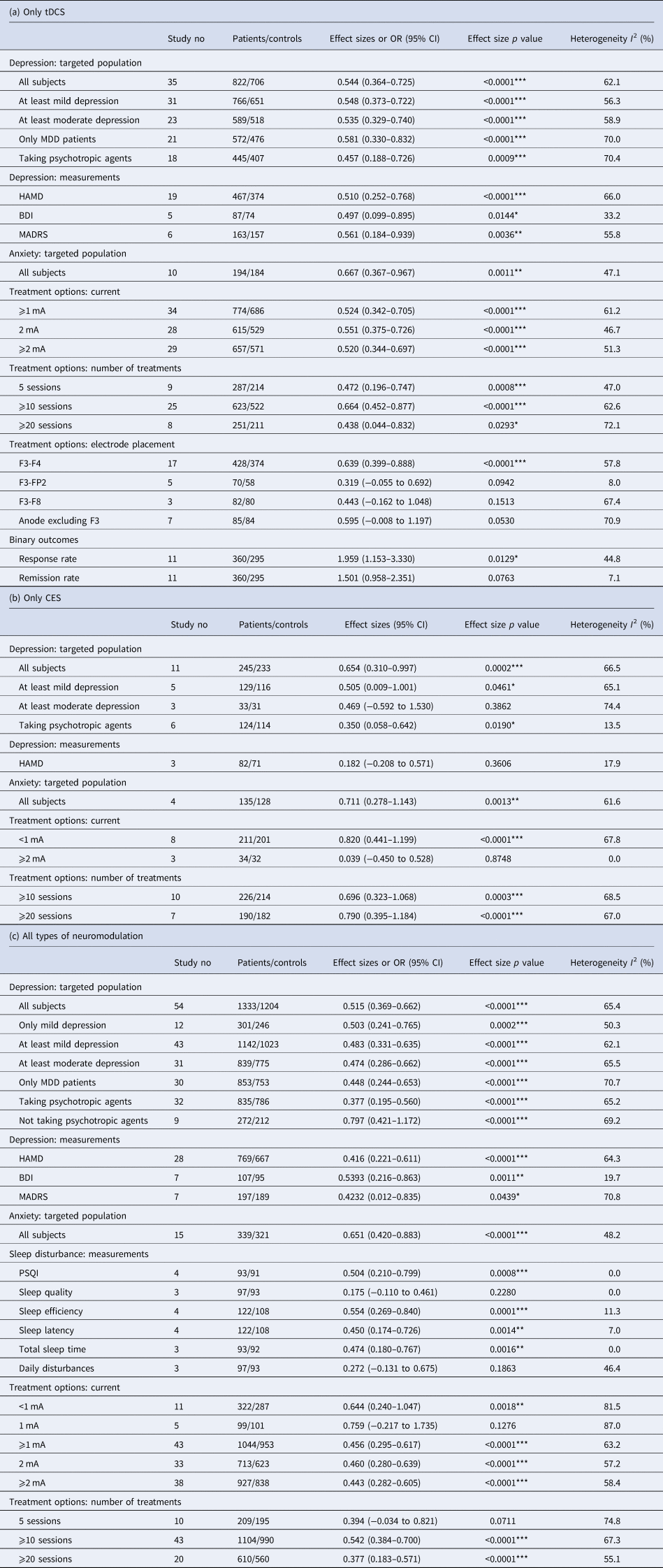
tDCS, transcranial direct current stimulation; CES, cranial electrotherapy stimulation; MDD, major depressive disorder; HAMD, Hamilton Rating Scale for depression; BDI, Beck Depression Inventory; MADRS, Montgomery-Asberg Depression Rating Scale; PSQI, Pittsburgh Sleep Quality Index.
*p < 0.05, **p < 0.01, ***p < 0.001.
For subgroup analysis of mild and moderate depression and of major depressive disorder, the active tDCS group was statistically superior to the sham group on depressive symptoms. For subgroup analysis of specific depression measurements, the active tDCS group was superior to the sham group on the Hamilton Rating Scale for Depression (HAMD), the Beck Depression Inventory (BDI) and the Montgomery-Asberg Depression Rating Scale (MADRS). For subgroup analysis of different current intensities and treatment sessions, the active tDCS group showed a superior effect compared to the sham group. When separating different positions of electrodes, only F3–F4 revealed a significantly higher effect in the tDCS group than in the sham group.
Pooled effects of CES on depression and anxiety
Meta-analysis examining the active CES group showed a significant effect on depressive symptoms compared to the sham group (Hedges' g = 0.654, p < 0.0001) (Fig. 2b). Regarding secondary study outcomes, the active CES group showed a significant effect on anxiety (Hedges' g = 0.711, p = 0.001) (Table 2b).
Subgroup analysis of different severities of depression showed that the active CES group had a significant effect on mild depression but not on moderate depression. For subgroup analysis of different stimulation intensities, the active CES group had a large effect size for intensities of less than 1 mA. For subgroup analysis of the number of treatment sessions, the active CES group revealed a superior effect when the number of treatment sessions was greater than 10.
Treatment effect of all types of neuromodulation on depression, anxiety and sleep disturbance
Combining all types of NINCEN, pooled analysis of the active NINCEN group showed a significant effect on depressive symptoms (Hedges' g = 0.515, p < 0.0001). For secondary outcomes, the active NINCEN group showed a superior effect on anxiety compared to the sham group (Hedges' g = 0.651, p < 0.0001). Regarding sleep disturbance, the active NINCEN group showed a significant effect on the Pittsburgh Sleep Quality Index (PSQI) score, sleep efficiency, sleep latency and total sleep time, whereas it had no significant effect on sleep quality or daily disturbance (Table 2c).
For subgroup analysis of mild and moderate depression and of major depressive disorder, the active NINCEN group was statistically superior to the sham group on depressive symptoms. For subgroup analysis of specific depression measurements, the active NINCEN group was superior to the sham group on the HAMD, BDI and MADRS. For subgroup analysis of different stimulation intensities, the active NINCEN group had no significant effect on the 1 mA subgroup. On stratifying analysis into different treatment sessions, most subgroup analyses remained significant apart from the subgroup with five sessions (Hedges' g = 0.394, p = 0.07).
Sensitivity analysis
The stability of the meta-analysis and subgroup analyses was tested through a leave-one-out sensitivity analysis. The pooled estimate of the following meta-analyses was influenced by a single study: the MADRS for all types of NINCEN; the BDI for tDCS; at least 20 sessions for tDCS; remission rate for tDCS; at least mild depression for only CES; total sleep time for all types of NINCEN; and daytime disturbance for all types of NINCEN (online Supplementary Table S3).
Publication bias
In the above analyses regarding depression, anxiety and sleep disturbance, visual inspection of the funnel plots revealed symmetry and thus no evidence of publication bias (online Supplementary Fig. S3). Egger's regression tests also indicated no publication bias (online Supplementary Table S4). Furthermore, there was no significant publication bias in the subgroup analyses.
Meta-regression
Analyses of study-level covariates showed a negative association between age and depressive symptoms (Table 3). The results indicated that age and medication status could have a moderating effect on the treatment effects of NINCEN (older subjects and subjects taking concomitant psychotropic agents tended to have lower efficacy). Other continuous variables across the studies, such as current, current density, number of treatment sessions and female proportion, did not show significant moderating effects on the meta-analytical result.
Table 3. Meta-regression of pre-defined variables of interest

tDCS, transcranial direct current stimulation; CES, cranial electrotherapy stimulation.
*p < 0.05.
Discussion
The major findings of the present study include:
(1) tDCS had a significant effect on the improvement of depression and anxiety. Regarding depression, the effects of tDCS on patients with different levels of depression and the effects using a distinct scale were all significantly beneficial. With regard to binary outcome response and remission, tDCS revealed a significantly higher odds ratio for a response but not for remission. For tDCS using different levels of current, a number of treatment sessions all showed a significant effect.
(2) The effects of CES on depression and anxiety in all subjects were significant. However, when considering the severity of depression, individuals with less severe depression seem to benefit more from CES. CES with a different number of treatment sessions all had a significant effect and CES using a low current had a relatively high therapeutic effect.
(3) There are few study data for sleep disturbance. Combining the results of these studies, NINCEN had significant effects on the PSQI scores, sleep efficiency, sleep latency and total sleep time, whereas the effects on sleep quality and daily disturbance were non-significant. Combining all the NINCEN studies, treatment with at least 10 sessions seemed to be more promising.
(4) Meta-regression analysis revealed that age and taking psychotropic agents had significant impacts on the results.
The most robust finding in our analysis is that tDCS is an effective treatment for depression and anxiety over a wide range of conditions, regardless of its severity or method of measurement. The effect size for treating depression in all subjects is 0.54 (a medium to large level), which varied little in the subgroup analyses. The results are compatible with previous meta-analyses exploring the effect of tDCS on depression (Berlim, Van den Eynde, & Daskalakis, Reference Berlim, Van den Eynde and Daskalakis2013; Kalu, Sexton, Loo, & Ebmeier, Reference Kalu, Sexton, Loo and Ebmeier2012; Shiozawa et al., Reference Shiozawa, Fregni, Bensenor, Lotufo, Berlim, Daskalakis and Brunoni2014; Zhang et al., Reference Zhang, Lam, Peng, Zhang, Zhang, Huang and Lee2021). Considering that depression and anxiety are frequently comorbid, tDCS may be beneficial for both. On a microscopic level, the therapeutic effect of tDCS on emotional disturbance may be associated with the resting membrane potential, spontaneous neuronal firing rates and synaptic strength (Arul-Anandam & Loo, Reference Arul-Anandam and Loo2009). When focusing on the activation of brain regions, the DLPFC and anterior cingulate cortex are often considered the main targets of tDCS (Bai, Dokos, Ho, & Loo, Reference Bai, Dokos, Ho and Loo2014; Jog et al., Reference Jog, Kim, Anderson, Kubicki, Kayathi, Jann and Narr2021; Mehrsafar et al., Reference Mehrsafar, Rosa, Zadeh and Gazerani2020; Wolkenstein & Plewnia, Reference Wolkenstein and Plewnia2013; Wolkenstein et al., Reference Wolkenstein, Zeiller, Kanske and Plewnia2014). Clarification of the mechanism would be helpful for the optimization of tDCS in the future.
With regard to the discrepancy of tDCS efficacy on the response and remission of depression, several points can be discussed. First, the result may be understood as ‘tDCS is helpful but does not show very high efficacy on depression’. This viewpoint is supported by some well-designed studies: for example, tDCS did not reveal non-inferiority to escitalopram in one randomized-controlled study (Brunoni et al., Reference Brunoni, Moffa, Sampaio-Junior, Borrione, Moreno, Fernandes and Bensenor2017). Second, in most included studies of our analysis tDCS was an add-on treatment, which means that these depressive patients are more likely to be treatment-resistant; this may underestimate the efficacy of tDCS. Such an explanation was supported by our meta-regression result regarding medication status: individuals without concomitant psychotropic agent use revealed better efficacy. Third, the result regarding remission did not pass the sensitivity analysis, therefore the non-significant efficacy of remission should be interpreted with caution.
The therapeutic effect of CES on depression was considered to be associated with the homeostasis of the limbic, hypothalamic and reticular activating system (Gunther & Phillips, Reference Gunther and Phillips2010). In our analysis, when separating subjects into different levels of depression, those with more severe depression showed relatively low improvement after receiving CES. However, when considering all subjects, the effect sizes for depression and anxiety were 0.65 and 0.71, respectively, which are higher than the effect size with tDCS. A recent meta-analysis points out that CES has a significant effect on treating depression, with a small to medium effect size (Price et al., Reference Price, Briley, Haltiwanger and Hitching2021); our result is similar, although the effect size in our study was a little higher. These results indicate that when facing emotional problems that are not very severe, the use of CES is a worthy option.
With regard to the results of the treatment setting, the tDCS and CES findings on current could be discussed separately. Most CES studies adopted a low current (less than 1 mA), which is effective for improving emotion; a higher current did not show significant efficacy. On the other hand, the current in tDCS studies was often higher than 1 mA and the efficacy of tDCS in most current ranges was significant. This discrepancy may imply distinct neurophysiological mechanisms for tDCS and CES. A recent meta-analysis (Zhang et al., Reference Zhang, Lam, Peng, Zhang, Zhang, Huang and Lee2021) points out that a current of 2 mA is effective in tDCS for treating depression; however, in our analysis, the effect sizes for ‘2 mA’ and ‘at least 1 mA’ were similar. Regarding all NINCEN studies, at least 10 treatment sessions seemed more promising because 20 or more sessions did not reveal any additional effect size. Furthermore, the electrode placement may be associated with treatment efficacy; F3–F4 seems the most promising and was also the most commonly adopted placement in the included studies. DLPFC is usually considered to be the targeted brain region of F3–F4 placement (Brighina et al., Reference Brighina, Curatolo, Cosentino, De Tommaso, Battaglia, Sarzi-Puttini and Fierro2019; Lloyd, Wittkopf, Arendsen, & Jones, Reference Lloyd, Wittkopf, Arendsen and Jones2020).
Our meta-regression indicates that the age of subjects affects the analysis results. The elderly showed a relatively low response to NINCEN and similar features were often found in pharmacotherapy studies (Knochel et al., Reference Knochel, Alves, Friedrichs, Schneider, Schmidt-Rechau, Wenzler and Oertel-Knochel2015). Based on the information above, if we view the ‘enhancing activity of specific brain regions’ as the main mechanism of NINCEN, then a rational hypothesis would be that ‘individuals with a higher level of brain degeneration have a higher resistance to be activated’. But the meta-regression may only disclose a ‘tendency’ and does not mean that NINCEN was ineffective in the elderly. Several studies in the elderly and in patients post-stroke have revealed NINCEN to be beneficial for both emotional and cognitive function (Li et al., Reference Li, Zhu, Klomparens, Xu, Wang, Wang and Song2019a; Lu et al., Reference Lu, He, Zhang, Wang, Zhang, Dong and Yang2020; Valiengo et al., Reference Valiengo, Goulart, de Oliveira, Bensenor, Lotufo and Brunoni2017; Wong et al., Reference Wong, Chan, Wong, Wong, Yung, Wong and Cheng2019).
Some analyses on depression and sleep disturbance did not pass the leave-one-out sensitivity analysis, which means that the significance levels of these analyses would change when one included study was removed. Several tDCS analyses had this feature, such as using the BDI score as the outcome, treatment with at least 20 sessions and using the remission rate as the outcome, therefore these results should be interpreted cautiously; it may also explain the difference in meta-analyses on a similar topic. On the other hand, the results of analyses regarding publication bias revealed high robustness. For analyses with enough included studies, no significant effects were found in Egger's tests, which indicates that the therapeutic effects and size of the studies were not significantly correlated in our analyses.
Several limitations of this study should be discussed. First, the heterogeneity of the incorporated studies still needs to be viewed cautiously. Although we have used subgroup analyses to manage the different treatment settings, targeted populations and measurements, some issues cannot be analyzed using this approach. Second, tDCS and CES were adopted in most included studies, therefore the meta-analyses for these two techniques had higher values; the meta-analysis results for all types of NINCEN are thus highly influenced by the tDCS and CES data. Analysis of all types of NINCEN was for comprehensiveness and for extracting other effective components of this kind of therapy; however, our findings for the latter were not very impressive. Third, we only analyzed the effects of ‘active NINCEN stimulation v. sham’ in this study. Some studies compared the efficacy of NINCEN and other types of interventions (Park et al., Reference Park, Choi, Kim, Kim, Son, Roh and Park2020); however, if we had performed this analysis the article would have become too complicated. Fourth, depression, anxiety and sleep disturbance actually have some overlapping presentations (e.g. sleep disturbance is sometimes one symptom of depression/anxiety). This cannot be managed in the current meta-analysis because of a lack of detailed symptom profiles. Finally, our analysis could not provide more insight about the conditions contraindicated for receiving NINCEN because individuals with contraindications were excluded from the studies, thus no available data could be used for incorporation. Common contraindications of NINCEN in these studies included a history of seizures, use of a pacemaker and scalp conditions that could be affected by NINCEN (Cleland, Galick, Huckstep, Lenhart, & Madhavan, Reference Cleland, Galick, Huckstep, Lenhart and Madhavan2020; Russo, Souza Carneiro, Bolognini, & Fregni, Reference Russo, Souza Carneiro, Bolognini and Fregni2017).
The results for tDCS and CES in this analysis have clinical meaning. In summary, tDCS is effective for both depression and anxiety; the effects are robust when considering different populations and treatment parameters but the rational expectation of the effect of tDCS should be ‘response’ rather than ‘remission’. CES also has a significant effect on depression and anxiety; in addition, it is relatively effective for patients with mild depression. Electrode placement and medication status are also factors that could affect treatment efficacy. Both tDCS and CES may be used for patients with depression/anxiety who are not suitable receiving pharmacotherapy. To determine whether other NINCEN options are safe and effective, further large and well-designed trials are required.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291721005560
Acknowledgements
The authors thank Ms. Yi-Ling Lin, Ms. Huei-Mei Ma and Ms. Yi-Mei Huang for their administrative work during manuscript preparation.
Financial support
None.
Conflict of interest
None.
Ethical standards
This systematic review/meta-analysis has been approved by PROSPERO (ID No. CRD42021227132).