Schizophrenia is sexually dimorphicFootnote 1 in its incidence, onset, and course. During the primary reproductive years, women tend to show a lower cumulative incidence, later onset (by ~3–5 years), and milder course than men (Häfner, Reference Häfner2019; Jones, Reference Jones2013; Riecher-Rössler, Butler, & Kulkarni, Reference Riecher-Rössler, Butler and Kulkarni2018), yet dramatic shifts in sex-differentiated patterns of risk occur at midlife (~age 40–50 years old). Moreover, while the peak onset age in both men and women is in early adulthood (Häfner, Maurer, Loffler, & Riecher-Rossler, Reference Häfner, Maurer, Loffler and Riecher-Rossler1993), women show a second, midlife peak in the incidence of schizophrenia. Indeed, most midlife onset cases of schizophrenia occur in women (Riecher-Rossler, Loffler, & Munk-Jorgnsen, Reference Riecher-Rossler, Loffler and Munk-Jorgnsen1997), and first hospital admission rates for psychosis after age 40 have been found to be twice as high in women (21%) than men (10%) (Häfner et al., Reference Häfner, Maurer, Loffler and Riecher-Rossler1993; Riecher-Rossler et al., Reference Riecher-Rossler, Loffler and Munk-Jorgnsen1997). In addition, women with an existing diagnosis experience midlife increases in illness severity, which persist into later stages of adulthood. These midlife changes in incidence and severity are not observed in men of similar ages (Kirkbride et al., Reference Kirkbride, Errazuriz, Croudace, Morgan, Jackson, Boydell and Jones2012; Ochoa, Usall, Cobo, Labad, & Kulkarni, Reference Ochoa, Usall, Cobo, Labad and Kulkarni2012; Polachek, Manor, Baumfeld, & Bagadia, Reference Polachek, Manor, Baumfeld and Bagadia2017; Van Der Werf et al., Reference Van Der Werf, Hanssen, Köhler, Verkaaik, Verhey, Van Winkel and Allardyce2014).
Although a handful of studies have failed to find strong age effects on risk for psychosis in women (likely due to methodological limitations, such as truncating data collection at age 45; e.g. Kühl, Laursen, Thorup, & Nordentoft, Reference Kühl, Laursen, Thorup and Nordentoft2016), findings from systematic reviews and the vast majority of empirical studies confirm a female-specific increase in psychosis during midlife (Kirkbride et al., Reference Kirkbride, Errazuriz, Croudace, Morgan, Jackson, Boydell and Jones2012; Ochoa et al., Reference Ochoa, Usall, Cobo, Labad and Kulkarni2012; Van Der Werf et al., Reference Van Der Werf, Hanssen, Köhler, Verkaaik, Verhey, Van Winkel and Allardyce2014). This female predominance of midlife risk is striking and may provide critical clues to etiologic underpinnings of psychosis, particularly sex-specific factors. Indeed, it has been posited that these epidemiological patterns are tied to pathophysiological processes unique to women and the midlife period of life (Castle, Sham, & Murray, Reference Castle, Sham and Murray1998; Häfner et al., Reference Häfner, Maurer, Loffler and Riecher-Rossler1993). Naturally, the predominant theory over the past 30 years has focused on the menopausal transition (i.e. perimenopause) and its associated changes in ovarian hormones as potential mechanisms of midlife psychosis risk in women (Häfner, Reference Häfner2019; Häfner et al., Reference Häfner, Maurer, Loffler and Riecher-Rossler1993; Kulkarni, Hayes, & Gavrilidis, Reference Kulkarni, Hayes and Gavrilidis2012; Riecher-Rössler & Häfner, Reference Riecher-Rössler and Häfner1993; Riecher-Rössler, Häfner, Stumbaum, Maurer, & Schmidt, Reference Riecher-Rössler, Häfner, Stumbaum, Maurer and Schmidt1994).
Nonetheless, menopause and its related hormonal changes are not the only possible explanation for midlife psychosis in women. Other factors relevant to midlife, such as life stressors (e.g. changes in work, health/illness, marital status/residence), could also contribute to increased risk for psychosis (Holtzman et al., Reference Holtzman, Trotman, Goulding, Ryan, MacDonald, Shapiro and Walker2013; Thomas, Mitchell, & Woods, Reference Thomas, Mitchell and Woods2019). Although these factors are not sex-specific, i.e. men endure these same midlife changes/stressors, gender differences in the prevalence of some types of stressors and in responsivity to stress have been described (see Almeida, Reference Almeida2005; Bangasser & Wiersielis, Reference Bangasser and Wiersielis2018; Ter Horst, Wichmann, Gerrits, Westenbroek, & Lin, Reference Ter Horst, Wichmann, Gerrits, Westenbroek and Lin2009; Thomas, Mitchell, & Woods, Reference Thomas, Mitchell and Woods2018). Understanding why the midlife period is a time of heightened risk for psychosis in women may substantially advance etiologic theories, treatment approaches, and women's health by providing a clearer understanding of key mechanisms of risk and identifying promising targets for more tailored and effective treatment. Such etiological advances may also contribute to discussions of equifinality in schizophrenia – whereby a diversity of pathways may lead to mechanistically disparate but clinically similar syndromes (Green & Glausier, Reference Green and Glausier2016). A better understanding of the etiologic significance of midlife, presumably via its associated hormonal and psychosocial correlates, may help unpack mechanistic heterogeneity in the illness and could have broad implications for understanding differential risk across age groups, genders, and sexes.
In this narrative review, we review clinical and pre-clinical data examining the potential role of menopause and ovarian hormones in heightening midlife psychosis risk in women. A narrative, rather than systematic, review was conducted because of the paucity of data directly examining the midlife/menopause period and our intent to provide a broad topical overview and thematic synthesis of data obtained from an array of methodological approaches (e.g. experimental animal studies, correlational data, pharmaceutical trials). We focus our review on the diagnosis of schizophrenia as well as psychotic symptoms, which are a defining feature of the disorder. Psychosis is broadly characterized by a gross departure from consensual reality and includes delusions (e.g. false beliefs) and hallucinations (e.g. false perceptions), whereas schizophrenia is a heterogeneous disorder that encompasses a number of symptoms, including positive psychotic symptoms (e.g. delusions, hallucinations), negative symptoms (e.g. lack of motivation, flat affect), and disorganized symptoms (American Psychiatric Association, 2013). In addition, we review the influence of ovarian hormones on schizophrenia-like phenotypes in animal studies, which offer a translational perspective on midlife psychosis risk in women. We begin by providing an overview of the menopausal transition, and then we focus on evidence for a role of the menopausal transition and ovarian hormones in psychosis risk. We end by discussing current gaps in the literature and important areas for future research.
Overview of search strategy and criteria
Although this is a narrative review, we believe it is important to outline our broad approach to article identification and selection. Articles were obtained by searching PubMed, PsychInfo, and Google Scholar databases for key terms: psychosis, psychotic disorder, psychotic-like, schizophrenia, midlife, menopause, perimenopause, postmenopause, estrogen, estradiol, progesterone, ovarian hormones, ovarian function. Articles were also identified from references within published articles. Article abstracts were screened for relevance, and studies were included if the study design and findings addressed midlife (ages 40–60), menopausal, and/or ovarian hormone effects on psychotic symptoms in women. Sixty-four manuscripts, including some systematic reviews and meta-analyses, met these criteria and were included in our review.
Defining menopausal stages
Broadly speaking, there are four main reproductive stages during midlife in women: premenopause, perimenopause, menopause, and postmenopause. Women are considered to be in premenopause (typically up to ~age 45) when they are having regular menstrual cycles and are not experiencing the vasomotor symptoms (e.g. hot flashes, flushes, night sweats) that are common during later menopausal stages (see below) (Gold, Reference Gold2011; Harlow et al., Reference Harlow, Gass, Hall, Lobo, Maki, Rebar and De Villiers2012). During premenopause, estradiol and progesterone levels are relatively high and fluctuate in predictable patterns that track with periods of ovulation (e.g. increased estradiol prior to ovulation; increased progesterone after ovulation) (see Fig. 1; Harlow et al., Reference Harlow, Gass, Hall, Lobo, Maki, Rebar and De Villiers2012; Prior, Reference Prior2006). Following premenopause, the female hypothalamic-pituitary-gonadal axis undergoes dramatic changes, particularly in the production and secretion of ovarian hormones and variability in ovarian follicle development, ovulation, and bleeding patterns. Moreover, as a result of ovarian follicle depletion, the ovary no longer responds to key pituitary hormones [i.e. follicle-stimulating hormone (FSH); luteinizing hormone] that regulate the menstrual cycle, and the production of ovarian estrogen and progesterone eventually ceases (Harlow et al., Reference Harlow, Gass, Hall, Lobo, Maki, Rebar and De Villiers2012). These changes associated with reproductive aging occur over the course of the menopause transition (i.e. perimenopause), menopause, and postmenopausal stages.
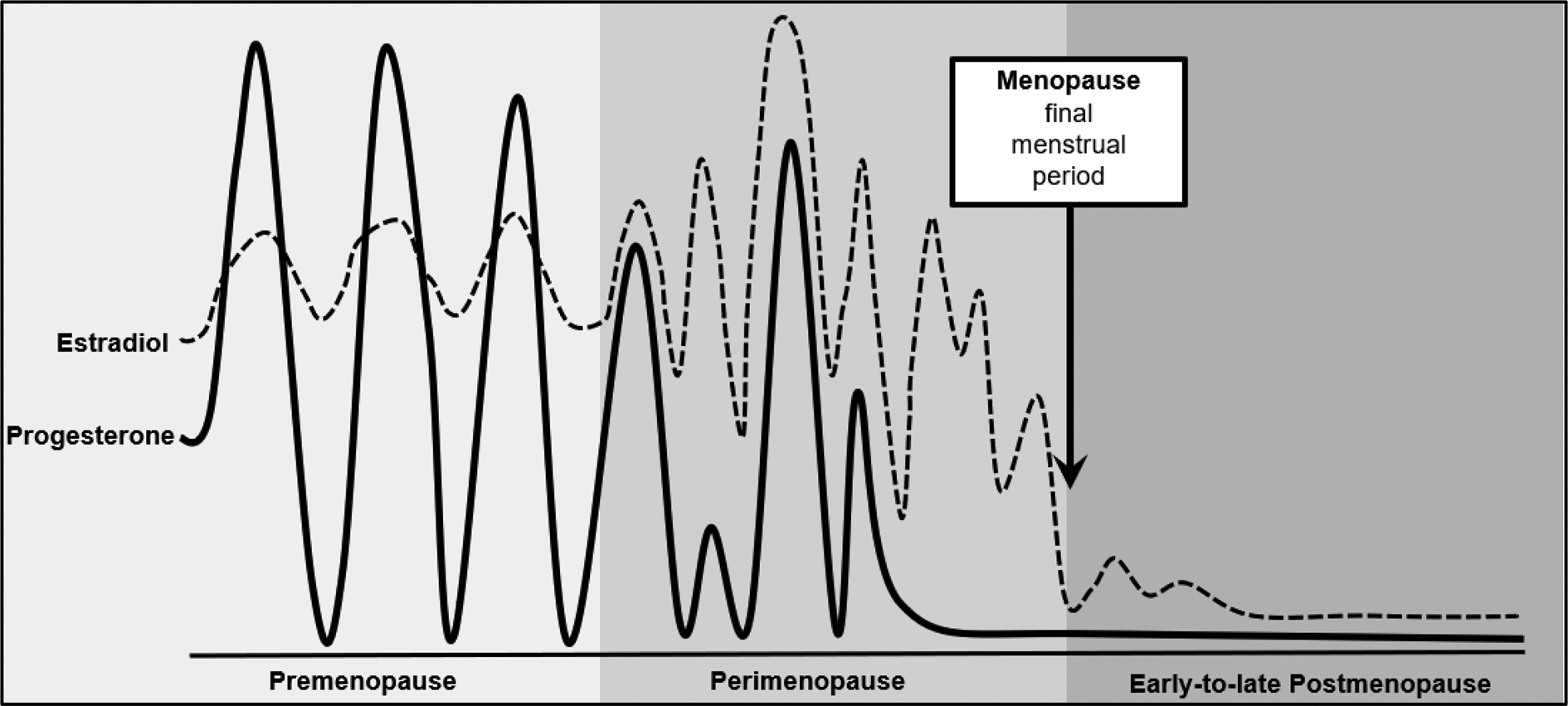
Fig. 1. Changes in ovarian hormones across reproductive stages. Figure modified from source: Prior (Reference Prior2006).
Most women begin perimenopause in their 40s; the perimenopause transition is marked by changes in menstrual cycle timing and frequency, decreased fertility, and in the vast majority of women (~80%), the onset of vasomotor symptoms and insomnia (Harlow et al., Reference Harlow, Gass, Hall, Lobo, Maki, Rebar and De Villiers2012; Williams et al., Reference Williams, Kalilani, Dibenedetti, Zhou, Granger and Fehnel2008). Specifically, women shift from premenopausal status to perimenopause once they experience a cycle length change that is ≥7 days and occurs for at least two consecutive cycles and/or have two consecutively skipped cycles (Harlow et al., Reference Harlow, Gass, Hall, Lobo, Maki, Rebar and De Villiers2012). The culmination of these reproductive patterns and symptoms largely arises from the increase in FSH (as the number of ovarian follicles drop) and changes in estrogen. Although estrogen declines during perimenopause, levels can dramatically flux – rising and falling unevenly, and in some cases, rising to levels even higher than in premenopause (see Fig. 1; Prior, Reference Prior2006). Progesterone secretion is dependent upon ovulation; in cycling women, progesterone levels rise during the post-ovulatory/luteal phase of the menstrual cycle to prepare the uterus for a fertilized egg and to help maintain early pregnancy. Thus, during the perimenopausal period, progesterone production becomes irregular and will eventually stop, as ovulation ceases (Harlow et al., Reference Harlow, Gass, Hall, Lobo, Maki, Rebar and De Villiers2012; Prior, Reference Prior2006).
Women reach menopause when they have gone 12 consecutive months without menses (Harlow et al., Reference Harlow, Gass, Hall, Lobo, Maki, Rebar and De Villiers2012), typically between the ages of 45 and 55 (Boldsen & Jeune, Reference Boldsen and Jeune1990; Chan, Gomes, & Singh, Reference Chan, Gomes and Singh2020; Gold, Reference Gold2011). Following this event, women are considered to be in the last reproductive phase – postmenopause. The postmenopausal period is characterized by low levels of estradiol and progesterone; however, some continued (albeit more intermittent) fluctuations in estradiol (see Fig. 1; Harlow et al., Reference Harlow, Gass, Hall, Lobo, Maki, Rebar and De Villiers2012; Prior, Reference Prior2006) and vasomotor symptoms continue to occur during the early postmenopause phase in most women (Williams et al., Reference Williams, Kalilani, Dibenedetti, Zhou, Granger and Fehnel2008). Leading guidelines (e.g. STRAW + 10) therefore consider the initial early postmenopause period (e.g. first year after final menses) to be a part of perimenopause (Harlow et al., Reference Harlow, Gass, Hall, Lobo, Maki, Rebar and De Villiers2012).
Psychosis and menopausal stage
As noted by others (e.g. Häfner, Reference Häfner2019; Kulkarni et al., Reference Kulkarni, Hayes and Gavrilidis2012; Riecher-Rössler, Reference Riecher-Rössler, Soares and Warren2009), the striking overlap between the midlife spike in schizophrenia/psychosis and age of onset of the menopausal transition (both between ages 40 and 50 years old) strongly suggests that processes involved in menopause may contribute to midlife psychosis risk in women. Remarkably, however, studies generally have not directly examined menopausal stage but have instead have relied on age as an indirect marker of the menopausal transition. Unfortunately, age as a proxy for menopausal changes is problematic since menopausal status cannot be reliably predicted by chronological age (Boldsen & Jeune, Reference Boldsen and Jeune1990; Chan et al., Reference Chan, Gomes and Singh2020; Gold, Reference Gold2011; Greer, Sandridge, & Chehabeddine, Reference Greer, Sandridge and Chehabeddine2003; Stanford, Hartge, Brinton, Hoover, & Brookmeyer, Reference Stanford, Hartge, Brinton, Hoover and Brookmeyer1987). Although most women begin experiencing perimenopausal symptoms in their 40s, there is significant variability in the age at onset and length of perimenopause (mean length = 4 years; range 6 months – 8 years) and the age at which postmenopause is reached (median ~51 years; range 30–62 years of age) (Boldsen & Jeune, Reference Boldsen and Jeune1990; Chan et al., Reference Chan, Gomes and Singh2020; Gold, Reference Gold2011; Greer et al., Reference Greer, Sandridge and Chehabeddine2003; Harlow et al., Reference Harlow, Gass, Hall, Lobo, Maki, Rebar and De Villiers2012; Stanford et al., Reference Stanford, Hartge, Brinton, Hoover and Brookmeyer1987). Consequently, during midlife (i.e. ages 40–60), women of the same age can be in very different menopausal stages (i.e. premenopause, perimenopause, and postmenopause). This variability makes it difficult to conclude that midlife age effects are, de facto, menopausal stage effects.
Only one prior study has explicitly tested whether the menopausal transition is associated with increased psychotic symptoms (e.g. paranoid ideation, psychotism scores; Rössler, Ajdacic-Gross, Riecher-Rössler, Angst, & Hengartner, Reference Rössler, Ajdacic-Gross, Riecher-Rössler, Angst and Hengartner2016). This study examined women from the community who were not selected for psychosis risk and found no significant associations between psychotic symptoms and menopausal stage. However, this study also did not find consistent midlife age effects for psychotic symptoms or well-replicated age/menopausal status effects for other psychiatric symptoms (e.g. depression). These null findings lead to concerns that this smaller (N ~ 50 women per menopausal stage) unselected and community sample of participants made it difficult to detect changes in psychosis and psychiatric risk. Furthermore, the broad measure of ‘psychoticism’ employed in this study is better described by two factors (Pedersen, Urnes, Kvarstein, & Karterud, Reference Pedersen, Urnes, Kvarstein and Karterud2016): one describing schizotypal signs and one describing core features of clinical psychosis; it is possible that combining all items into a unitary measure may have obscured true relationships between psychotic symptoms and menopausal stage.
Clearly, additional large-scale studies are needed to directly tie menopausal status to midlife psychosis risk in women. In addition to conducting in-depth staging of menopausal status, it will be important for these studies to rule out other factors that could influence or account for increased psychosis risk in women during midlife (e.g. changes in relationship status, employment, stress and distress levels, history of psychopathology; Holtzman et al., Reference Holtzman, Trotman, Goulding, Ryan, MacDonald, Shapiro and Walker2013; Rössler et al., Reference Rössler, Ajdacic-Gross, Riecher-Rössler, Angst and Hengartner2016; Thomas et al., Reference Thomas, Mitchell and Woods2019). Arguing against a psychosocial explanation are findings from Castle et al. (Reference Castle, Sham and Murray1998), who showed that the increased incidence of psychotic symptoms in women during midlife were present even after adjusting for marital status and premorbid work functioning. Nonetheless, other data suggest that psychosocial distress (e.g. discontent in employment, relationships, health) and a history of psychopathology (e.g. psychotic symptoms, depression) prior to age 41 may significantly contribute to individual differences in risk for psychotic-like symptoms in midlife women (Rössler et al., Reference Rössler, Ajdacic-Gross, Riecher-Rössler, Angst and Hengartner2016). Given the scant number of studies conducted, more research is needed to examine menopausal risk for psychosis in women and for whom risk may be most amplified. Moreover, men also experience changes/stressors during midlife, yet the midlife shift in psychosis risk is not observed among men. The relative influence of midlife changes/stress on psychosis risk may therefore occur via sex-specific processes (e.g. heightened female responsivity to stress that occurs because of sex-differentiated factors, like ovarian hormones; Bangasser & Wiersielis, Reference Bangasser and Wiersielis2018; Ter Horst et al., Reference Ter Horst, Wichmann, Gerrits, Westenbroek and Lin2009) that would render women more vulnerable to midlife experiences of stress – a possibility that has yet to be examined but warrants further attention.
Ovarian hormones
Perhaps not surprisingly, theories of menopausal risk have focused quite heavily on the potential role of ovarian hormones (i.e. estrogen, progesterone) in midlife psychosis risk in women. The notion that ovarian hormones might contribute to psychotic symptomatology dates back to the late 1800s and early 1900s when psychiatrists first described chronic hypoestrogenism, ‘menstrual psychosis’, and increases in psychosis following changes in ovarian functioning (via postpartum or ovarian surgery) (see Bergemann, Parzer, Runnebaum, Resch, & Mundt, Reference Bergemann, Parzer, Runnebaum, Resch and Mundt2007; Riecher-Rössler & Häfner, Reference Riecher-Rössler and Häfner1993).
Since that time, there has been increasing acceptance of the ‘estrogen protection hypothesis’ that posits that estradiol's known neuroprotective effects on cognition, presumably via its effects on neurotransmitter systems (e.g. dopamine, serotonin, glutamate, GABA), decreases female risk for schizophrenia from puberty to the start of perimenopause (Kulkarni et al., Reference Kulkarni, Hayes and Gavrilidis2012; Riecher-Rössler et al., Reference Riecher-Rössler, Häfner, Stumbaum, Maurer and Schmidt1994, Reference Riecher-Rössler, Butler and Kulkarni2018; Seeman, Reference Seeman1996). Estradiol has potent genomic (e.g. regulation of the expression of target genes) and rapid non-genomic (e.g. activation of signaling cascades) neuroprotective properties that have far-reaching effects on hormone receptors, regulation of signaling systems, promotion of cell survival and antioxidant processes, enhancement of synaptic plasticity and connectivity, and modulation of inflammatory response (Fuentes & Silveyra, Reference Fuentes and Silveyra2019; Villa, Vegeto, Poletti, & Maggi, Reference Villa, Vegeto, Poletti and Maggi2016). Thus, the dramatic fluctuations and/or drops in estradiol during the menopausal transition may leave the brain vulnerable to age-related changes in function, particularly in neurotransmitter systems that are implicated in psychosis (e.g. dopamine, serotonin, glutamate, GABA; Kulkarni et al., Reference Kulkarni, Hayes and Gavrilidis2012; Stahl, Reference Stahl2018). The dopaminergic system may be particularly relevant given evidence of dopamine dysregulation in schizophrenia and the fact that the majority of antipsychotics drugs act via antagonism of dopamine D2 receptor or interact with serotonin receptors (5-HT1A and 5-HT2A) in combination with D2 receptor antagonism (Horacek et al., Reference Horacek, Bubenikova-Valesova, Kopecek, Palenicek, Dockery, Mohr and Cyril2006; Kulkarni et al., Reference Kulkarni, Hayes and Gavrilidis2012; Oyamada, Horiguchi, & Rajagopal, Reference Oyamada, Horiguchi and Rajagopal2015).
Surprisingly, to date, no studies have examined whether changes in ovarian hormones across the menopausal transition contribute to psychosis risk in midlife. The paucity of data is striking given past theories and the central role for ovarian hormones in menopause and the regulation of neurobiological systems in women. Nonetheless, animal and human data across other reproductive stages (e.g. menstruation) and studies of treatment effectiveness (e.g. estrogen enhancement of neuroleptics) in women show strong associations between lower estradiol levels and psychosis risk.
In terms of reproductive phase effects, animal studies provide strong experimental evidence of a causal link between ovarian hormones and behavioral phenotypes relevant to positive symptoms of schizophrenia. In intact cycling female rats, schizophrenia-like phenotypes (e.g. deficits in sensorimotor gating, latent inhibition) are reduced during proestrous – the estrous cycle phase that immediately precedes estrus and corresponds to the rise and peak in estradiol (Arad & Weiner, Reference Arad and Weiner2008; Koch, Reference Koch1998; Perez, Donegan, & Lodge, Reference Perez, Donegan and Lodge2019). Similarly, ovariectomy of adult female rats, which removes the primary source of estrogen and progesterone, leads to increased expression of schizophrenia-like phenotypes (e.g. attenuated latent inhibition), whereas exogenous estradiol reverses these effects (Arad & Weiner, Reference Arad and Weiner2009; Sbisa, Van Den Buuse, & Gogos, Reference Sbisa, Van Den Buuse and Gogos2018; Vaillancourt, Cyr, Rochford, Boksa, & Di Paolo, Reference Vaillancourt, Cyr, Rochford, Boksa and Di Paolo2002; Van den Buuse & Eikelis, Reference Van den Buuse and Eikelis2001).
Studies in humans provide direct and indirect evidence for a link between ovarian hormones and psychosis risk in women. Women with schizophrenia have high rates of menstrual irregularities and anovulation and are more likely to have circulating levels of estradiol that fall below the normal reference range (Bergemann et al., Reference Bergemann, Mundt, Parzer, Jannakos, Nagl, Salbach and Resch2005; Huber, Emrich, & Schneider, Reference Huber, Emrich and Schneider2001; Riecher-Rössler et al., Reference Riecher-Rössler, Häfner, Stumbaum, Maurer and Schmidt1994). For example, compared to healthy controls, women with schizophrenia had lower estradiol levels across menstrual cycle days and showed less robust shifts in estradiol concentrations across menstrual cycle phases (Bergemann et al., Reference Bergemann, Mundt, Parzer, Jannakos, Nagl, Salbach and Resch2005; Riecher-Rössler et al., Reference Riecher-Rössler, Häfner, Stumbaum, Maurer and Schmidt1994). These lower estradiol levels are unlikely to be explained by hyperprolactinemia from antipsychotic drug treatment, as some studies documenting reduced estradiol levels were conducted before the era of antipsychotics (for review, see Gogos et al., Reference Gogos, Sbisa, Sun, Gibbons, Udawela and Dean2015); however, whether low estradiol or cycle irregularities/anovulation are driven by other co-occurring factors (e.g. extreme stress, nutritional deficits, low weight from anhedonia; Huhmann, Reference Huhmann2020; Toufexis, Rivarola, Lara, & Viau, Reference Toufexis, Rivarola, Lara and Viau2014) that are associated with schizophrenia and are known to blunt ovarian hormone production remains to be determined.
Additional indirect evidence of ovarian hormone contributions to psychosis risk come from studies of menarche, pregnancy, the postpartum period, and menstrual cycle phases. Late onset of menarche can be indicative of an underlying disturbance or delay in estrogen availability (Klein, Emerick, Sylvester, & Vogt, Reference Klein, Emerick, Sylvester and Vogt2017). Investigators have therefore explored whether delayed puberty is predictive of schizophrenia and its associated neural abnormalities (e.g. connectivity impairments) that are known to be regulated/mediated by estradiol (Bähner & Meyer-Lindenberg, Reference Bähner and Meyer-Lindenberg2017; Gould, Woolley, Frankfurt, & McEwen, Reference Gould, Woolley, Frankfurt and McEwen1990; Woolley, Reference Woolley1998). Consistent with possible estrogen disturbances, later onset of menarche has been associated with increased risk for schizophrenia as well as hippocampal dysconnectivity (Cohen, Seeman, Gotowiec, & Kopala, Reference Cohen, Seeman, Gotowiec and Kopala1999; Damme, Ristanovic, Vargas, & Mittal, Reference Damme, Ristanovic, Vargas and Mittal2020; Riecher-Rössler et al., Reference Riecher-Rössler, Butler and Kulkarni2018). While these effects are promising, the potential mechanisms by which patients with schizophrenia experience late onset of menarche are unclear. Age of onset of menarche is dependent upon a number of genetic and environmental factors, and thus, delayed timing could arise from constitutional growth delays, health/physical abnormalities leading to hypogonadism, and/or life conditions (e.g. stress, inadequate nutrition) that disrupt hormone function (Huhmann, Reference Huhmann2020; Klein et al., Reference Klein, Emerick, Sylvester and Vogt2017).
Additionally, pregnancy, the postpartum period, and the menstrual cycle produce clear shifts in ovarian hormone production that map onto shifts in psychosis risk. Women with schizophrenia show improvements in psychotic symptoms and reductions in relapse during pregnancy – a life stage when women experience substantial elevations in estradiol and progesterone (Chang & Renshaw, Reference Chang and Renshaw1986; Jones, Chandra, Dazzan, & Howard, Reference Jones, Chandra, Dazzan and Howard2014; Kendell, Chalmers, & Platz, Reference Kendell, Chalmers and Platz1987). Conversely, increased vulnerability to new onset or relapse of psychosis has been observed during the postpartum period when levels of ovarian hormones dramatically drop (Brockington, Kelly, Hall, & Deakin, Reference Brockington, Kelly, Hall and Deakin1988; Jones et al., Reference Jones, Chandra, Dazzan and Howard2014; Kendell et al., Reference Kendell, Chalmers and Platz1987; Mahe & Dumaine, Reference Mahe and Dumaine2001; Mota et al., Reference Mota, Chartier, Ekuma, Nie, Hensel, MacWilliam and Bolton2019; Perry, Gordon-Smith, Jones, & Jones, Reference Perry, Gordon-Smith, Jones and Jones2021; VanderKruik et al., Reference VanderKruik, Barreix, Chou, Allen, Say, Cohen and von Dadelszen2017). Similar symptom shifts have been observed across the menstrual cycle in women with schizophrenia, even with the blunted hormone levels described above. An exacerbation of psychotic symptoms and risk for relapse (e.g. rate of hospital admissions) was found during menstrual cycle phases marked by lower estradiol levels (e.g. premenstrual/early follicular), whereas reductions in risk have been observed during phases marked by higher estradiol levels (i.e. mid-luteal) (Bergemann et al., Reference Bergemann, Parzer, Runnebaum, Resch and Mundt2007; Brockington et al., Reference Brockington, Kelly, Hall and Deakin1988; Endo, Daiguji, Asano, Yamashita, & Takahashi, Reference Endo, Daiguji, Asano, Yamashita and Takahashi1978; Gattaz, Vogel, Riecher-Rössler, & Soddu, Reference Gattaz, Vogel, Riecher-Rössler and Soddu1994; Hallonquist, Seeman, Lang, & Rector, Reference Hallonquist, Seeman, Lang and Rector1993; Huber et al., Reference Huber, Emrich and Schneider2001; Riecher-Rössler et al., Reference Riecher-Rössler, Häfner, Stumbaum, Maurer and Schmidt1994; Rubin et al., Reference Rubin, Carter, Drogos, Pournajfi-Nazarloo, Sweeney and Maki2010; Seeman, Reference Seeman1996).
Phase-based symptom fluctuations have also been tied directly to circulating estradiol levels, providing even stronger evidence of hormone-driven changes in psychosis risk. The severity of patients' psychotic symptoms is inversely correlated with levels of estradiol at different phases of the menstrual cycle. Specifically, in normally cycling, pre-menopausal women with schizophrenia, higher estradiol levels are associated with a reduction in psychotic symptoms, whereas lower estradiol levels exacerbate symptom severity (Bergemann et al., Reference Bergemann, Parzer, Runnebaum, Resch and Mundt2007; Huber et al., Reference Huber, Emrich and Schneider2001; Riecher-Rössler et al., Reference Riecher-Rössler, Häfner, Stumbaum, Maurer and Schmidt1994). Because menstrual cycle fluctuations in ovarian hormones occur in a natural and predictive manner among women with normal cycles, it is likely that these hormone–symptom relationships reflect estradiol effects on psychotic symptom expression rather than the reverse.
Finally, studies of shifts in the effectiveness of neuroleptics by estradiol levels have provided additional support for associations between estradiol levels and psychosis. Age-based reductions in antipsychotic medication effectiveness have been observed in women, but not men; before age 40, women with schizophrenia require lower doses of antipsychotic medication yet the dose required for an effective response often increases during midlife (González-Rodríguez & Seeman, Reference González-Rodríguez and Seeman2019; Kulkarni, Reference Kulkarni2009; Kulkarni et al., Reference Kulkarni, Hayes and Gavrilidis2012). Key neurobiological targets of antipsychotic medications (e.g. dopaminergic, serotoninergic, and glutamatergic systems) are modulated by estradiol (Becker & Chartoff, Reference Becker and Chartoff2019; Horacek et al., Reference Horacek, Bubenikova-Valesova, Kopecek, Palenicek, Dockery, Mohr and Cyril2006; Kulkarni et al., Reference Kulkarni, Hayes and Gavrilidis2012), leading researchers to explore whether estrogen can enhance the effects of antipsychotic medications. In ovariectomized female rats, administration of estradiol facilitated the efficacy of antipsychotic medications and reversed schizophrenic-like phenotypes (e.g. attenuated latent inhibition; Arad & Weiner, Reference Arad and Weiner2009). A similar pattern of effects was observed according to ovarian hormone fluctuations across the estrous cycle – gonadally intact female rats displayed schizophrenic-like symptoms and a decreased response to antipsychotic at low hormonal levels (metestrus–diestrus) whereas antipsychotic medication was effective in disrupting the schizophrenic-like behavior when given in a cycle phase associated with high hormonal levels (i.e. proestrus) (Arad & Weiner, Reference Arad and Weiner2008).
Similarly, several clinical trials have provided strong evidence that treatment with estrogens (e.g. estradiol) or selective estrogen receptor modulators (e.g. Raloxifene) in conjunction with antipsychotic medication is beneficial for schizophrenia, in that significant improvements in positive and/or negative psychotic symptoms as well as cognitive performance have been observed in premenopausal and postmenopausal women (Begemann, Dekker, Van Lunenburg, & Sommer, Reference Begemann, Dekker, Van Lunenburg and Sommer2012; Brand, de Boer, & Sommer, Reference Brand, de Boer and Sommer2021; Ghafari et al., Reference Ghafari, Fararouie, Shirazi, Farhangfar, Ghaderi and Mohammadi2013; Gogos et al., Reference Gogos, Sbisa, Sun, Gibbons, Udawela and Dean2015; González-Rodríguez & Seeman, Reference González-Rodríguez and Seeman2019; Kulkarni et al., Reference Kulkarni, Gavrilidis, Wang, Worsley, Fitzgerald, Gurvich and Burger2015; Lindamer, Buse, Lohr, & Jeste, Reference Lindamer, Buse, Lohr and Jeste2001; Wang, Dong, Wang, & Li, Reference Wang, Dong, Wang and Li2018; Weiser et al., Reference Weiser, Levi, Zamora, Biegon, SanGiovanni, Davidson and Davis2019; Zhu et al., Reference Zhu, Zheng, Li, Cai, Yang, Ungvari and Xiang2021). Moreover, findings from a recent double-blind, randomized, placebo-controlled trial revealed age-based effects. Specifically, compared with placebo, women who received estradiol showed significant improvements in psychotic symptoms, but this effect was limited to those who were in a perimenopausal age range (38–46 years old); symptom improvements were not observed in younger women (≤37 years old; Weiser et al., Reference Weiser, Levi, Zamora, Biegon, SanGiovanni, Davidson and Davis2019). Dose–response effects have also been found – women show greater reductions in psychotic symptoms at higher doses of estrogen (González-Rodríguez & Seeman, Reference González-Rodríguez and Seeman2019; Kulkarni et al., Reference Kulkarni, Riedel, De Castella, Fitzgerald, Rolfe, Taffe and Burger2001, Reference Kulkarni, Gavrilidis, Wang, Worsley, Fitzgerald, Gurvich and Burger2015). Together, these findings advance understanding of the clinical relevance of estrogen deficiency in the pathophysiology of psychosis and the likely importance of estrogen augmentation for neuroleptic drug efficacy, especially for women experiencing low estradiol or estradiol declines.
In summary, animal and human data during earlier reproductive stages or across neuroleptic treatment provide initial support for the effects of lower estradiol levels on increased risk for psychosis in women. These data align with the estrogen protection hypothesis and longstanding theories proposing that the decreasing estradiol levels that characterize perimenopause could contribute to midlife psychosis risk in women. However, to date, studies have been relatively few in number, most studies have not directly examined estradiol levels, and no studies have examined whether estrogen contributes to the observed increased risk for psychosis in midlife or during the critical menopausal transition.
Importantly, there has also been a lack of consideration of progesterone in psychosis risk (Bergemann et al., Reference Bergemann, Parzer, Runnebaum, Resch and Mundt2007; González-Rodríguez & Seeman, Reference González-Rodríguez and Seeman2019; Sun, Walker, Dean, van den Buuse, & Gogos, Reference Sun, Walker, Dean, van den Buuse and Gogos2016), including the potential interplay between estrogen and progesterone. Progesterone exerts neuroprotective effects within the CNS (e.g. promotion of cell survival, synaptic plasticity and connectivity; enhanced cognitive function; Singh & Su, Reference Singh and Su2013; Sun et al., Reference Sun, Walker, Dean, van den Buuse and Gogos2016) via its involvement in the modulation of neurotrophic expression (e.g. BDNF) and several neurotransmitter systems (e.g. dopamine, serotonin, glutamate, GABA; Singh & Su, Reference Singh and Su2013) implicated in psychotic disorders. Progesterone also has strong, antagonistic effects on estrogen that alters estradiol action (Asarian & Geary, Reference Asarian and Geary2006; Miner et al., Reference Miner, Martini, Smith, Brunt, Kaplan, Halliwill and Minson2011) and its associated effects on behavior (e.g. binge eating, anxiety; Graham & Daher, Reference Graham and Daher2016; Klump et al., Reference Klump, Keel, Racine, Burt, Neale, Sisk and Hu2013). Because women with schizophrenia have high rates of anovulation and the progesterone surge is dependent on ovulation, at least a subgroup of women with schizophrenia experience persistently low levels of progesterone. Menstrual cycle phase differences in psychotic symptom expression, as well as symptom changes associated with pregnancy and the postpartum period, also indirectly support the possibility that low progesterone could also be risky. Indeed, progesterone is elevated (in addition to estradiol) in reproductive phases (i.e. pregnancy; midluteal cycle phase) associated with reductions in psychotic symptoms, and both progesterone and estradiol are low in phases (e.g. postpartum period; premenstrual/early follicular cycle phases) associated with increased risk. Given progesterone's effects within the CNS and the dramatic decreases in progesterone levels across the menopausal transition, future studies should aim to elucidate the potential joint effects of estrogen and progesterone on psychosis risk.
Discussion
Women show striking midlife increases in psychosis incidence and severity, which are not observed in men. These sex-differentiated patterns provide initial support for a role for the menopausal transition and ovarian hormones in psychosis risk in women. However, the paucity of data directly examining these mechanisms is surprising given extensive theories that have proposed these factors as causal. Thus, there are many gaps in the literature that are critical areas for future research.
The inability of age to reliably predict menopausal status in women highlights the strong need for studies to directly assess menopausal status. There are straightforward criteria available for staging women (e.g. STRAW + 10; Harlow et al., Reference Harlow, Gass, Hall, Lobo, Maki, Rebar and De Villiers2012) that do not require biological samples. These criteria rely on readily identifiable, objective physical signs of menopause (e.g. skipped periods, changes in cycle length, vasomotor symptoms). There are also easy to administer self-report questionnaires that can be used to identify menopausal stage and evaluate the severity of menopausal symptoms, which may offer additional information on the impact of the menopausal transition on psychosis risk. Future studies are strongly encouraged to directly assess menopausal status rather than rely on age as a proxy measure. Age is correlated with other factors (e.g. midlife stressors and role transitions; Thomas et al., Reference Thomas, Mitchell and Woods2019) that could enhance risk for psychosis, especially in vulnerable women (Myin-Germeys, Delespaul, & Van Os, Reference Myin-Germeys, Delespaul and Van Os2005; Reininghaus et al., Reference Reininghaus, Gayer-Anderson, Valmaggia, Kempton, Calem, Onyejiaka and Morgan2016). Further, although the midlife shift in risk (~ages 40–50) aligns with the general timing of perimenopausal period, there could be continuing risk into postmenopause given its hormonal milieu of chronically low estradiol (and progesterone) levels. Directly examining menopausal status and other factors associated with midlife (e.g. stressors; role transitions) would substantially increase understanding of psychosis risk in middle-aged women.
Studies directly assessing ovarian hormone mechanisms are desperately needed as well. To date, no studies have examined links between natural physiological changes in estrogen in midlife or the critical menopausal transition and psychotic symptoms. Although animal and human data during earlier reproductive stages suggest that decreasing estradiol levels contribute to psychosis risk in women, it is unclear whether effects at earlier life stages (e.g. menstrual cycle, pregnancy, and postpartum) directly translate to midlife and the menopausal transition. Additionally, not all women experience perimenopausal/postmenopausal hormonal changes in the same way, so assessing menopausal stages, in the absence of capturing individual differences in ovarian hormone effects, could obscure or hinder the detection of potential menopause–psychosis relationships. Moving forward, it will be important for future studies to use comprehensive and multiple/longitudinal measures of hormones to accurately capture the dramatic hormonal fluctuations and drops (see Fig. 1) that characterize this stage of development. Ovarian hormones can now reliably be assessed in saliva multiple times per day and/or across several days, making the characterization of menopausal hormone profiles much more feasible (and accurate). These saliva sampling techniques have been used to examine ovarian hormone effects across the menstrual cycle for other phenotypes (e.g. worry, binge eating; Gloe, Kashy, Jacobs, Klump, & Moser, Reference Gloe, Kashy, Jacobs, Klump and Moser2021; Klump et al., Reference Klump, Keel, Racine, Burt, Neale, Sisk and Hu2013) and would be an incredibly useful addition to the literature examining hormone influences on psychosis, particularly during menopause. In addition, there are well-documented sex differences in responsivity to stress, including heightened stress sensitivity in women, that are at least partially modulated by ovarian hormones and may help explain observed midlife increases in risk among women but not men (Bangasser & Wiersielis, Reference Bangasser and Wiersielis2018; Ter Horst et al., Reference Ter Horst, Wichmann, Gerrits, Westenbroek and Lin2009; Toufexis et al., Reference Toufexis, Rivarola, Lara and Viau2014). The hormonal/biological changes and symptoms (e.g. hot flashes/night sweats, sleep disturbances) that accompany the menopausal transition are also stressful and decrease wellbeing, which could further contribute to psychosis risk in women. Thus, concurrent measurement of ovarian hormones, menopausal status, and stress responsivity will likely be important for fully elucidating female-specific vulnerability to psychosis in midlife.
Finally, it would also be helpful for future studies to characterize population-level risk for psychosis during menopause and hormonal transitions. There is a surprising lack of research on psychosis risk during midlife in non-treatment seeking, community-based samples. We therefore lack critical data on midlife, perimenopausal, and population-level risk. Data indicate that it is possible to examine psychotic symptoms in population-based and community samples – even symptoms that fall short of diagnostic thresholds and affect a much larger proportion of women (McGrath et al., Reference McGrath, Saha, Al-Hamzawi, Alonso, Bromet, Bruffaerts and Kessler2015; Nuevo et al., Reference Nuevo, Chatterji, Verdes, Naidoo, Arango and Ayuso-Mateos2012). Studies of population-based samples during the menopausal transition would provide critical data on psychosis risk that can be used in mental health and physician screenings to increase identification of midlife psychosis in women. These public health benefits, along with the potential treatment implications of estrogen modulation of psychosis risk during midlife (see section on neuroleptic treatment above), could substantially decrease the burden of mental illness in this critical area of women's health.
Financial support
This research was supported by the National Institute of Mental Health (R01 MH128196) grant awarded to K. L. K., K. M. C., and K. N. T.
Conflict of interest
None.




