Introduction
Preclinical studies suggest that the 5-HT4 receptor is a promising therapeutic target for the treatment of cognitive impairment and depression. 5-HT4 receptors are postsynaptic receptors that are expressed in abundance in the basal ganglia, hippocampus, amygdala and prefrontal cortex (Roychowdhury et al., Reference Roychowdhury, Haas and Anderson1994; Compan et al., Reference Compan, Daszuta, Salin, Sebben, Bockaert and Dumuis1996; Cai et al., Reference Cai, Flores-Hernandez, Feng and Yan2002; Vilaró et al., Reference Vilaró, Cortés and Mengod2005; Beliveau et al., Reference Beliveau, Ganz, Feng, Ozenne, Hojgaard, Fisher, Svarer, Greve and Knudsen2017). 5-HT4 receptor activation has an excitatory effect, enhancing the release of acetylcholine in the frontal cortex and hippocampus (Consolo et al., Reference Consolo, Arnaboldi, Giorgi, Russi and Ladinsky1994; Siniscalchi et al., Reference Siniscalchi, Badini, Beani and Bianchi1999), increasing long term potentiation in the hippocampus (Marchetti et al., Reference Marchetti, Chaillan, Dumuis, Bockaert, Soumireu-Mourat and Roman2004) and inducing a rapid and sustained increase in basal firing of 5-HT cells in the dorsal raphe nucleus (Lucas and Debonnel, Reference Lucas and Debonnel2002; Lucas et al., Reference Lucas, Compan, Charnay, Neve, Nestler, Bockaert, Barrot and Debonnel2005). 5-HT4 receptor activation has also been shown to upregulate hippocampal cell proliferation and increase the expression of neuroplasticity-related proteins such as cyclic AMP response element binding and brain-derived neurotrophic factor (Lucas et al., Reference Lucas, Rymar, Du, Mnie-Filali, Bisgaard, Manta, Lambas-Senas, Wiborg, Haddjeri, Piñeyro, Sadikot and Debonnel2007; Pascual-Brazo et al., Reference Pascual-Brazo, Castro, Díaz, Valdizán, Pilar-Cuéllar, Vidal, Treceño and Pazos2012), effects which mirror those seen with clinically effective antidepressants (Harmer et al., Reference Harmer, Duman and Cowen2017).
Consistent with this neurobiological profile, 5-HT4 partial agonists have a facilitatory effect on rodent behavioural tests of learning and memory (King et al., Reference King, Marsden and Fone2008; Hagena and Manahan-Vaughan, Reference Hagena and Manahan-Vaughan2017), and in particular hippocampal-dependent learning and memory (Hagena and Manahan-Vaughan, Reference Hagena and Manahan-Vaughan2017), including pro-cognitive effects on spatial learning (Fontana et al., Reference Fontana, Daniels, Wong, Clark and Eglen1997; Lelong et al., Reference Lelong, Dauphin and Boulouard2001), place recognition (Lamirault and Simon, Reference Lamirault and Simon2001; Orsetti et al., Reference Orsetti, Dellarole, Ferri and Ghi2003) and object recognition (Lamirault and Simon, Reference Lamirault and Simon2001; Levallet et al., Reference Levallet, Hotte, Boulouard and Dauphin2009). These memory enhancing properties are often attributed to a facilitation of central cholinergic activity, which is supported by studies showing that 5-HT4 receptor agonists reverse scopolamine- and atropine-induced cognitive impairments (Fontana et al., Reference Fontana, Daniels, Wong, Clark and Eglen1997; Marchetti-Gauthier et al., Reference Marchetti-Gauthier, Roman, Dumuis, Bockaert and Soumireu-Mourat1997; Cachard-Chastel et al., Reference Cachard-Chastel, Lezoualc'h, Dewachter, Deloménie, Croes, Devijver, Langlois, Van Leuven, Sicsic and Gardier2007; Lo et al., Reference Lo, De Maeyer, Vermaercke, Callaerts-Vegh, Schuurkes and D'Hooge2014).
More recently, preclinical studies have also highlighted the 5-HT4 receptor as a novel antidepressant target. An increase in basal firing of 5-HT cells in the dorsal raphe nucleus is seen within 30 min of 5-HT4 receptor agonist administration (Lucas and Debonnel, Reference Lucas and Debonnel2002; Lucas et al., Reference Lucas, Compan, Charnay, Neve, Nestler, Bockaert, Barrot and Debonnel2005), which is in striking contrast to the decrease in firing induced by acute selective serotonin reuptake inhibitor (SSRI) administration (Blier et al., Reference Blier, Piñeyro, el Mansari, Bergeron and de Montigny1998), and suggests that 5-HT4 receptor agonists may hold potential as rapid onset antidepressants. Consistent with this, studies in rodents have demonstrated that 5-HT4 receptor agonists exert antidepressant-like effects on behavioural paradigms that mirror those seen with conventional SSRIs, but with a more rapid onset of action (Lucas et al., Reference Lucas, Rymar, Du, Mnie-Filali, Bisgaard, Manta, Lambas-Senas, Wiborg, Haddjeri, Piñeyro, Sadikot and Debonnel2007; Pascual-Brazo et al., Reference Pascual-Brazo, Castro, Díaz, Valdizán, Pilar-Cuéllar, Vidal, Treceño and Pazos2012; Mendez-David et al., Reference Mendez-David, David, Darcet, Wu, Kerdine-Römer, Gardier and Hen2014). For example, the 5-HT4 receptor partial agonist RS67333 reduces immobility in the forced swim test (Lucas et al., Reference Lucas, Rymar, Du, Mnie-Filali, Bisgaard, Manta, Lambas-Senas, Wiborg, Haddjeri, Piñeyro, Sadikot and Debonnel2007) and reverses the anhedonic-like behaviour produced by both chronic mild stress and corticosterone, as well as the hyperlocomotion that follows olfactory bulbectomy (Mendez-David et al., Reference Mendez-David, David, Darcet, Wu, Kerdine-Römer, Gardier and Hen2014). Further, blockade of 5-HT4 receptors prevents the anxiolytic and antidepressant-like effects of the SSRI fluoxetine in animal models (Mendez-David et al., Reference Mendez-David, David, Darcet, Wu, Kerdine-Römer, Gardier and Hen2014).
Positron Emission Tomography (PET) studies provide some initial support for a role of the 5-HT4 receptor in human cognition and depression; for example, lower striatal 5-HT4 receptor binding has been associated with a family history of depression (Madsen et al., Reference Madsen, Torstensen, Holst, Haahr, Knorr, Frokjaer, Brandt-Larsen, Iversen, Fisher and Knudsen2014) and hippocampal 5-HT4 receptor binding has been negatively associated with memory performance (Haahr et al., Reference Haahr, Fisher, Holst, Madsen, Jensen, Marner, Lehel, Baaré, Knudsen and Hasselbalch2013). Until recently, further elucidation of the role of the 5-HT4 receptor in humans has been difficult because of the lack of suitable compounds to manipulate 5-HT4 receptor function. The selective high-affinity 5-HT4 partial agonist, prucalopride, has recently been licensed for the treatment of constipation and offers a unique opportunity to investigate the role of the 5-HT4 receptor in human cognition. Prucalopride has good brain penetration in rodents (Johnson et al., Reference Johnson, Drummond, Grimwood, Sawant-Basak, Miller, Tseng, McDowell, Vanase-Frawley, Fisher, Rubitski, Stutzman-Engwall, Nelson, Horner, Gorczyca, Hajos and Siok2012), and has pro-cognitive and antidepressant-like effects in animal models (Lucas et al., Reference Lucas, Rymar, Du, Mnie-Filali, Bisgaard, Manta, Lambas-Senas, Wiborg, Haddjeri, Piñeyro, Sadikot and Debonnel2007; Cachard-Chastel et al., Reference Cachard-Chastel, Devers, Sicsic, Langlois, Lezoualc'h, Gardier and Belzung2008).
Here we use prucalopride to characterise, for the first time, the cognitive effects of selective 5-HT4 receptor activation in humans. In order to investigate the translation of the behavioural effects of 5-HT4 agonism seen in animals, we measured the effects of prucalopride on standard tests of learning and memory, including verbal memory (Rey Auditory Verbal Learning Task (RAVLT)), working memory (N-back Task) and implicit contextual learning (Contextual Cueing Task). In addition, we used measures of emotional processing (Emotional Test Battery) and reward processing (Probabilistic Instrumental Learning Task) to investigate whether prucalopride has an antidepressant-like profile in healthy volunteers. Depression is known to be associated with negative biases in information processing (Roiser and Sahakian, Reference Roiser and Sahakian2013) and decreased reward sensitivity (Pizzagalli et al., Reference Pizzagalli, Iosifescu, Hallett, Ratner and Fava2008) and we have previously demonstrated that acute administration of clinically active antidepressants reverses these biases in healthy volunteers (Harmer et al., Reference Harmer, Duman and Cowen2017). Assessing the effect of novel putative antidepressants on measures of emotional and reward processing in healthy volunteers is, therefore, a useful way of translating putative antidepressant effects from animal models to humans. Based on the profile of behavioural effects of 5-HT4 receptor agonism in animals, we predicted that acute prucalopride would enhance learning and memory, induce a positive shift in emotional information processing, and increase sensitivity to reward on our battery of cognitive measures. Such findings would represent an important translation of the effects of 5-HT4 agonism seen in preclinical studies.
Materials and methods
Participants
Forty one healthy participants, aged 19–40 years, were included in this study (19 male, 22 female). Participants were fluent in English and were screened for contraindication to prucalopride administration. Exclusion criteria included: psychoactive medication use in the past 3 months (excluding the contraceptive pill); current or past DSM-5 psychiatric disorder [assessed with the Structured Clinical Interview for DSM-5, Clinician Version, (First et al., Reference First, Williams, Karg and Spitzer2015)]; first degree relative with bipolar disorder; prior exposure to task battery; lactose intolerance; pregnancy/breastfeeding; consumption of >20 units of alcohol per week and smoking >5 cigarettes per day. The study was approved by the University of Oxford Central University Research Ethics Committee (MSD-IDREC reference R44853/RE004). Written informed consent was obtained from participants.
Design
The study had a between-subject, double-blind and placebo-controlled design. Participants were randomly assigned to a single dose of prucalopride (Resolor; 1 mg oral dose) or placebo (lactose tablets; Rayonex Biomedical), encapsulated in identical white capsules. Randomisation was stratified for sex. Prucalopride is rapidly absorbed (T max after single 2 mg dose: 2–3 h; terminal half-life: 24–30 h) and cognitive testing, therefore, commenced 2 h after administration in order to maximise drug levels during testing (Frampton, Reference Frampton2009; Flach et al., Reference Flach, Scarfe, Dragone, Ding, Seymour, Pennick, Pankratz, Troy and Getsy2016). Female participants were not tested during the premenstrual week. The following cognitive tests were administered in a fixed order: Emotional Test Battery, Auditory Verbal Learning Task, Probabilistic Instrumental Learning, Contextual Cueing and N-Back. A brief description of each task is given below and further details can be found in the Supplemental Information.
Questionnaire measures
Participants completed the following self-report questionnaires to obtain baseline measures of mood, anxiety and personality: Beck Depression Inventory (Beck et al., Reference Beck, Steer and Brown1996), Spielberger State-Trait Anxiety Inventory, Trait Version (STAI-T; Spielberger et al., Reference Spielberger, Gorssuch, Lushene, Vagg and Jacobs1983) and Eysenck Personality Questionnaire (Eysenck and Eysenck, Reference Eysenck and Eysenck1975). Affect (assessed using the Positive and Negative Affect Scale, PANAS; Watson et al., Reference Watson, Clark and Tellegen1988), anxiety (assessed using the Spielberger State-Trait Anxiety Inventory, State Version, STAI-S; Spielberger et al., Reference Spielberger, Gorssuch, Lushene, Vagg and Jacobs1983) and side effects were measured at three times during the test session (baseline, pre-cognitive testing and post-cognitive testing). Side effects were measured using a scale in which participants were required to rate the extent to which they were experiencing each of the most commonly reported side effects of prucalopride (Sajid et al., Reference Sajid, Hebbar, Baig, Li and Philipose2016): headache, abdominal pain, nausea, diarrhoea, decreased appetite, dizziness, vomiting, flatulence, fatigue and gastrointestinal sounds. At the end of the study visit, participants were asked to guess which group they were in with a forced-choice question.
Rey auditory verbal learning task (RAVLT)
Participants were read a list of 15 concrete nouns (List A) and asked to immediately verbally recall as many items as they could. This was repeated five times before a list of unrelated words was presented (List B) and participants were again asked to recall them. Participants were then asked to recall List A immediately (short-delay) and after a delay of ~15 min (long-delay). Number of words correct, repetitions (correct words recalled more than once in the same acquisition trial) and intrusions (incorrect words not present in the list) were measured. Participants then completed a recognition task where they were required to indicate which of a list of words (15 List A words, 35 distractors) had previously been presented. Number of hits and false alarms were measured.
N-back
In this task [adapted from Mannie et al., (Reference Mannie, Harmer, Cowen and Norbury2010)] participants indicated whether a letter presented on the screen (‘target’) matched a previously presented letter (‘cue’) which was presented n trials ago (where n was one, two or three in the 1-back, 2-back and 3-back conditions, respectively). The performance was compared to a control, 0-back, condition where participants were asked to press ‘Same’ if they saw the letter ‘X’, and ‘Different’ in response to any other letter. Mean accuracy and response latency for each condition were recorded.
Contextual cueing
In this task (adapted from Klinge et al., Reference Klinge, Shuttleworth, Muglia, Nobre, Harmer and Murphy2018) participants reported the orientation of a target (‘T’) which was hidden amongst an array of distractor stimuli (‘L's). On half of the trials, the array was a repeat of one previously presented and on the other half of trials the array was novel. Median reaction time and mean accuracy were calculated for repeat and novel array trials separately. In order to measure contextual learning, difference scores (novel v. repeat) were computed for the first (five blocks) and second (five blocks) half of the task.
Emotional test battery
The ETB (Harmer et al., Reference Harmer, O'Sullivan, Favaron, Massey-Chase, Ayres, Reinecke, Goodwin and Cowen2009) (P1vital, Oxford, UK) is designed to assess the processing of a variety of affectively valenced stimuli and comprises five validated, computerised cognitive tasks: Facial Expression Recognition Task (FERT), Emotional Categorisation Task (ECAT), Facial Dot-Probe Task (FDOT), Emotional Recall Task (EREC) and Emotional Recognition Memory Task (EMEM). The FERT comprises a series of facial expressions associated with six basic emotions: anger, disgust, fear, happy, sad and surprise in a range of different intensity levels. Participants were required to identify the emotion of the face. Accuracy and mean reaction times were recorded. The ECAT comprises a series of positively and negatively valenced self-referent words, and participants were required to indicate whether they would like or dislike to be referred to as each word. Accuracy and mean reaction times were recorded. In the FDOT, participants reported the orientation of two dots which appeared in a position on the screen previously occupied by an emotional face (fearful or happy) or a neutral face. Attentional vigilance scores were calculated by subtracting the mean reaction time from trials when probes appeared in the same position as the emotional face (congruent trials) from trials when probes appeared in the opposite position to the emotional face (incongruent trials). The EREC is a surprise free recall task during which participants were required to remember as many of the positively and negatively valenced self-referent words from the ECAT as they could in 4 min. The number of words correctly recalled (hits) and falsely recalled (false alarms) were recorded. The EMEM comprises self-referent words from the ECAT, and previously unseen words that participants were required to classify as familiar or novel. Reaction time, accuracy and false alarms were recorded.
Probabilistic instrumental learning task (PILT)
In this task [Fig. S1, (adapted from Pessiglione et al., Reference Pessiglione, Seymour, Flandin, Dolan and Frith2006)], participants were required on each trial to choose one of two pairs of symbols. One pair was associated with win outcomes (win 20p or no change) and the other with loss outcomes (lose 20p or no change). Each symbol in the pair corresponded to reciprocal probabilities (0.7 or 0.3) of the associated outcomes occurring. Participants were instructed to pick the symbol they believed was most likely to win (or least likely to lose), with the aim of maximising their monetary pay off. Feedback on the outcome of each trial was given after a choice was made. Participants completed three runs of the task, each with a new set of four symbols. The total amount won, total amount lost, end total, symbol choice and choice consistency were recorded. In order to provide temporal information about reward learning in the two groups, learning curves were created, displaying the proportion of participants on each trial that chose the correct (high probability) symbol in the win condition and the correct (low probability) symbol in the loss condition (Fig. 3). Lastly a reinforcement learning model was fit to participant choice data (Pessiglione et al., Reference Pessiglione, Seymour, Flandin, Dolan and Frith2006). This model contained separate learning rate and decision temperature parameters for the win and loss outcomes, and was used to complement the non-model based analysis (see Supplemental Information).

Fig. 3. Performance on the PILT. Learning curves for each group depicting trial-by-trial the proportion of participants who chose the correct symbol in (a) the win condition of the PILT (associated with high-probability win) and (b) the loss condition of the PILT (associated with low-probability loss). (c) Proportion of participants in each group who chose the correct symbol across the last 20 win and loss trials of the PILT, where learning had plateaued. Asterisk represents the main effect of group across win and loss conditions (p = 0.05). Error bars: ±1 s.e.
Statistical analysis
Statistical analyses were carried out using IBM SPSS Statistics (version 22). To assess changes in subjective mood and side effects before and after prucalopride/placebo administration, repeated measure analyses of variance (ANOVAs) were performed with time as a within-subject factor. Behavioural task data were analysed using repeated measures ANOVA with a between-subjects factor of the treatment group (prucalopride or placebo) and within-subjects factors of facial emotional expression (FERT and FDOT), word valence (ECAT, EREC and EMEM), acquisition block (AVLT), condition (N-back) and trial type (contextual cueing and probabilistic instrumental learning task). Additional within-subjects factors were included for the FDOT (mask condition), EREC (recall accuracy) and contextual cueing task (block). Where assumptions of equality of variances were broken, degrees of freedom were corrected with the Greenhouse–Geisser procedure. Post hoc analyses using independent samples t tests were performed to follow-up interactions observed. These are reported with the effect size (Cohen's d) and with corrected degrees of freedom where equal variances between groups cannot be assumed.
Results
Demographic data and subjective mood and side effects
The groups were well matched for age, gender and baseline scores of depression, anxiety and affect (Table 1). There were no significant effects of prucalopride on subjective measures of anxiety, or positive and negative affect (Table 2). There were no significant differences between groups at baseline or pre-testing (2 h post-drug administration) in frequency of side effects reported. At the post-testing time point (~5 h post-drug administration), there were significantly more reports of headache [χ(1) = 9.95, p = 0.002, OR = 8.96, 95% CI = 2.14–37.52] and nausea [χ(1) = 4.97, p = 0.026, OR = 9.23, 95% CI = 0.99–85.78] in the prucalopride group, and an increase in reports of diarrhea in the prucalopride group, although this was not significant [χ(1) = 3.59, p = 0.058]. Randomisation guesses suggested that participants were better than chance at guessing group allocation (correct guess: placebo 61.9%, prucalopride 63.2%). One participant (prucalopride) withdrew prior to cognitive testing due to feeling nauseous (this participant also reported mild nausea at baseline). Data is therefore presented for 40 participants (21 placebo and 19 prucalopride).
Table 1. Demographics and psychometrics of participants
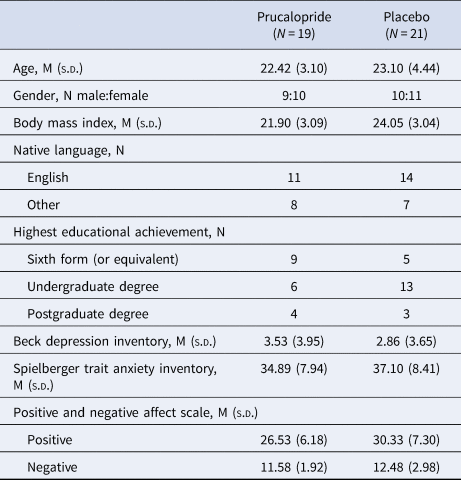
Gender and native language values represent the number of participants. Remaining questionnaire data represent the mean with standard deviation in parentheses
Table 2. Subjective measures of anxiety, mood and side effects

Values represent the mean with standard deviation in parentheses. Questionnaire data were analysed using repeated measures analysis of variance (ANOVA) with group as a between subjects factor (placebo, prucalopride) and timepoint as a within groups factor (baseline, pre-cognitive testing and post-cognitive testing).The p values displayed are for the group × timepoint interaction
Auditory verbal learning task
As expected, there was a significant effect of block on word recall [F(2.28, 86.65) = 119.7, p < 0.001], indicating that participants’ recall of List A words improved across the five acquisition blocks in both groups (Fig. 1). In addition, there was a significant interaction between the block and group [F(2.28, 86.65) = 2.29, p = 0.049]. Follow up analyses revealed that this interaction was driven by improved recall in the prucalopride group compared with the placebo group in block 1 [t(38) = 2.61, p = 0.01, d = 0.8, 95% CI = 0.43–3.37], block 2 [t(38) = 2.79, p = 0.008, d = 0.9, 95% CI = 0.32–1.99] and block 3 [t(27.9) = 2.73, p = 0.01, d = 0.8, 95% CI = 0.28–1.97], see Fig. 1. There was no difference in the number of words accurately recalled after a short [t(27.43) = 1.57, p = 0.128, CI = −0.27 to 2.03] and long delay [t(33.02) = −1.58, p = 0.114, 95% CI = −0.22 to 1.94] or in the number of words correctly recalled from List B [t(32.37) = 1.60, p = 0.12, 95% CI = −0.36 to 2.97], although the placebo group did have significantly more intrusions in their recall of List B (M = 0.48, s.d. = 0.60) than the prucalopride group [M = 0, s.d. = 0; t(20) = 3.63, p = 0.002, d = 1.1, 95% CI = 0.20–0.75].

Fig. 1. Performance on the RAVLT. Mean number of words recalled by the placebo and prucalopride groups immediately following List A and B presentation, and following a short and long delay. Error bars: ±1 s.e. Asterisks represent where there was a significant difference between group in recall (*p < 0.05, **p < 0.01).
N-back
There was a main effect of task condition on accuracy [F(3,108) = 103.2, p < 0.001] and reaction time [F(2.13, 76.6) = 74.57, p < 0.001], which reflected the expected effect of reduced accuracy and increased reaction times as the number of trials participants had to hold in mind increased. However, there was no significant main effect of prucalopride on accuracy [F(1, 36) = 0.16, p = 0.694, 95% CIs (0.83–0.88), (0.82–0.88)] or reaction time [F(1, 36) = 0.23, p = 0.632, 95% CIs (563.53–645.80), (549.69–631.96)] and no condition × group interaction on accuracy [F(1.35, 48.42) = 0.67, p = 0.461] or reaction time [F(2.03, 72.99) = 1.10, p = 0.34].
Contextual cueing task
For both accuracy and reaction time there was a main effect of trial type [accuracy: F(1,38) = 4.3, p = 0.05, reaction time: F(1,38) = 27.64, p < 0.001] and block [accuracy: F(4.9,186.1) = 3.32, p < 0.01, reaction time: F(2.35, 84.32) = 33.9, p < 0.001], indicating that across both groups, participants were more accurate and faster to respond to the target on repeated arrays compared with novel arrays, and on later blocks. Prucalopride administration had no significant effect on accuracy to identify the target [main effect of group: F(1, 38) = 0.006, p = 0.941, trial type × block × group interaction F(6.7,253.7) = 0.67, p = 0.69]. There was also no significant effect of prucalopride on reaction time measures [main effect of group: F(1, 38) = 0.026, p = 0.872, trial type × block × group interaction F(1.00, 38.00) = 0.26, p = 0.617].
Emotional test battery
There were no significant effects of prucalopride administration on facial expression recognition (FERT), attentional vigilance to emotional faces (FDOT) or speed to classify positive and negative personality characteristics (ECAT) all p's > 0.2, Table S1.
There was, however, an effect of prucalopride on recall and recognition of the emotional words. On the emotional word recall task (EREC), there was a significant interaction between the group and recall accuracy [F(1,38) = 7.96, p = 0.008], which was driven by a significant decrease in false alarms [t(38) = 2.7, p = 0.011, d = 0.9, 95% CI = 0.32–2.27] and a (non-significant) increase in hits [t(38) = 1.9, p = 0.065, d = 0.6, 95% CI = −2.55 to 0.08] in the prucalopride group compared with the placebo group (Fig. 2). Importantly, there was no significant three-way group × valence × accuracy interaction [F(1,38) = 2.43, p = 0.12], indicating that the improvements in recall accuracy in the prucalopride group were not specific to positive or negative words.

Fig. 2. Performance on emotional memory tasks. Mean number of hits and false alarms for positive and negative personality descriptors, as compared between the drug and placebo group in the (a) Emotional Recall Task [EREC] and (b) Emotional Recognition Task [EMEM]. Error bars: ±1s.e.. Asterisk represents the significant interaction between group and recall accuracy (p < 0.01).
On the emotional word recognition task (EMEM) there was a near significant recognition accuracy × group interaction [F(1,38) = 3.92, p = 0.055], which was again driven by relatively increased hits and decreased false alarms in the prucalopride group compared with the placebo group (Fig. 2), although neither of these individual contrasts reached significance [hits: t(38) = 1.16, p = 0.26, 95% CI = −11.02 to 3.01; false alarms: t(38) = 1.37, p = 0.18, 95% CI = −6.08 to 31.45].
Probabilistic instrumental learning taskFootnote †Footnote 1
There was no significant effect of prucalopride on the amount won [t(37) = 0.31, p = 0.760, 95% CI = −1.68 to 1.24], the amount lost [t(31.09) = 1.54, p = 0.134, 95% CI = −0.25 to 1.81] or the total monetary amount earned [t(37) = 0.85, p = 0.399, 95% CI = −1.69 to 0.69].
Learning curves were produced for each group depicting trial-by-trial the proportion of participants who chose the correct symbol in the win condition (associated with high-probability win, Fig. 3a) and the loss condition (associated with low-probability loss, Fig. 3b). Both groups learnt to choose the high-probability win symbol and the low-probability loss symbol by approximately trial 10. To assess reward and loss sensitivity after learning, the proportion of participants choosing the correct symbol was averaged over the last 20 trials of the task for win and loss conditions separately (Walsh et al., Reference Walsh, Browning, Drevets, Furey and Harmer2018a, Reference Walsh, Huneke, Brown, Browning, Cowen and Harmer2018b). There was a main effect of group [F(1,37) = 3.96, p = 0.054, 95% CIs (0.77–0.89), (0.85–0.97)], which did not significantly interact with outcome valence [group × valence interaction F(1,37) = 0.06, p = 0.804]. This main effect of group reflected an increased probability of choosing the correct symbol in the prucalopride group compared with the placebo group (mean probability of choosing correct symbol prucalopride: 0.91, placebo: 0.86), Fig. 3c. Analyses of the parameters derived from the computational model are consistent with the pattern of results seen in the learning curves: there was no effect of prucalopride on learning rates generally [main effect of group: F(1,37) = 0.27, p = 0.87, 95% CIs (−1.99 to −1.20), (−1.95 to −1.14)] or as a function of outcome valence [group × valence: F(1,37) = 1.29, p = 0.26]. However there was a significant effect of group on the inverse decision temperature parameter [main effect of group: F(1,37) = 3.33, p = 0.035, 95% CIs (1.44–1.97), (1.84–2.39)] which was not modified by outcome valence [group × valance: F(1,37) = 0.38, p = 0.54].
Discussion
This is the first study to investigate the cognitive effects of acute activation of the 5-HT4 receptor in humans. Animal studies have suggested that 5-HT4 receptor agonists have pro-cognitive and antidepressant-like effects, and this study represents an important step in translating these findings to humans using an experimental medicine approach. Consistent with the effects of 5-HT4 agonism in animals, acute prucalopride had a pro-cognitive effect in healthy volunteers across three separate tasks: increasing word recall in an explicit verbal learning task; increasing the accuracy of recall and recognition of words in an incidental emotional memory task; and increasing the probability of choosing a symbol associated with high probability of reward or absence of loss in a probabilistic instrumental learning task. These findings are a translation of the memory enhancing effects of 5-HT4 receptor agonism seen in animals, and lend weight to the idea that the 5-HT4 receptor is a useful target for the treatment of cognitive deficits associated with depression and other disorders. Contrary to the effects reported in animal models, prucalopride did not have an antidepressant profile on our measures of emotional processing.
Preclinical animal studies have established that the 5-HT4 receptor plays a central role in a range of behavioural learning and memory tasks (King et al., Reference King, Marsden and Fone2008; Hagena and Manahan-Vaughan, Reference Hagena and Manahan-Vaughan2017) and the findings of the current study are consistent with this. There was a clear pattern of improved performance on the RAVLT; whilst both groups reached ceiling by later acquisition trials, the prucalopride group learnt the word list faster, recalling significantly more words on earlier acquisition blocks than placebo. Prucalopride also acted to increase memory accuracy. On the emotional recall and recognition tasks, as well as the second wordlist of the AVLT, there was a pattern of decreased false alarms/intrusions in the prucalopride group, suggesting that 5-HT4 receptor agonism increases the precision of memory. This pattern of increased hits and decreased false alarms is opposite to that typically seen in people with cognitive impairment (Schacter et al., Reference Schacter, Verfaellie and Pradere1996; Bennett et al., Reference Bennett, Golob, Parker and Starr2006). The finding that prucalopride improved performance on word recall and recognition tasks are interesting in light of a previous PET study which reported an inverse correlation of 5-HT4 receptor binding in the hippocampus and performance on the AVLT (Haahr et al., Reference Haahr, Fisher, Holst, Madsen, Jensen, Marner, Lehel, Baaré, Knudsen and Hasselbalch2013). The direction of this effect is opposite to the memory enhancing effects of 5-HT4 receptor agonism seen in the current study and preclinical studies, which may be due to the PET binding potential representing a composite measure of receptor density and affinity. Whilst this previous PET evidence was important in establishing a possible relationship with 5-HT4 binding and memory function in humans, the current study extends this by demonstrating that memory function can be directly facilitated by experimental manipulation of 5-HT4 receptor function.
There was no effect of prucalopride on working memory or implicit contextual learning. 5-HT4 receptor function has been particularly associated with hippocampal-dependent learning and memory (Hagena and Manahan-Vaughan, Reference Hagena and Manahan-Vaughan2017) and this may explain why prucalopride did not have an effect on these tasks, which are typically associated with neural circuitry outside of the hippocampus (Preston and Gabrieli, Reference Preston and Gabrieli2008; Nee and D'Esposito, Reference Nee and D'Esposito2018). However, 5-HT4 receptor agonism has previously been reported to affect working memory in animals, although typically only when the agonist is administered at higher doses, and when impairment in working memory performance has been induced with scopolamine (Lelong et al., Reference Lelong, Lhonneur, Dauphin and Boulouard2003). Healthy volunteers can be insensitive to cognition enhancing effects on working memory (Husain and Mehta, Reference Husain and Mehta2011), and it may be that a higher dose of prucalopride and/or participants with lower baseline performance would be needed to detect an effect of prucalopride on a standard working memory task.
Studies in rodents have demonstrated that 5-HT4 receptor agonists exert antidepressant-like effects on a range of behavioural paradigms, which mirror those seen with conventional SSRIs, and often occur with a more rapid onset of action (Lucas et al., Reference Lucas, Rymar, Du, Mnie-Filali, Bisgaard, Manta, Lambas-Senas, Wiborg, Haddjeri, Piñeyro, Sadikot and Debonnel2007; Pascual-Brazo et al., Reference Pascual-Brazo, Castro, Díaz, Valdizán, Pilar-Cuéllar, Vidal, Treceño and Pazos2012; Mendez-David et al., Reference Mendez-David, David, Darcet, Wu, Kerdine-Römer, Gardier and Hen2014). We have previously demonstrated that acute administration of clinically active antidepressants in healthy volunteers and depressed patients produces positive shifts in emotional processing that are predictive of later therapeutic effects (Harmer et al., Reference Harmer, Duman and Cowen2017). Assessing the effect of novel putative antidepressants on measures of emotional processing in healthy volunteers is therefore a useful way of translating to humans effects from animal models, which are known to often lack reliable predictive validity (Hyman, Reference Hyman2012). The emotional processing tasks used in this study are validated experimental medicine measures of antidepressant drug treatment; they are sensitive to a wide range of different antidepressant treatments, they show discriminative effects from other treatments that are not antidepressant, and they are not affected by putative antidepressants that have subsequently failed clinical trials (Harmer et al., Reference Harmer, Cowen and Goodwin2011).
Using this experimental medicine approach here, we have demonstrated that, despite the antidepressant profile of 5-HT4 receptor agonists in animals, a single dose of prucalopride did not have an antidepressant profile in humans on tests of emotional processing. Acute prucalopride did not alter the recognition of facial expressions of emotion or attentional vigilance to emotional faces. Whilst there were some effects of prucalopride on the recall of emotional words, these were seen for both positive and negative words, rather than the specific increase in positive recall and a decrease in negative recall that is typically seen with clinically effective antidepressants (Harmer et al., Reference Harmer, Hill, Taylor, Cowen and Goodwin2003; Harmer et al., Reference Harmer, Heinzen, O'Sullivan, Ayres and Cowen2008; Arnone et al., Reference Arnone, Horder, Cowen and Harmer2009). Similarly on the PILT, we did not see a specific increase in reward sensitivity, which would have been suggestive of an antidepressant profile, given the decreases in reward sensitivity that are typically associated with anhedonia and depression (Pizzagalli et al., Reference Pizzagalli, Iosifescu, Hallett, Ratner and Fava2008; Vrieze et al., Reference Vrieze, Pizzagalli, Demyttenaere, Hompes, Sienaert, de Boer, Schmidt and Claes2013). Rather, prucalopride acted to increase the proportion of correct choices on the PILT, which was confirmed using a computational model demonstrating a significant increase in the inverse decision temperature parameter. This increase in inverse temperature is consistent with the pro-cognitive profile of prucalopride shown in the memory task and suggests that prucalopride acts to facilitate the utilisation of the expected values associated with each symbol during decision making.
There are a number of potential explanations for the lack of antidepressant profile seen with acute prucalopride. First, it may be that the effects seen in animals do not translate, and 5-HT4 receptor agonists do not have antidepressant effects in humans. Indeed, the emotional processing tasks used in this study have successfully distinguished putative antidepressant agents that, while promising in animal models, lack clinical therapeutic efficacy (Pringle et al., Reference Pringle, McTavish, Williams, Smith, Cowen and Harmer2011). An alternative explanation is that prucalopride needs to be administered at a higher dose or for longer in order to have antidepressant-like effects. The 1 mg dose of prucalopride used in the current study is half of the standard clinical dose used in the treatment of constipation and, whilst there is evidence of antidepressant like effects of acute 5-HT4 receptor agonism in rats (Tamburella et al., Reference Tamburella, Micale, Navarria and Drago2009), the majority of preclinical studies have used a longer administration schedule of 3–7 days (Lucas et al., Reference Lucas, Rymar, Du, Mnie-Filali, Bisgaard, Manta, Lambas-Senas, Wiborg, Haddjeri, Piñeyro, Sadikot and Debonnel2007; Pascual-Brazo et al., Reference Pascual-Brazo, Castro, Díaz, Valdizán, Pilar-Cuéllar, Vidal, Treceño and Pazos2012; Mendez-David et al., Reference Mendez-David, David, Darcet, Wu, Kerdine-Römer, Gardier and Hen2014). Future studies should, therefore, investigate the effect of higher doses and longer prucalopride administration on emotional processing.
The findings of this study should be interpreted in light of a number of potential limitations. First, there were increased side effects reported by the prucalopride group, in particular headache, nausea and diarrhoea. These side effects were not unexpected given the high expression of 5-HT4 receptors in the gastrointestinal system. Indeed pilot work in our group established that a higher 2 mg dose of prucalopride was not satisfactorily tolerated by healthy volunteers, which informed our decision to use a lower 1 mg dose in the current study. Importantly, it is unlikely that these side effects were responsible for driving the effects seen on cognition, given that an improvement rather than an impairment was seen. It is however important to note that the blinding of the study was not successful, which is likely to be a consequence of the side effect profile of prucalopride. Second, it is important to note that this study was exploratory in nature. Since this was the first study to experimentally manipulate 5-HT4 receptor function, it was necessary to take a broad cognitive profiling approach and measure the effect of prucalopride on a range of cognitive tasks. Whilst this has been useful in characterising the cognitive effects of 5-HT4 receptor activation, it has the disadvantage of requiring multiple statistical comparisons and therefore an increased chance of Type I errors. Importantly, the overlapping effects of improved recall across multiple tasks increase confidence in the findings, although it remains important to replicate the effects seen here in future studies.
Taken together, the results of this study are a significant translation of work on the 5-HT4 receptor from animals to humans, and suggest that 5-HT4 agonists may provide a novel approach for the treatment of the cognitive deficits associated with many disorders, including depression. 5-HT4 receptor agonists might be a particularly promising adjunct to SSRIs, which typically do not improve cognitive functioning. Indeed in animal models of depression, 5-HT4 receptor agonists can potentiate the antidepressant effects of SSRIs, again hinting at a possible useful adjunctive role.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291719002836.
Acknowledgements
The study was funded by the Medical Research Council (MR/K022202/1) and supported by the NIHR Oxford Health Biomedical Research Centre. The research materials supporting this publication can be accessed by contacting the corresponding author.
Disclosures
SEM has received consultancy fees from P1Vital and Johnson & Johnson (J&J). She holds grant income from UCB and J&J. MB is employed on a part time basis by P1vital Ltd. He has received travel expenses from Lundbeck and acted as a consultant for J&J. CJH has received consultancy fees from P1vital, Lundbeck, Servier and J&J. She holds grant income from UCB and J&J. The other authors report no conflicts of interest.








