Introduction
Multiple studies implicate reward and dopaminergic system dysfunction in the pathogenesis of major depressive disorder (MDD). Yet, only few studies use experimentally controlled designs to probe the role of these systems in MDD. Here, we examine the acute effects of lurasidone, a novel D2 antagonist with adjunctive antidepressant properties, on neural responding to reward in depression using two functional imaging modalities.
Depressed patients display alterations across several key phases of reward processing. Blunting of neural responses when anticipating or obtaining rewards (Knutson et al., Reference Knutson, Bhanji, Cooney, Atlas and Gotlib2008; Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan, Dougherty, Iosifescu, Rauch and Fava2009; Keren et al., Reference Keren, O'Callaghan, Vidal-Ribas, Buzzell, Brotman, Pan, Meffert, Kaisera, Wolke, Pine and Stringaris2018) is associated with anhedonia, while increased reactivity to losses may underlie the behavioural avoidance that is characteristic of depression (Stringaris et al., Reference Stringaris, Vidal-Ribas Belil, Artiges, Lemaitre, Gollier-Briant, Wolke, Vulser, Miranda, Penttila, Struve, Fadai, Kappel, Grimmer, Goodman, Poustka, Conrod, Cattrell, Banaschewski, Bokde, Bromberg, Buchel, Flor, Frouin, Gallinat, Garavan, Gowland, Heinz, Ittermann, Nees, Papadopoulos, Paus, Smolka, Walter, Whelan, Martinot, Schumann and Paillere-Martinot2015; Luking et al., Reference Luking, Pagliaccio, Luby and Barch2016; Engelmann et al., Reference Engelmann, Berns and Dunlop2017; Hevey et al., Reference Hevey, Thomas, Laureano-Schelten, Looney and Booth2017). Recently, a direct link has been found between reduced mid-brain dopamine transporter density and neural activity during reward processing within the mesolimbic pathway in healthy and depressed human participants (Dubol et al., Reference Dubol, Trichard, Leroy, Sandu, Rahim, Granger, Tzavara, Karila, Martinot and Artiges2018).
These findings make reward processing an attractive treatment target. Dopaminergic compounds provide a promising way to manipulate fronto-striatal reward pathways (Pessiglione et al., Reference Pessiglione, Seymour, Flandin, Dolan and Frith2006; Jocham et al., Reference Jocham, Klein and Ullsperger2011, Reference Jocham, Klein and Ullsperger2014; Chowdhury et al., Reference Chowdhury, Guitart-Masip, Lambert, Dayan, Huys, Düzel and Dolan2013; Dean et al., Reference Dean, Horndasch, Giannopoulos and McCabe2016; Harmer et al., Reference Harmer, Duman and Cowen2017). Surprisingly, however, very few studies have used dopaminergic drugs to probe the association between neural reward signalling and depression. Recently, Admon et al. (Reference Admon, Kaiser, Dillon, Beltzer, Goer, Olson, Vitaliano and Pizzagalli2017) showed that a single-dose of the dopamine receptor antagonist amisulpride normalised reward processing by increasing reward-related striatal activation and corticostriatal connectivity in depressed individuals. This effect is thought to result from transient increases in dopamine signalling at low amisulpride doses (Schoemaker et al., Reference Schoemaker, Claustre, Fage, Rouquier, Chergui, Curet, Oblin, Gonon, Carter, Benavides and Scatton1997; Admon et al., Reference Admon, Kaiser, Dillon, Beltzer, Goer, Olson, Vitaliano and Pizzagalli2017). Strengthening of striatal functioning through dopamine antagonists has been shown before in healthy volunteers (Mehta et al., Reference Mehta, McGowan, Lawrence, Aitken, Montgomery and Grasby2003; Handley et al., Reference Handley, Zelaya, Reinders, Marques, Mehta, O'Gorman, Alsop, Taylor, Johnston, Williams, McGuire, Pariante, Kapur and Dazzan2013) and is presumed to occur through presynaptic D2/D3 autoreceptor blockade (Fernandez-Seara et al., Reference Fernandez-Seara, Aznarez-Sanado, Mengual, Irigoyen, Heukamp and Pastor2011, Goozee et al., Reference Goozee, Handley, Kempton and Dazzan2014).
It may seem counterintuitive that some antipsychotics are antidepressant in augmentation treatment for bipolar and MDD, given that D2 antagonism (a central feature of all antipsychotics) is known to suppress reward-related striatal activation, for example, with haloperidol (Pessiglione et al., Reference Pessiglione, Seymour, Flandin, Dolan and Frith2006; Pleger et al., Reference Pleger, Ruff, Blankenburg, Kloppel, Driver and Dolan2009; Oei et al., Reference Oei, Rombouts, Soeter, van Gerven and Both2012). However, olanzapine, quetiapine and lurasidone, which are efficacious adjunctive antidepressants [olanzapine (Tohen et al., Reference Tohen, Vieta, Calabrese, Ketter, Sachs, Bowden, Mitchell, Centorrino, Risser, Baker, Evans, Beymer, Dube, Tollefson and Breier2003, Reference Tohen, Kanba, McIntyre, Fujikoshi and Katagiri2014), quetiapine (Suppes et al., Reference Suppes, Datto, Minkwitz, Nordenhem, Walker and Darko2014; Suttajit et al., Reference Suttajit, Srisurapanont, Maneeton and Maneeton2014), lurasidone (Loebel et al., Reference Loebel, Cucchiaro, Silva, Kroger, Hsu, Sarma and Sachs2014a, Reference Loebel, Cucchiaro, Silva, Kroger, Sarma, Xu and Calabrese2014b; Nelson et al., Reference Nelson, Suppes, Tsai, Mao, Pikalov and Loebel2015; Suppes et al., Reference Suppes, Kroger, Pikalov and Loebel2016a, Reference Suppes, Silva, Cucchiaro, Mao, Targum, Streicher, Pikalov and Loebel2016b)] differ from haloperidol in their broader profile, including greater serotonergic action. Indeed, blockade of serotonergic 5-HT receptors (5-HT1A, 5-HT2A, 5-HT7) stimulates striatal dopamine release and in addition to this, serotonergic neurons directly impact upon reward (and predominantly aversive) processing (Boureau and Dayan, Reference Boureau and Dayan2011; Huang et al., Reference Huang, Horiguchi, Felix and Meltzer2012; Inaba et al., Reference Inaba, Mizuhiki, Setogawa, Toda, Richmond and Shidara2013; Liu et al., Reference Liu, Zhou, Li, Hu, Lu, Ma, Feng, Zhang, Wang, Zeng, Bao, Kim, Chen, El Mestikawy and Luo2014; Cohen et al., Reference Cohen, Amoroso and Uchida2015; Hayashi et al., Reference Hayashi, Nakao and Nakamura2015; Li et al., Reference Li, Zhong, Wang, Feng, Liu, Zhou, Jia, Hu, Zeng, Guo, Fu and Luo2016). However, there are few studies that have assessed modulation of loss anticipation and feedback with antidepressant drugs. The evidence thus far points to a pattern of blunting of aversive events with acute administration of selective serotonin reuptake inhibitors (SSRIs) (McCabe et al., Reference McCabe, Mishor, Cowen and Harmer2010; Macoveanu et al., Reference Macoveanu, Rowe, Hornboll, Elliott, Paulson, Knudsen and Siebner2013, Reference Macoveanu, Fisher, Haahr, Frokjaer, Knudsen and Siebner2014; Macoveanu, Reference Macoveanu2014), but crucially also with D2 antagonists that have anti-depressant properties [amisulpride (Admon et al., Reference Admon, Kaiser, Dillon, Beltzer, Goer, Olson, Vitaliano and Pizzagalli2017) and aripiprazole (Bolstad et al., Reference Bolstad, Andreassen, Groote, Server, Sjaastad, Kapur and Jensen2015)]. These findings raise the intriguing possibility that dopamine antagonists with adjunctive antidepressant properties may exert their effects via reward and/or penalty signal normalisation.
In this paper, we test whether an acute dose of 20 mg lurasidone, a D2 receptor antagonist (Loebel and Citrome, Reference Loebel and Citrome2015) with demonstrated antidepressant properties in monotherapy and in combination treatment (Loebel et al., Reference Loebel, Cucchiaro, Silva, Kroger, Hsu, Sarma and Sachs2014a, Reference Loebel, Cucchiaro, Silva, Kroger, Sarma, Xu and Calabrese2014b; Suppes et al., Reference Suppes, Kroger, Pikalov and Loebel2016a; Goldberg et al., Reference Goldberg, Ng-Mak, Siu, Chuang, Rajagopalan and Loebel2017), influences reward and penalty signal in depression. Lurasidone was selected because it is the most recently licensed dopamine antagonist with antidepressant properties and there is no information with regards to its effects on brain reward and penalty signalling (Loebel et al., Reference Loebel, Cucchiaro, Silva, Kroger, Hsu, Sarma and Sachs2014a, Reference Loebel, Cucchiaro, Silva, Kroger, Sarma, Xu and Calabrese2014b; Nelson et al., Reference Nelson, Suppes, Tsai, Mao, Pikalov and Loebel2015; Nierenberg et al., Reference Nierenberg, Tsai, Mao, Pikalov, Suppes and Loebel2015; Suppes et al., Reference Suppes, Kroger, Pikalov and Loebel2016a, Reference Suppes, Silva, Cucchiaro, Mao, Targum, Streicher, Pikalov and Loebel2016b; Goldberg et al., Reference Goldberg, Ng-Mak, Siu, Chuang, Rajagopalan and Loebel2017). We employ a randomised, placebo-controlled cross-over design with functional magnetic resonance imaging (fMRI) and arterial spin labelling (ASL) imaging acquired on two separate occasions per individual. This design overcomes the limitations of correlational studies through randomisation and experimental manipulation. Since symptoms of MDD fall on a continuous dimension (Angst et al., Reference Angst, Sellar and Merikangas2000; Ayuso-Mateos et al., Reference Ayuso-Mateos, Nuevo, Verdes, Naidoo and Chatterji2010), we recruited medication-naïve subjects across the range of depression severity, including healthy volunteers, as well as people meeting full-criteria for MDD. This research approach is in line with the Research Domain Criteria framework (Morris and Cuthbert, Reference Morris and Cuthbert2012) [e.g. as in Stringaris et al. (Reference Stringaris, Vidal-Ribas Belil, Artiges, Lemaitre, Gollier-Briant, Wolke, Vulser, Miranda, Penttila, Struve, Fadai, Kappel, Grimmer, Goodman, Poustka, Conrod, Cattrell, Banaschewski, Bokde, Bromberg, Buchel, Flor, Frouin, Gallinat, Garavan, Gowland, Heinz, Ittermann, Nees, Papadopoulos, Paus, Smolka, Walter, Whelan, Martinot, Schumann and Paillere-Martinot2015) where symptom levels are related to the brain measurements]. It also does justice to findings concerning the genetic underpinnings of common mental illness (Plomin et al., Reference Plomin, Haworth and Davis2009) as well as current approaches to understanding neural system perturbation in a dimensional way (Matthews and Hampshire, Reference Matthews and Hampshire2016).
Depression is characterised by hyporeactivity to reward (Knutson et al., Reference Knutson, Bhanji, Cooney, Atlas and Gotlib2008; Forbes et al., Reference Forbes, Hariri, Martin, Silk, Moyles, Fisher, Brown, Ryan, Birmaher, Axelson and Dahl2009; Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan, Dougherty, Iosifescu, Rauch and Fava2009; Gotlib et al., Reference Gotlib, Hamilton, Cooney, Singh, Henry and Joormann2010; Admon et al., Reference Admon, Nickerson, Dillon, Holmes, Bogdan, Kumar, Dougherty, Iosifescu, Mischoulon, Fava and Pizzagalli2015; Luking et al., Reference Luking, Pagliaccio, Luby and Barch2016; Keren et al., Reference Keren, O'Callaghan, Vidal-Ribas, Buzzell, Brotman, Pan, Meffert, Kaisera, Wolke, Pine and Stringaris2018) and hyperactivity to aversive stimuli (Gotlib et al., Reference Gotlib, Hamilton, Cooney, Singh, Henry and Joormann2010; Admon et al., Reference Admon, Nickerson, Dillon, Holmes, Bogdan, Kumar, Dougherty, Iosifescu, Mischoulon, Fava and Pizzagalli2015; Luking et al., Reference Luking, Pagliaccio, Luby and Barch2016; Engelmann et al., Reference Engelmann, Berns and Dunlop2017), and thus an antidepressant effect could be brought about by increasing reward, decreasing salience to negative events, or, both simultaneously. Given the relative paucity of literature on processing of losses (Keren et al., Reference Keren, O'Callaghan, Vidal-Ribas, Buzzell, Brotman, Pan, Meffert, Kaisera, Wolke, Pine and Stringaris2018), our study is designed to interrogate both anticipation and feedback of rewards and penalties. We hypothesise a normalisation of fronto-striatal reward and/or penalty function following acute-dose administration in depression. More specifically, we anticipate that subjects scoring high on depression will show a baseline difference in fronto-striatal activity which will be reverted by acute-dose lurasidone. We first explore the expectation that the dopamine antagonist lurasidone will show striatal blunting during the anticipation phase, in line with numerous findings with D2 antagonist drugs (Pessiglione et al., Reference Pessiglione, Seymour, Flandin, Dolan and Frith2006; Pleger et al., Reference Pleger, Ruff, Blankenburg, Kloppel, Driver and Dolan2009). Although, we note that a structurally similar drug, amisulpride has shown opposite effects (Admon et al., Reference Admon, Kaiser, Dillon, Beltzer, Goer, Olson, Vitaliano and Pizzagalli2017). An intriguing question is whether any blunting in reward processing that occurs with these drugs could have beneficial effects when dealing with loss. This is important given findings from serotonergic drugs that show on the one hand blunting of reward processing and on the other, amelioration of negative feedback (McCabe et al., Reference McCabe, Mishor, Cowen and Harmer2010; Macoveanu et al., Reference Macoveanu, Rowe, Hornboll, Elliott, Paulson, Knudsen and Siebner2013, Reference Macoveanu, Fisher, Haahr, Frokjaer, Knudsen and Siebner2014; Macoveanu, Reference Macoveanu2014), which could underlie its antidepressant effects. In addition, we seek to address a key concern in pharmacoimaging studies, namely that shifts in global or regional cerebral blood flow (CBF) could underlie changes observed in a blood oxygenated level dependent (BOLD) fMRI signal. We therefore also use ASL, an imaging modality that allows the quantification of CBF at rest, to disentangle global and regional CBF changes from a BOLD fMRI signal.
Materials and methods
Participants
Forty-three participants (28 female, 15 male) were recruited using the research volunteer recruitment webpage at King's College London, social media and posters at university counselling services across London.
We recruited young people across a range of depression and anhedonia scores in the community as symptoms of MDD are known to fall on a continuum (Angst et al., Reference Angst, Sellar and Merikangas2000; Ayuso-Mateos et al., Reference Ayuso-Mateos, Nuevo, Verdes, Naidoo and Chatterji2010), allowing us to assess the role of symptom level in reward processing on and off lurasidone (see text and online Figs S2–S4 in the Supplementary Methods). Inclusion criteria restricted recruitment to right-handed individuals 18–25 years of age with no contraindications to MRI, no serious medical conditions and no lifetime substance dependence. Please refer to the online Supplementary Methods for full details of inclusion and exclusion criteria. Table 1 provides demographic and clinical information for the entire sample (n = 43). Online Table S1 in the Supplementary Methods provides demographic and clinical characteristics of recruited participants according to depression severity cut-off scores from the Beck's Depression Inventory-II (BDI-II). Participants received £230 in compensation for attending the assessment appointment and both scanning visits, in addition to their winnings from the fMRI task. All participants provided written informed consent, as approved by the Ethics Subcommittee of Psychiatry, Nursing & Midwifery Research (RESC reference number: PNM/13/14-122).
Table 1. Demographic and clinical characteristics of participants in a study investigating the effect of lurasidone on reward and penalty processing

Design and procedure
Depression and anhedonia scores were assessed using the BDI-II (Beck et al., Reference Beck, Steer, Ball and Ranieri1996) and the Snaith–Hamilton Pleasure Scale (SHAPS) (Snaith et al., Reference Snaith, Hamilton, Morley, Humayan, Hargreaves and Trigwell1995). On the basis of BDI-II scores, participants who were eligible following this screening procedure were invited to the assessment appointment.
Figure 1 illustrates the procedure and timeline of the study. At the assessment appointment, participants first completed a pre-MRI safety screening. Participants then completed questionnaires to assess handedness (Edinburgh Handedness Inventory) and IQ (National Adult Reading Test) (Nelson and Willison, Reference Nelson and Willison1991). This was followed by the Mini International Neuropsychiatric Interview version 6.0.0 (M.I.N.I.) (Sheehan et al., Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller, Hergueta, Baker and Dunbar1998) which assessed past and present mental health disorders. Participants’ height, weight, heart rate, blood pressure and electrocardiogram (ECG) were measured by the experimenter and blood samples (for Full Blood Count and Liver Function Tests) were taken by a study physician. Participants provided a urine sample for drug testing and for pregnancy testing in female participants. Participants were guided through the scanning procedure in a mock scanner and completed training for the Monetary Incentive Delay (MID) task.

Fig. 1. Procedure and timeline for a study investigating the effect of lurasidone on reward and penalty processing.
If participants fulfilled the inclusion criteria after the assessment appointment, they were invited to take part in two scan days. There was a 97% retention rate in the study and this is illustrated in online Fig. S1 in the Supplementary Methods. Participants were randomised into one of two drug administration orders: placebo-lurasidone (placebo at visit one and lurasidone at visit two), or lurasidone-placebo. Both scan days followed the same schedule. On arrival at the imaging centre, participants had their heart rate and blood pressure measured and filled in two brief questionnaires to measure sedation [Visual Analogue Scale (VAS) (Herbert et al., Reference Herbert, Johns and Doré1976) and state-anxiety (State Trait Anxiety Inventory; STAI) (Spielberger et al., Reference Spielberger, Gorsuch and Lushene1970)]. Next, the experimenter administered a capsule of either lurasidone (20 mg) or placebo. This dose was selected to minimise post-synaptic D2 blockade (la Fougere et al., Reference la Fougere, Meisenzahl, Schmitt, Stauss, Frodl, Tatsch, Hahn, Moller and Dresel2005), as in similar studies of related medications (Admon et al., Reference Admon, Kaiser, Dillon, Beltzer, Goer, Olson, Vitaliano and Pizzagalli2017). Given the pharmacokinetic profile of lurasidone, the pill was consumed, followed by a 350 calorie meal (Greenberg and Citrome, Reference Greenberg and Citrome2017). Peak plasma levels of lurasidone are reached at approximately 3 h after tablet ingestion and the plasma half-life is 18 h (Greenberg and Citrome, Reference Greenberg and Citrome2017). In order to align the study assessments with peak plasma levels the MRI scan took place 3 h after tablet consumption (Fig. 1). Prior to the MRI scan, 2 h 45 min after drug administration, the experimenter measured participants’ heart rate and blood pressure again, and participants completed the VAS and STAI questionnaires. The scan lasted approximately 1.5 h and included structural scans, ASL and a functional scan acquisition while completing the MID task. After the scan, and approximately 4.5 h after drug administration, the experimenter assessed the participants’ heart rate and blood pressure, the VAS/STAI questionnaires were completed and ECG was collected. Participants were paid in cash for their winnings from the MID task and were discharged.
fMRI task
The MID task used in the current study was an adaptation of the task from, for example Knutson and colleagues (Knutson et al., Reference Knutson, Adams, Fong and Hommer2001). The task involves anticipation and receipt of monetary rewards and penalties. The task elicits robust fronto-striatal responses in healthy individuals and has high scan-rescan reliability (Plichta et al., Reference Plichta, Schwarz, Grimm, Morgen, Mier, Haddad, Gerdes, Sauer, Tost, Esslinger, Colman, Wilson, Kirsch and Meyer-Lindenberg2012; Wu et al., Reference Wu, Samanez-Larkin, Katovich and Knutson2014). During the anticipation and receipt of monetary reward and penalties, several studies using this task have demonstrated altered fronto-striatal activation in depressed individuals compared with healthy controls (Knutson et al., Reference Knutson, Bhanji, Cooney, Atlas and Gotlib2008; Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan, Dougherty, Iosifescu, Rauch and Fava2009; Carl et al., Reference Carl, Walsh, Eisenlohr-Moul, Minkel, Crowther, Moore, Gibbs, Petty, Bizzell, Dichter and Smoski2016). This makes the MID task well-suited for the current study and further details are provided in the online Supplementary Methods.
MRI acquisition parameters
The MRI acquisition parameters are described in the online Supplementary Methods.
fMRI data analysis
ASL pre-processing
Spatial normalisation of the CBF maps was achieved using Automated Software for ASL Processing (ASAP; Mato Abad et al., Reference Mato Abad, Garcia-Polo, O'Daly, Antonio Hernandez-Tamames and Zelaya2016). This pipeline employs the Statistical Parametric Mapping suite (SPM, Functional Imaging Laboratory, University College London, London, UK, version 12 – https://www.fil.ion.ucl.ac.uk/spm). Full details are provided in the online Supplementary Methods.
fMRI pre-processing
fMRI data were preprocessed and quality assured using SPM12 in Matlab version (R2016b). This consisted of reorientation to the AC-PC line, slice timing correction, motion correction (Friston et al., Reference Friston, Williams, Howard, Frackowiak and Turner1996), multi-channel segmentation and co-registration to each participant's structural image. The normalise estimate & write function within SPM12 was used, with the Montreal Neurological Institute template (MNI152). Smoothing was completed using a Gaussian kernel of 4 mm full-width half-maximum.
ASL statistical analysis
To test for statistical significant changes in resting CBF we carried out a paired-sample t test, which compared the CBF maps collected after administration of lurasidone against those acquired after placebo. Quantitative measures of global CBF and striatal CBF were extracted for each participant after placebo and lurasidone. The striatal region-of-interest (ROI) was formed by combining anatomically defined binary masks of the caudate, putamen and nucleus accumbens (NAcc) (see online Fig. S7 in the Supplement) (O’Doherty et al., Reference O'Doherty, Dayan, Schultz, Deichmann, Friston and Dolan2004). A repeated-measures analysis of covariance (ANCOVA) was performed for global and striatal CBF with the following factors: Medication (placebo, lurasidone) as the within-subject variable, Medication Order (placebo-lurasidone, lurasidone-placebo) as the between-subject factor and Depression Severity (total BDI-II score) as the covariate of interest. To test if changes in baseline CBF were related to the BOLD findings, the change in CBF between the two sessions was entered as covariates in all subsequent analyses. Specifically, the change in CBF values for a given region was used as covariates for the same region in the fMRI analyses.
fMRI first-level model
The BOLD signal was modelled with a canonical haemodynamic response function that was convolved with the onset times of task regressors to compute parameter estimates using the general linear model (GLM) at the single-subject level. The GLM included nine task-related regressors: passive condition, three cues (neutral, win, loss) and five outcomes [with (win outcome following win cue), missed win (no-change outcome following a win cue), loss (penalty outcome following a loss cue), avoided loss (no-change outcome following a loss cue) and neutral outcome (no-change outcome following a neutral/no-incentive cue)]. High-pass temporal filtering (128 s cut-off) was used to remove low-frequency artefacts. Estimated movement parameters were added to the design matrix. These included six rigid-body movement parameters, a regressor accounting for frame-wise displacement (i.e. the 3D movement from volume 1–2, 2–3 etc.), and additional binary regressors to indicate image volumes with spikes greater than 1 mm, and images either side of the spike (i.e. motion scrubbing and padding). Movement analyses are described in the online Supplementary Methods.
fMRI statistical analysis
Anticipation and outcome
Following previous findings that depression is associated with differential fronto-striatal abnormalities in response to anticipation v. receipt of monetary outcomes (Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan, Dougherty, Iosifescu, Rauch and Fava2009) statistical analyses were separately conducted for the cue and outcome phases of the task.
To test a priori hypotheses regarding fronto-striatal responses to the anticipation and outcome of reward and penalty, we conducted a ROI analysis. Mean activations were extracted from seven bilateral anatomical masks of the caudate, putamen, NAcc, orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), insula and amygdala for each participant for the following contrasts of interest: (i) anticipation neutral > baseline, (ii) anticipation win > baseline, (iii) anticipation loss > baseline, (iv) Reward Outcome: feedback win > missed win and (v) Penalty Outcome: feedback loss > avoided loss. This analytic approach has been used previously (Admon et al., Reference Admon, Kaiser, Dillon, Beltzer, Goer, Olson, Vitaliano and Pizzagalli2017) and mitigates possible spillover effects of cue type on the neural responses to outcomes. Masks were collapsed across hemispheres because hemispheric effects on task activation were non-significant and because of the high correlation between hemispheric ROIs. To avoid circular analysis (Kriegeskorte et al., Reference Kriegeskorte, Simmons, Bellgowan and Baker2009), whole regions from atlas toolboxes in SPM12 were used (see online Fig. S7 in the supplementary data). These ROIs were chosen in accordance with meta-analytical findings of the neural correlates of reward and penalty processing (Diekhof et al., Reference Diekhof, Kaps, Falkai and Gruber2012; Bartra et al., Reference Bartra, McGuire and Kable2013; Zhang et al., Reference Zhang, Chang, Guo, Zhang and Wang2013).
For the anticipation phase of the task, a repeated-measures ANCOVA was performed for each ROI with the following factors: Medication (placebo, lurasidone) and Anticipation Cue (neutral, win, loss) as within-subject variables, Medication Order as the between-subject factor, and Depression Severity (total BDI-II score) as the covariate of interest.
To test our hypothesis regarding normalisation of reward and/or penalty responses, we conducted a repeated measures ANCOVA for each ROI. This included the factors: Medication (placebo, lurasidone) and Outcome Type (reward, penalty) as within-subject variables, Medication Order as the between-subject factor, and Depression Severity (total BDI-II score) as the covariate of interest. We predicted that normalisation responses in depressed individuals on lurasidone would be captured by a Medication-by-Depression Severity-by-Outcome Type interaction. We expected to find no effect of Medication Order.
In order to examine further the drug effects on the neural signal, we examined how the difference in neural activity (Δ neural activity) between placebo and lurasidone in each ROI varied across depression scores. For this, Pearson correlation coefficients were estimated.
To complement our primary dimensional analyses (using a continuous measure of depression), we also examined our hypothesis regarding normalisation of responses using categorical groups in a repeated measures ANOVA model (Fig. 4). We used severity cut-off scores for the BDI-II (Beck et al., Reference Beck, Steer, Ball and Ranieri1996, Krefetz et al., Reference Krefetz, Steer, Gulab and Beck2002, Kumar et al., Reference Kumar, Steer, Teitelman and Villacis2002) to compare individuals with low depressive symptoms [total BDI-II score: 0–16 (normal-mild mood disturbance), n = 24] to individuals with high depressive symptoms [total BDI-II score: 17–43 (borderline-severe depression), n = 18] on placebo and lurasidone (Strober et al., Reference Strober, Green and Carlson1981; Barrera and Garrisonjones, Reference Barrera and Garrisonjones1988; Whitaker et al., Reference Whitaker, Johnson, Shaffer, Rapoport, Kalikow, Walsh, Davies, Braiman and Dolinsky1990; Ambrosini et al., Reference Ambrosini, Metz, Bianchi, Rabinovich and Undie1991; Marton et al., Reference Marton, Churchard, Kutcher and Korenblum1991; Canals et al., Reference Canals, Blade, Carbajo and Domenech-Llaberia2001).
For all of the above ROI analyses, the threshold for statistical significance was set at (p < 0.007) following Bonferroni adjustment for seven multiple ROI comparisons. We also tested the association between dimensional anxiety scores and brain activation in an ANCOVA, with Anxiety Severity (total score on the anxiety subscale of the Hospital Anxiety and Depression Scale) as the covariate of interest.
In order to model the effects of lurasidone and depression status beyond the fronto-striatal network targeted in the ROI analyses, exploratory whole brain analyses were also conducted (see the online Supplementary Methods and Results).
Results
Behavioural results
A repeated measures ANCOVA with Medication (placebo or lurasidone) and Cue Type (reward, penalty, neutral) as the within-subject variables, Medication Order (placebo-lurasidone, lurasidone-placebo) as the between-subject variable and Depression Severity (total BDI-II score) as the covariate of interest was completed for (i) Total Winnings, (ii) Mean Reaction Time (RT) and (iii) Accuracy. Performance data are presented in online Table S2 in the Supplementary Results. In all analyses, there were no effects of Medication Order or interactions with Medication Order (all p values > 0.050). In all analyses there were no significant three-way interactions between either (i) Total Winnings, (ii) Mean RT or (iii) Accuracy and Medication and Depression Severity. Significant two way interactions between Cue Type and Mean RT, and Cue Type and Accuracy are presented in the online Supplementary Results. We also examined the effect of Medication, Medication Order and Depression Severity on the change in Sedation ratings (total VAS scores) and State-anxiety ratings (total STAI score) from pre-drug administration (Measure 1) to peak-of-drug (Measure 2). There were no significant main effects or interactions (all p values > 0.050) (please refer to the online Supplementary Results).
Reward processing (blood-oxygen-level dependent signal) results
Response to outcomes
Primary analyses
These primary analyses are conducted with depression measured as a continuous variable. In order to test the hypothesis that lurasidone would increase activation to reward outcomes and decrease responses to penalties in depressed individuals, we conducted a repeated-measures ANCOVA. Medication (placebo, lurasidone) and Outcome Type (Reward Outcome v. Penalty Outcome) were the within-subject variables, Medication Order was the between-subject factor and Depression Severity (total BDI-II score) was the covariate of interest (n = 40). Three participants were excluded from the analyses (please refer to the online Supplementary Results). The repeated measures ANCOVA revealed a significant Medication-by-Depression Severity-by-Outcome Type interaction in the ACC (F = 8.10, df = 1, 37, p = 0.007), after passing Bonferroni adjustment for seven multiple ROI comparisons. The interaction fell short of Bonferroni-adjusted significance in the OFC (F = 4.47, df = 1, 37, p = 0.041) and insula (F = 4.90, df = 1, 37, p = 0.033). There were no significant interactions with Medication Order (all p values >0.050).
To understand the significant three-way interaction, we conducted two repeated-measures ANCOVAs for Reward Outcome (n = 41) and Penalty Outcome separately (n = 41 after excluding outliers, please refer to online Supplementary Results).
This revealed a significant Medication-by-Depression Severity-by-Penalty Outcome interaction in the ACC (F = 11.98, df = 1, 38, p = 0.001). Figure 2 demonstrates that under placebo, individuals with higher depressive symptoms had greater ACC activity during penalty outcomes. However, this trend was not found under lurasidone. Put simply, brain activity to penalties in the ACC in individuals with elevated depression scores under lurasidone, but not placebo, resembles brain activity of individuals with low depressive symptoms. In keeping with this result, we found that ΔACC (the difference between neural activity under lurasidone and placebo) was negatively correlated with depression severity. Figure 3 illustrates the finding that the absolute difference in neural activity between lurasidone and placebo increased as a function of depression scores.
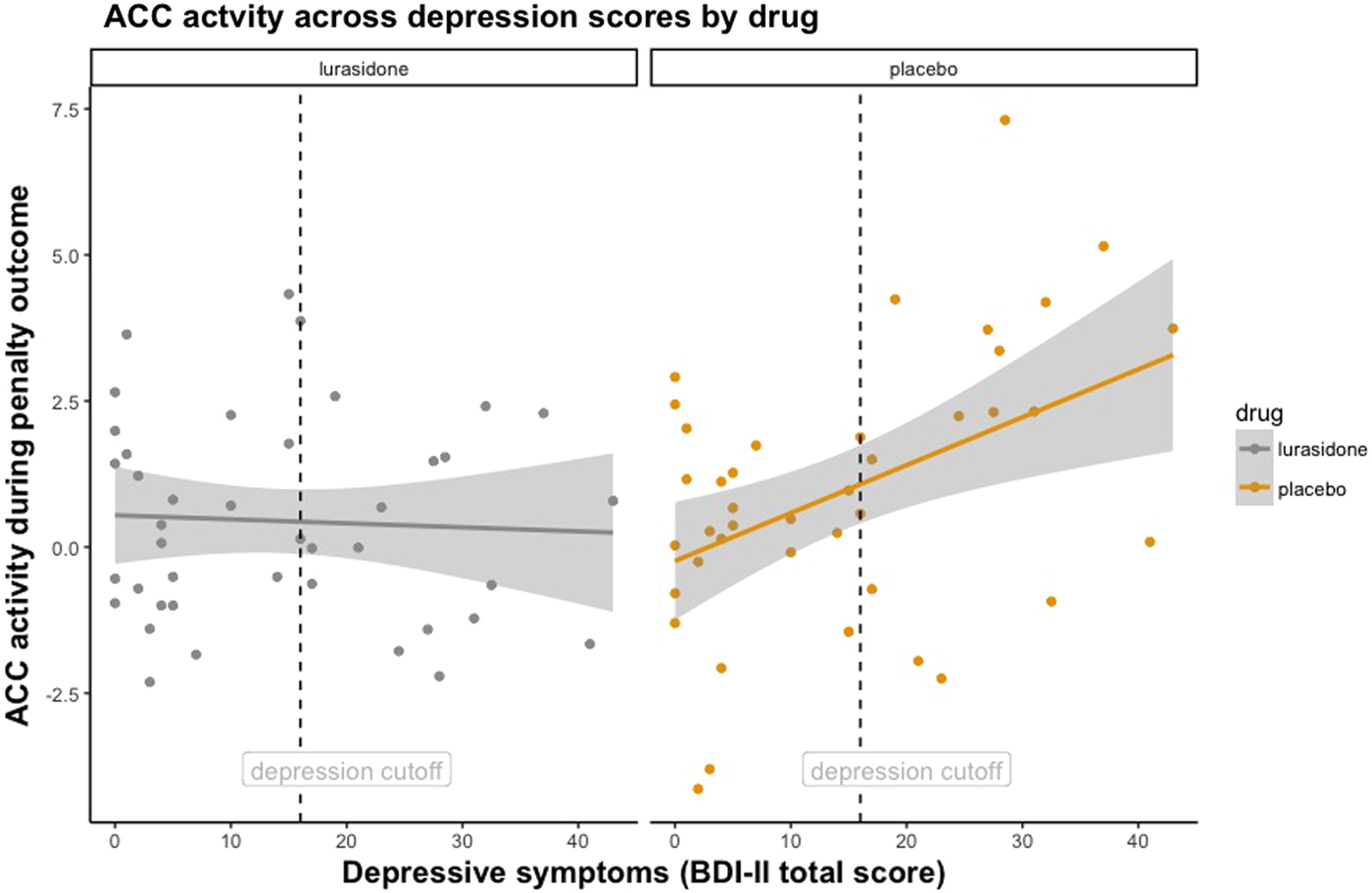
Fig. 2. Facet plot illustrating ACC response during Penalty Outcome across continuous depression scores under lurasidone and placebo. Dashed vertical line denotes depression severity cut-off score on the BDI-II.

Fig. 3. Intra-individual change in penalty related ACC activity (the difference between neural activity under lurasidone and placebo) as a function of continuous depression scores.
A similar pattern of results, namely a signal normalisation, was found in the OFC (F = 4.94, df = 1, 37, p = 0.032), but the interaction fell short of significance after Bonferroni adjustment (see the online Supplementary Results).
We then examined the Medication-by-Depression Severity-by-Reward Outcome interaction across the seven ROIs. This also displayed a pattern of signal normalisation, although in an opposite direction to Penalty Outcome, as lurasidone had its strongest effect of increasing responses to reward outcomes in individuals with high depression severity. This trend fell short of significance in the NAcc (F = 4.87, df = 1, 38, p = 0.033) and ACC (F = 5.92, df = 1, 37, p = 0.020) following Bonferroni correction.
Secondary analyses
Complementing the primary (continuous variable) analyses, we sought to replicate our results using categorical analyses. A repeated-measures ANOVA with Medication (placebo, lurasidone) and Outcome Type (Reward Outcome v. Penalty Outcome) as the within-subject variables and Depression Group (low v. high depressive symptoms) and Medication Order as the between-subject factors (n = 40), revealed a significant Medication-by-Depression Group-by-Outcome Type interaction in the ACC (F = 8.68, df = 1, 38, p = 0.005).
Figure 4 illustrates these findings using BDI-II cut-off scores, with individuals with low depressive symptoms (total BDI-II score: 0–16, n = 24) v. high depressive symptoms (total BDI-II score: 17–43, n = 18). Post-hoc t tests showed that participants with high depressive symptoms receiving placebo had significantly greater ACC activation to Penalty Outcomes than participants with high depressive symptoms receiving lurasidone (T = 2.17, df = 19, p = 0.043), and participants with low depressive symptoms receiving placebo (T = 2.32, df = 37, p = 0.026). There was no significant difference between individuals with high BDI-II scores on lurasidone and individuals with low BDI-II scores on placebo (T = 0.48, df = 37, p = 0.634). Together, these findings indicate that brain activity to penalties in the ACC in individuals with elevated depression scores under lurasidone, but not placebo, resembles brain activity of healthy volunteers.

Fig. 4. Box plot illustrating ACC Response to Penalty Outcomes (loss > avoided loss). Depression severity cut-off scores from the BDI-II, with individuals with low depressive symptoms (total BDI-II score: 0–16, n = 24) v. high depressive symptoms (total BDI-II score: 17–43, n = 18).
To summarise, across reward and penalty outcomes, lurasidone had its strongest effect of increasing responses to reward outcomes and decreasing responses to penalty outcomes in individuals with high depression severity (Figs 2–4). The pattern and significance of the results remained when the outliers were included in the analysis (see the online Supplementary Results).
Response to cues
In contrast to the outcome results, there were no significant interactions with depression in the anticipation phase of the task. Instead, the repeated measures ANCOVA revealed a significant Medication-by-Anticipation Cue interaction in the ACC (F = 8.16, df = 2, 72, p = 0.001) and caudate (F = 7.78, df = 2, 72, p = 0.001). Post-hoc tests show that lurasidone reduced responses to win and loss cues v. placebo, and increased responses for neutral cues in the ACC and caudate. This fell short of significance in the OFC (F = 3.94, df = 2, 72, p = 0.024) and amygdala (F = 3.85, df = 2, 72, p = 0.026).
Anxiety severity analyses and exploratory whole-brain findings for the anticipatory and outcome phases of the task are presented in the online Results Section of the Supplementary data.
Cerebral blood flow (CBF)
In order to ensure that the BOLD results in the ACC were independent of changes in underlying CBF, we tested the effects of acute lurasidone administration on global and regional blood flow. As shown in Fig. 5, a paired-samples t test across the whole-brain showed that lurasidone increased CBF in bilateral putamen relative to placebo during rest in the whole sample (n = 43). Significant increases in blood flow were not observed in the ACC. The repeated measures ANCOVA revealed that the extracted global and striatal CBF values were not related to Depression Severity (F = 0.02, df = 1, 40, p = 0.903), Medication Order (F = 0.44, df = 1, 40, p = 0.903), or any three-way interactions with these respective factors (F = 0.01, df = 1, 40, p = 0.952); (F = 1.10, df = 1, 40, p = 0.300). The change in CBF values for each of the seven ROIs were extracted and used as covariates for the same region in the fMRI BOLD analyses. This did not lead to any changes in the results: non-significant results remained non-significant and significant results remained significant. In particular, the Medication-by-Depression Severity-by-Outcome Type interaction in the ACC (F = 8.13, df = 1, 36, p = 0.007).

Fig. 5. Increased CBF in bilateral putamen for lurasidone relative to placebo during rest in the whole sample (n = 43). Significant at the peak level whole-brain analyses, family-wise error-corrected (left putamen x = −26, y = −4, z = 2, t = 6.15: p = 0.002, right putamen x = 28, y = −2, z = 2, t = 5.50: p = 0.015). Bar represents T-value.
Discussion
In this study, we compared the effects of lurasidone and placebo on neural responding to reward and penalties in medication-naïve young-adult subjects across the range of depression severity. During the anticipation phase of the task, we found that lurasidone reduced responses to win and loss cues v. placebo, and increased responses for neutral cues in the ACC and caudate across the entire sample (i.e. regardless of depression severity). We found that brain activity in the ACC to Penalty Outcomes in individuals with high symptoms of depression under lurasidone, but not placebo, resembled brain activity of individuals with low symptoms of depression. Specifically, lurasidone reduced ACC signalling to negative feedback in young people with elevated depressive symptoms. Increased regional and global blood flow under lurasidone did not drive the BOLD findings. These results provide evidence for abnormalities in neural reward-penalty systems in depression and highlight the potential of targeted pharmacological treatments (dopaminergic agents) to normalise penalty related processing in depression.
Our findings are consistent with the notion that acute dose of drugs with antidepressant properties, either as used in monotherapy or combination treatment, can have an effect on brain processes implicated in depression (Harmer et al., Reference Harmer, Duman and Cowen2017). For example, SSRIs reduce negative bias and amygdala response to negative emotional stimuli (Harmer et al., Reference Harmer, O'Sullivan, Favaron, Massey-Chase, Ayres, Reinecke, Goodwin and Cowen2009; Murphy et al., Reference Murphy, Norbury, O'Sullivan, Cowen and Harmer2009). In our study, the effects of normalisation where localised to the ACC, a region that integrates diverse striatal and prefrontal functions (Haber and Knutson, Reference Haber and Knutson2010). For example, the ACC and ventral striatum (VS) show functional connectivity at rest (Pan et al., Reference Pan, Sato, Salum, Rohde, Gadelha, Zugman, Mari, Jackowski, Picon, Miguel, Pine, Leibenluft, Bressan and Stringaris2017) and input from the ACC to the VS allows for flexible deployment and adaptation of behaviour to changing circumstances (Holroyd and Coles, Reference Holroyd and Coles2002; Walton et al., Reference Walton, Croxson, Behrens, Kennerley and Rushworth2007; Alexander and Brown, Reference Alexander and Brown2011; Holroyd and Yeung, Reference Holroyd and Yeung2012; Walsh and Anderson, Reference Walsh and Anderson2012; Holroyd and Umemoto, Reference Holroyd and Umemoto2016; Umemoto and Holroyd, Reference Umemoto and Holroyd2016; Shahnazian and Holroyd, Reference Shahnazian and Holroyd2017). Electrophysiological (electroencephalogram) studies have shown that the feedback negativity (FRN), an event-related potential which indicates the early appraisal of feedback and appears larger following the presentation of negative feedback, has its origins in the ACC (Gehring and Willoughby, Reference Gehring and Willoughby2002; Holroyd and Coles, Reference Holroyd and Coles2002; Holroyd et al., Reference Holroyd, Larsen and Cohen2004; Hajcak et al., Reference Hajcak, Holroyd, Moser and Simons2005; Yeung et al., Reference Yeung, Holroyd and Cohen2005). Specifically, an FRN signal may be generated as ACC neurons shift from encoding expected to actual outcomes (i.e. a prediction error signal) (Hyman et al., Reference Hyman, Holroyd and Seamans2017). In our study, participants with higher depression severity on placebo showed greater ACC response to negative feedback. This is congruent with evidence of heightened sensitivity to negative outcomes in depression and its association with elevated loss-related signals in the ACC, and connected regions such as the anterior insula and striatum.
It has been postulated that increased ACC activity in depressed individuals to loss outcomes reflects biased stimuli representations that mediate choice behaviour, including preferential attention, planning and self-referential processing towards losses (Sylvester et al., Reference Sylvester, Wager, Lacey, Hernandez, Nichols, Smith and Jonides2003; Grimm et al., Reference Grimm, Ernst, Boesiger, Schuepbach, Hell, Boeker and Northoff2009; Gotlib et al., Reference Gotlib, Hamilton, Cooney, Singh, Henry and Joormann2010). A normalisation of ACC response in depressed individuals on lurasidone therefore suggests that lurasidone may act to decrease salience and processing of loss events.
Inter-individual differences between low and high depression severity subjects could account for the findings that lurasidone attenuated response to penalty outcomes in individuals with high depression severity only. Indeed, depression is associated with baseline differences in availability and function of 5-HT and/or D2 receptors and reductions in binding relative to healthy volunteers (Suhara et al., Reference Suhara, Nakayama, Inoue, Fukuda, Shimizu, Mori and Tateno1992; Yatham et al., Reference Yatham, Liddle, Dennie, Shiah, Adam, Lane, Lam and Ruth1999; Sheline et al., Reference Sheline, Mintun, Barch, Wilkins, Snyder and Moerlein2004; Yatham et al., Reference Yatham, Goldstein, Vieta, Bowden, Grunze, Post, Suppes and Calabrese2005). Thus, in accordance with previous findings that more divergent patterns of reward/penalty processing at baseline are associated with greater post-intervention change (Vrieze et al., Reference Vrieze, Pizzagalli, Demyttenaere, Hompes, Sienaert, de Boer, Schmidt and Claes2013; Rice et al., Reference Rice, Rawal, Riglin, Lewis and Dunsmuir2015; Burkhouse et al., Reference Burkhouse, Kujawa, Kennedy, Shankman, Langenecker, Phan and Klumpp2016; Walsh et al., Reference Walsh, Carl, Eisenlohr-Moul, Minkel, Crowther, Moore, Gibbs, Petty, Bizzell, Smoski and Dichter2016), it could be that subjects with more severe depressive symptoms have more ‘room for improvement’ following acute lurasidone administration.
In addition to attenuating penalty outcome responses, lurasidone reduced neural responses in the ACC and caudate during the anticipation of loss and reward cues across the entire sample (i.e. regardless of depression severity). This is in line with studies showing attenuated reward-related striatal activation during reward anticipation and decision making with D2 antagonist haloperidol (Pessiglione et al., Reference Pessiglione, Seymour, Flandin, Dolan and Frith2006; Pleger et al., Reference Pleger, Ruff, Blankenburg, Kloppel, Driver and Dolan2009). However, no effect has been reported for prediction of losses (Pessiglione et al., Reference Pessiglione, Seymour, Flandin, Dolan and Frith2006). There are various mechanisms which could account for the finding in this study, all of which are speculative at the moment. First, lurasidone may modulate tonic and phasic dopamine firing either directly by D2 antagonism or indirectly via antagonism at serotonergic 5-HT receptors (5-HT2A, 5-HT7). Antagonism at D2 receptors could act to block and reduce dopamine release, thereby also attenuating the BOLD signal. Alternatively, lurasidone may, at low doses, like amisulpride increase striatal dopamine release by preferentially blocking presynaptic dopamine auto-receptors. Increased dopamine availability may act to increase tonic levels of dopamine, in turn decreasing the phasic firing of dopamine neurons and the sensitivity of the dopamine reward system (Grace, Reference Grace1991), thereby potentially reducing BOLD signal to anticipation cues. Although it must be noted that ascribing the changes seen to one or more receptor systems is highly speculative as the precise mechanism by which BOLD signal is modulated cannot be determined with fMRI alone.
It is notable, that in line with previous studies utilising dopamine antagonists (Lahti et al., Reference Lahti, Holcomb, Weiler, Medoff and Tamminga2003; Lahti et al., Reference Lahti, Weiler, Medoff, Tamminga and Holcomb2005; Handley et al., Reference Handley, Zelaya, Reinders, Marques, Mehta, O'Gorman, Alsop, Taylor, Johnston, Williams, McGuire, Pariante, Kapur and Dazzan2013; Goozee et al., Reference Goozee, Handley, Kempton and Dazzan2014), we show here that lurasidone increased striatal CBF at rest. Increases in blood flow following antipsychotic lurasidone administration may be related to increased neuronal metabolism in striatal areas due to the large density of D2 receptors (Goozee et al., Reference Goozee, Handley, Kempton and Dazzan2014), with blockade of D2 receptors in the striatum potentially resulting in disinhibition of D2 receptor-containing medium spiny neurons (Fernandez-Seara et al., Reference Fernandez-Seara, Aznarez-Sanado, Mengual, Irigoyen, Heukamp and Pastor2011). Our results showed that the penalty and reward-related findings were unchanged after controlling for baseline shifts in global and striatal CBF, and highlight the utility of multi-modal fMRI in identifying if the effects of the drug administered are indeed neuronal.
Our study also showed a pattern in which lurasidone potentiated striatal (NAcc) activity to reward outcomes in young adults with elevated depressive symptoms. These findings did not survive stringent correction for multiple comparisons and should therefore be interpreted with appropriate caution. We note, that these results are in keeping with recent findings by Admon et al. (Reference Admon, Kaiser, Dillon, Beltzer, Goer, Olson, Vitaliano and Pizzagalli2017) who showed that a single-dose of the dopamine receptor antagonist amisulpride normalises reward processing by increasing reward-related striatal activation and connectivity between the striatum and mid-cingulate cortex in depressed individuals.
This study has several strengths. First, we tested the association between reward processing and depression using randomisation and experimental manipulation, thereby overcoming several of the limitations of correlational studies in drawing causal inferences. Second, the cross-over, within-subject design affords higher statistical power than a parallel design by minimising subject variance as each individual acts as their own control, and increasing the drug variance. Third, we recruited medication-naïve subjects across the range of depression severity, thus avoiding the confound of medication (Pessiglione et al., Reference Pessiglione, Seymour, Flandin, Dolan and Frith2006; Abler et al., Reference Abler, Erk and Walter2007).
This study also has limitations. Caution should be exercised with the interpretation of our results as ‘normalising’. In the absence of any behavioural effect there is no evidence for better performance of the task on lurasidone, there is no clear main effect of depressive symptoms on the task (i.e. no deficit to improve) and no other triangulating measure of response to negative outcomes which can be linked to function. Nevertheless, it could be argued that lurasidone changes an activity in the ACC that might be beneficial. Further studies need to address both the behavioural deficit and the neural changes in parallel. This has proven challenging as it requires alignment between different levels of explanation including task, neural, clinical and behavioural (Keren et al., Reference Keren, O'Callaghan, Vidal-Ribas, Buzzell, Brotman, Pan, Meffert, Kaisera, Wolke, Pine and Stringaris2018).
Our study was not designed to capture changes in depressive symptoms following lurasidone and therefore it is unclear how these would correlate with brain responses. However, our strategy of searching for the signal of an intervention in the first place is consistent with current recommendations to boost drug discovery (Krystal and State, Reference Krystal and State2014). The next piece of information which would be needed to infer causality, is whether lurasidone-induced neural changes (reduced penalty related ACC signalling and increased reward-related NAcc signalling) predict a decline in depressive and anhedonic symptoms (Shiroma et al., Reference Shiroma, Thuras, Johns and Lim2014; Godlewska et al., Reference Godlewska, Browning, Norbury, Cowen and Harmer2016). This would require longer-term lurasidone treatment in longitudinal studies with assessment of pre-post changes in behavioural and neural responses. Antidepressants seem to exacerbate reward deficits early in treatment (Kumar et al., Reference Kumar, Waiter, Ahearn, Milders, Reid and Steele2008; McCabe et al., Reference McCabe, Mishor, Cowen and Harmer2010; Marutani et al., Reference Marutani, Yahata, Ikeda, Ito, Yamamoto, Matsuura, Matsushima, Okubo, Suzuki and Matsuda2011) prior to normalisation following longer-term (2–6 week) treatment (Stoy et al., Reference Stoy, Schlagenhauf, Sterzer, Bermpohl, Haegele, Suchotzki, Schmack, Wrase, Ricken, Knutson, Adli, Bauer, Heinz and Stroehle2012; Scholl et al., Reference Scholl, Kolling, Nelissen, Browning, Rushworth and Harmer2017; Walsh et al., Reference Walsh, Browning, Drevets, Furey and Harmer2017). Thus, in line with longer-term dosing studies, repeated dosing with lurasidone could lead to increasing anticipation of rewards with more chronic exposure to the drug. Although speculative, one could predict a behavioural activation model of the antidepressant mechanism of action of lurasidone, with normalisation of responses to outcomes (consummation), prior to a normalisation of neural anticipatory signals with longer-term treatment (Dimidjian et al., Reference Dimidjian, Barrera, Martell, Munoz and Lewinsohn2011).
We note that we used two contrasts for the outcome type: reward and penalty outcome. Whilst this is standard in the literature in similarly designed studies (Admon et al., Reference Admon, Kaiser, Dillon, Beltzer, Goer, Olson, Vitaliano and Pizzagalli2017), an alternative modelling could be four levels: reward, missed reward, penalty and avoided penalty outcomes relative to no incentive outcomes. In addition, the recruitment was designed for analysis of depressive symptoms as a continuum, and as such any analysis of those with higher scores contrasted with lower scores may be underpowered. Interestingly we were able to replicate the results of Admon et al. (Reference Admon, Kaiser, Dillon, Beltzer, Goer, Olson, Vitaliano and Pizzagalli2017), as lurasidone potentiated striatal (NAcc) activity to reward outcomes using such categories but this did not survive correction for multiple comparisons.
In conclusion, our study shows that an acute dose of dopaminergic agent, lurasidone, transiently decreased penalty related ACC activity in individuals with high symptoms of depression. These findings suggest that modulation of dopamine transmission may help to normalise processing of negative outcomes in depressed individuals through the alteration of ACC signalling. Thus, ACC signalling may provide a new target for engagement in future drug development studies. Using an experimental medicine design such as the one used in this study, could help identify relevant compounds which could then be tested further in using longer-term follow-up.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718003306.
Acknowledgements
We thank all participants who have generously given their time to taking part in this study. We thank the Wellcome Trust (093909/Z/10/A) and National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) at South London and Maudsley NHS Foundation Trust and King's College London for funding this independent research project. We also thank the NIHR-BRC for funding Selina Wolke's Ph.D. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. We thank Miss Hazel Deacon for her assistance in administration and data entry. We also thank Brian Knutson for providing the images used in the MID task. Preliminary results from this study were presented (via poster) at the American Academy of Child and Adolescent Psychiatry (AACAP) 63rd Annual Meeting, New York, NY, USA, 24–29 October 2016 and the International Society for Bipolar Disorders Annual Conference, Washington DC, USA, 4–7 May 2017.
Funding
This study was funded by the Wellcome Trust (093909/Z/10/A) and National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) at South London and Maudsley NHS Foundation Trust and King’s College London.
Disclosure
Selina Wolke’s Ph.D. is funded through a 3-year National Institutes of Health Research (NIHR) Maudsley Biomedical Research Centre (BRC) Ph.D. Studentship based at the Institute of Psychiatry, Psychology and Neuroscience, King’s College London. Dr Argyris Stringaris has received funding from the Wellcome Trust and the UK National Institutes of Health Research, funds from University College London for a joint project with Johnson & Johnson, and royalties from Cambridge University Press and Oxford University Press. Prof. Mitul Mehta has received research funding from Eli Lilly, Roche and Takeda and has acted as a consultant for Cambridge Cognition and Lundbeck. Prof. Allan Young is employed by King’s College London and is an Honorary Consultant SLaM (NHS UK) and has received grant funding from the Medical Research Council and Wellcome Trust (UK). Prof. Young is part of advisory boards for all major pharmaceutical companies with drugs used in affective and related disorders and has no shareholdings in pharmaceutical companies. Prof. Young has initiated studies for Embolden Study (AZ), BCI Neuroplasticity study, Aripiprazole Mania Study, Eli Lilly, Lundbeck and Wyeth. Dr Owen O’Daly and Dr Fernando Zelaya are with the Institute of Psychiatry, Psychology and Neuroscience, King’s College London and report no potential conflicts of interest. Dr Nada Zahreddine is based at the Department of Psychiatry, Saint-Joseph University, Beirut, Lebanon and has nothing to declare. Drs Stringaris, Leibenluft, Pine, Keren and O’Callaghan are with the National Institute of Mental Health and have nothing to declare.
Author ORCIDs
Selina A. Wolke, 0000-0001-6762-0456; Allan H. Young, 0000-0003-2291-6952.








