Introduction
The human gut microbiome (GM), comprised of trillions of microorganisms, bacteria, viruses, fungi and other life forms, has been implicated in numerous aspects of human health and disease (Lai, Gao, & Zhang, Reference Lai, Gao and Zhang2020). The GM is diverse, personalized, dynamic and can be influenced, especially early in life, by factors such as vaginal/caesarean birth, diet, sleep, contact with other humans and stress (Gacesa et al., Reference Gacesa, Kurilshikov, Vich Vila, Sinha, Klaassen, Bolte and Weersma2022; Szeligowski, Yun, Lennox, & Burnet, Reference Szeligowski, Yun, Lennox and Burnet2020). However, genetic factors, especially immunological background also determine a person's microbiome composition (Thaiss, Zmora, Levy, & Elinav, Reference Thaiss, Zmora, Levy and Elinav2016). The GM partakes in a bidirectional communication with the central nervous system (CNS), called the gut–brain axis (Cryan et al., Reference Cryan, O'riordan, Cowan, Sandhu, Bastiaanssen, Boehme and Dinan2019). Disruptions to the GM have been associated with several neuropsychiatric disorders, including major depressive disorder (MDD), bipolar disorder (BD) and schizophrenia-spectrum disorders (e.g. schizophrenia, schizo-affective disorder and schizophreniform disorder, SSD) (Bastiaanssen et al., Reference Bastiaanssen, Cussotto, Claesson, Clarke, Dinan and Cryan2020; Genedi, Janmaat, Haarman, & Sommer, Reference Genedi, Janmaat, Haarman and Sommer2019; Nguyen, Hathaway, Kosciolek, Knight, & Jeste, Reference Nguyen, Hathaway, Kosciolek, Knight and Jeste2021; Szeligowski et al., Reference Szeligowski, Yun, Lennox and Burnet2020).
MDD, BD and SSD are heterogeneous psychiatric disorders, which place significant burden on a patient's well-being and global health (WHO, 2006). Worldwide, MDD, BD and SSD affect 264 million, 45 million and 20 million people, respectively (James et al., Reference James, Abate, Abate, Abay, Abbafati, Abbasi and Murray2018). Besides the overlap in psychiatric symptomatology, cognitive and biological functions, there is also a large genetic overlap between MDD, BD and SSD (Anttila et al., Reference Anttila, Bulik-Sullivan, Finucane, Walters, Bras, Duncan and Neale2018). Nowadays it is generally assumed that SSD, BD and MDD are disorders that are not entirely separated, but represent different stages of a continuum of clinical pictures (Haarman, Riemersma-Van der Lek, Burger, Drexhage, & Nolen, Reference Haarman, Riemersma-Van der Lek, Burger, Drexhage and Nolen2016).
Research into the role of the GM in MDD, BD and SSD is still in its infancy. This review is a narrative review which we have approached in a structured way and it presents data on the development and composition of the human gastrointestinal microbiota, and its interaction mechanisms in the gut–brain axis in MDD, BD and SSD. Furthermore, the therapeutic potential of pre/probiotics, diet and faecal microbiota transplantation (FMT) in these disorders will also be discussed. We begin by showing the different routes between the gut and brain and will then discuss the microbiome, after that we will discuss gut permeability and the GM in the psychiatric disorders. Finally, we will discuss potential mechanisms on how to adapt the GM to improve the clinical situation of patients.
Gut–brain axis
The gut and the brain communicate bidirectionally via several routes, including the vagal nerve, hypothalamic–pituitary–adrenal (HPA) axis, the production of bacterial metabolites, such as short-chain fatty acids (SCFAs), immune mediators and entero-endocrine signalling (Cryan et al., Reference Cryan, O'riordan, Cowan, Sandhu, Bastiaanssen, Boehme and Dinan2019; Golofast & Vales, Reference Golofast and Vales2020) (Fig. 1).
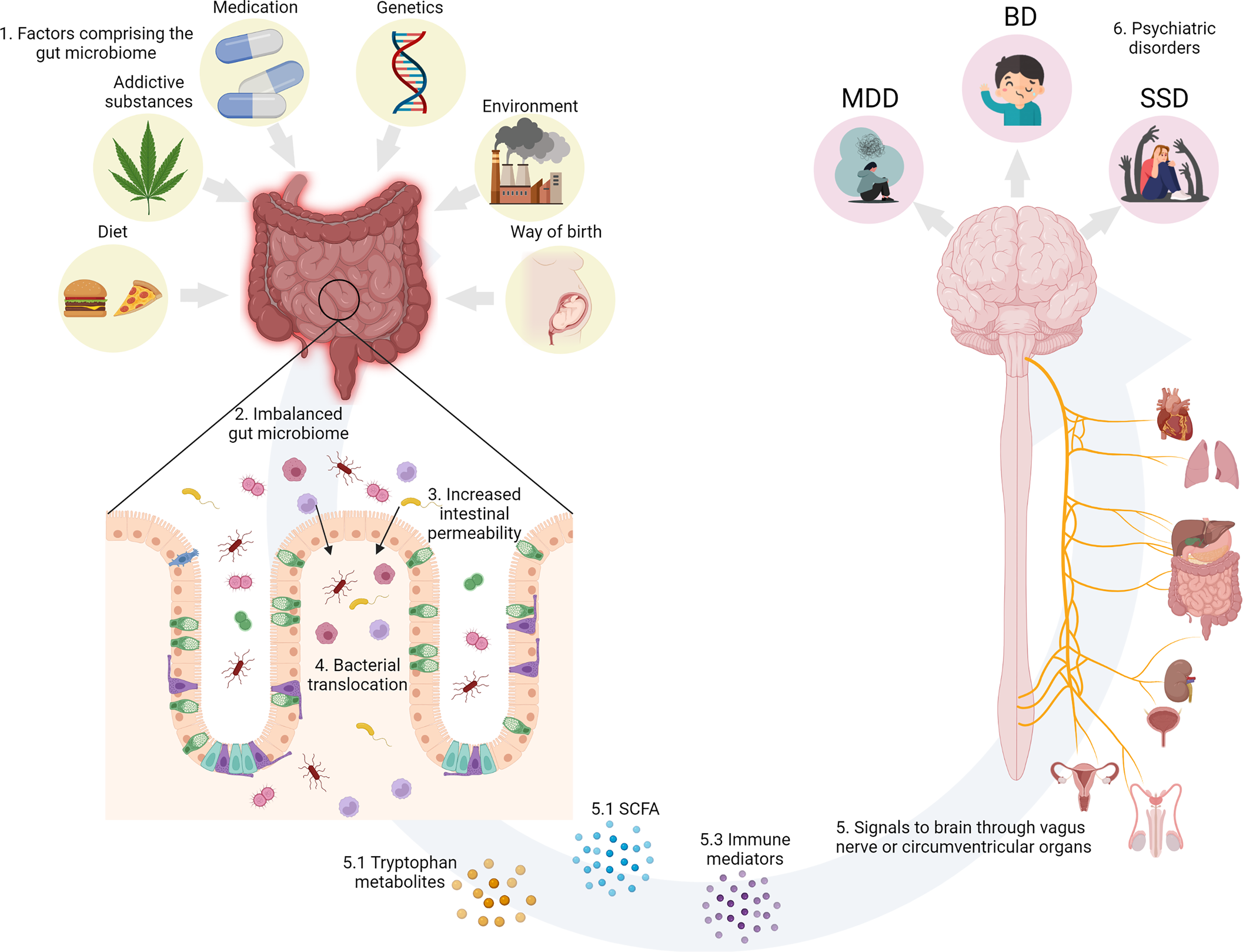
Fig. 1. (1) Environmental factors known to impinge on the human GM. (2) GM dysbiosis impairs intestinal permeability. (3) Increased intestinal permeability causes translocation of luminal components and reactivity of the intestinal immune system. (4) Bacterial translocation activates the gut–brain axis. (5) The gut and the brain communicate bidirectionally via several routes, including the vagal nerve, the HPA axis, immune mediators such as cytokines, and the production of bacterial metabolites, such as SCFAs. (6) The environmental factors, GM dysbiosis and increased permeability separately and in concert could contribute the development of psychiatric disorders. Created with BioRender.com.
Vagal nerve
The most direct route between the gut and the brain is the vagal nerve, roughly translated as the wandering nerve, and also known as the 10th cranial nerve. In reports from 1953 and 1961, ablation of the vagal nerve, formerly used for the treatment of peptic ulcers, resulted in an increase in the incidence of psychiatric disorders (Browning & Houseworth, Reference Browning and Houseworth1953; Whitlock, Reference Whitlock1961). Interestingly, this procedure recently turned out to reduce the incidence of Parkinson's disease (Svensson et al., Reference Svensson, Horváth-Puhó, Thomsen, Djurhuus, Pedersen, Borghammer and Sørensen2015). In animals, Sgritta et al. (Reference Sgritta, Dooling, Buffington, Momin, Francis, Britton and Costa-Mattioli2019) observed that the effects of Lactobacillus reuteri on social behaviour were dissipated after vagotomy in a genetic mouse model of autism (Shank3B−/− mouse).
Short-chain fatty acids
SCFAs are capable of signalling to the brain indirectly via nerve activation and can therefore influence behaviour (Sherwin, Sandhu, Dinan, & Cryan, Reference Sherwin, Sandhu, Dinan and Cryan2016). Ninety five per cent of SCFAs consist of acetate, propionate and butyrate. The primary source of SCFAs is microbial fermentation of dietary fibres in the gut, which is supported by the fact that germ-free animals and antibiotic treatment results in low SCFA levels (Cryan et al., Reference Cryan, O'riordan, Cowan, Sandhu, Bastiaanssen, Boehme and Dinan2019). SCFAs are capable of modulating neurotransmission. For example, propionic acid increases tryptophan hydroxylase expression which can reduce indoleamine serotonin, thereby influencing serotonergic neurotransmission (El-Ansary, Bacha, & Kotb, Reference El-Ansary, Bacha and Kotb2012; Nankova, Agarwal, MacFabe, & La Gamma, Reference Nankova, Agarwal, MacFabe and La Gamma2014). So far, only a few studies have investigated relations between neuropsychiatric disorders and SCFAs. Skonieczna-żydecka et al. (Reference Skonieczna-żydecka, Grochans, Maciejewska, Szkup, Schneider-Matyka, Jurczak and Stachowska2018) reported lower median content of acetate and higher isocaproic acid concentration in depressed women as compared to healthy women. Negative correlations were yielded between acetic, propionic and isocaproic acids and severity of depression as assessed with the Beck's Depression Inventory (BDI) scores. Szczesniak, Hestad, Hanssen, and Rudi (Reference Szczesniak, Hestad, Hanssen and Rudi2016) reported significantly higher isovaleric levels in the depressed patients as compared to healthy subjects. However, Kelly et al. (Reference Kelly, Borre, O’ Brien, Patterson, El Aidy, Deane and Dinan2016) found no differences in acetate, propionate, isobutyrate and butyrate between depressed patients and healthy controls. The limited sample sizes in all three studies could explain these inconsistent results. In animal models of mania, sodium butyrate reversed mania-like, e.g. behavioural hyperactivity, and depressive-like behaviour (Moretti et al., Reference Moretti, Valvassori, Varela, Ferreira, Rochi, Benedet and Quevedo2011; Resende et al., Reference Resende, Valvassori, Réus, Varela, Arent, Ribeiro and Quevedoa2013; Valvassori et al., Reference Valvassori, Resende, Budni, Dal-Pont, Bavaresco, Reus and Quevedo2015)
Immune mediators
Immune mediators are important intermediaries between the gut microbiota and the brain. Cytokines can signal to the brain from the periphery via the vagal nerve or via the circumventricular organs (Sherwin et al., Reference Sherwin, Sandhu, Dinan and Cryan2016). In the blood, bacteria or fragments from bacteria can bind lipopolysaccharide (LPS) binding protein (LBP), which is then connected to TLR-4, expressed on monocytes, macrophages and microglia, by soluble cluster of differentiation 14 (sCD14) (Kiecolt-Glaser et al., Reference Kiecolt-Glaser, Wilson, Bailey, Andridge, Peng, Jaremka and Belury2018; Kitchens & Thompson, Reference Kitchens and Thompson2005; Lim, Chang, & Wu, Reference Lim, Chang and Wu2019), which can lead to nuclear factor kappa-light-chain enhancer of activated B cells (NFκB) activation, inducing pro-inflammatory cytokine production (Genedi et al., Reference Genedi, Janmaat, Haarman and Sommer2019; Sherwin et al., Reference Sherwin, Sandhu, Dinan and Cryan2016). Thus, the presence of bacteria or small parts of bacteria can activate the immune system which also affects the brain. Blood levels of LBP, sCD14 and NFκB can reflect activity of this route. The group of Robert Yolken showed that these levels are increased in patients with BD and SSD, when patients had comorbid gastro-intestinal complaints (Severance et al., Reference Severance, Gressitt, Stallings, Origoni, Khushalani, Leweke and Yolken2013; Severance, Dickerson, & Yolken, Reference Severance, Dickerson and Yolken2020). Moreover, Foster, Baker, and Dursun (Reference Foster, Baker and Dursun2021) recently reviewed the relation of the GM and the immune system in MDD, where they draw attention to the importance of the immune system as an important player in the neurobiology combined with the GM in subtypes of depression.
Tryptophan metabolites
Along with influencing other metabolites, gut-bacteria influence tryptophan metabolism (Carlessi, Borba, Zugno, Quevedo, & Réus, Reference Carlessi, Borba, Zugno, Quevedo and Réus2021). Tryptophan is an essential amino acid, which is metabolized by two main pathways, namely, the serotonin (5-HT) pathway and the kynurenine pathway. According to a meta-analysis of 101 studies tryptophan and kynurenine are decreased across MDD, BD and SSD (Marx et al., Reference Marx, McGuinness, Rocks, Ruusunen, Cleminson, Walker and Fernandes2020). Conventional antidepressants enhance levels of central serotonin to produce a therapeutic effect. Most of the tryptophan is metabolized into kynurenine in the liver by tryptophan-2,3-dioxygenase for energy production, or following an inflammatory stimulus by indoleamine-2,3-dioxygenase. The presence of gut bacteria such as Clostridium perfringens can modulate gut production of 5-HT (Beaver & Wostmann, Reference Beaver and Wostmann1962; Yano et al., Reference Yano, Yu, Donaldson, Shastri, Ann, Ma and Hsiao2015). Studies in rats have shown that probiotic treatment can influence tryptophan, kynurenine and serotonin levels (Sherwin et al., Reference Sherwin, Sandhu, Dinan and Cryan2016). Rats treated with Bifidobacterium infantis 35624 had increased circulating tryptophan levels (Desbonnet, Garrett, Clarke, Bienenstock, & Dinan, Reference Desbonnet, Garrett, Clarke, Bienenstock and Dinan2009). Furthermore, rats treated with Lactobacillus johnsonii had lower circulating kynurenine levels with increased serotonin levels in the ileum and in the circulation (Valladares et al., Reference Valladares, Bojilova, Potts, Cameron, Gardner, Lorca and Gonzalez2013). Moreover, one study in MDD patients found decreases in the kynurenine/tryptophan ratio after treatment with probiotics (Lactobacillus helveticus and Bifidobacterium longum) (Kazemi, Noorbala, Azam, Eskandari, & Djafarian, Reference Kazemi, Noorbala, Azam, Eskandari and Djafarian2019). A meta-analysis studying the effect of probiotic supplementation on the tryptophan–kynurenine pathway observed significantly lower serum kynurenine and a decreased kynurenine/tryptophan ratio after treatment (Purton et al., Reference Purton, Staskova, Lane, Dawson, West, Firth and Marx2021). The results so far provide preliminary evidence that probiotics can modulate the tryptophan–kynurenine pathway.
Gut microbiome
The GM contains 1014 microorganisms, 2–20 million unique genes and over 1000 unique bacterial species. The GM is a perplexing genomic structure with many more genes than the human genome (Golofast & Vales, Reference Golofast and Vales2020). In contrast to the human genome, which is unalterable over lifetime, the GM is highly adaptable (Nguyen et al., Reference Nguyen, Hathaway, Kosciolek, Knight and Jeste2021). The GM is already influenced by the environment at the day of birth. Vaginally delivered neonates' microbiota resembles the maternal vaginal and faecal bacteria, whereas for infants born by caesarean section, their microbiota resembles the maternal skin and hospital environment (Bäckhed et al., Reference Bäckhed, Roswall, Peng, Feng, Jia, Kovatcheva-Datchary and Jun2015; Korpela et al., Reference Korpela, Helve, Kolho, Saisto, Skogberg, Dikareva and de Vos2020; Mitchell et al., Reference Mitchell, Mazzoni, Hogstrom, Bryant, Bergerat, Cher and Yassour2020). In Reference Cannon, Jones and Murray2002 Cannon, Jones and Murray already identified unplanned caesarean section as a risk factor for schizophrenia in their seminal meta-analysis. One epidemiological study found a weak association between birth by planned caesarean section, but not by unplanned emergency caesarean section and the risk of developing psychosis or BD (O'Neill et al., Reference O'Neill, Curran, Dalman, Kenny, Kearney, Clarke and Khashan2016). However, after correcting for matched siblings, this effect was no longer significant. The Finnish birth register data also showed an odds ratio (OR) of 2.5 for BD for birth by caesarean section (Chudal et al., Reference Chudal, Sourander, Polo-Kantola, Hinkka-Yli-Salomäki, Lehti, Sucksdorff and Brown2014).
Associations between antibiotic exposure and psychiatric disorders have been found in multiple large population-based studies (Köhler et al., Reference Köhler, Petersen, Mors, Mortensen, Yolken, Gasse and Benros2017; Liang et al., Reference Liang, Ye, Wen, Li, Cheng, Cheng and Zhang2021; Lurie, Yang, Hayne, Mamtani, & Boursi, Reference Lurie, Yang, Hayne, Mamtani and Boursi2015). Lurie et al. (Reference Lurie, Yang, Hayne, Mamtani and Boursi2015) found that treatment with a single antibiotic course was associated with higher risk for depression (n = 202,974) compared to a healthy control group (n = 803,961), with adjusted ORs of 1.23 [95% confidence level (CL) 1.18–1.29] for penicillin's and 1.25 (95% CL 1.15–1.35) for quinolones. Köhler et al. (Reference Köhler, Petersen, Mors, Mortensen, Yolken, Gasse and Benros2017) performed a large-scale study in individuals born in Denmark in 1985–2002 (n = 1,015,447), of which 5759 individuals were diagnosed with schizophrenia. The association of infections treated with anti-infective agents and the subsequent risk of schizophrenia and affective disorders during 1995–2013 was studied. Antibiotics, with a hazard rate ratio (HRR) of 1.44 (95% CL 1.25–1.66), and especially broad spectrum antibiotics (HRR = 1.53; 95% CL 1.32–1.71) were associated with increased risk for schizophrenia. However, Lurie et al. (Reference Lurie, Yang, Hayne, Mamtani and Boursi2015) did not find an association between antibiotic use and psychosis (n = 8487). Liang et al. (Reference Liang, Ye, Wen, Li, Cheng, Cheng and Zhang2021) observed positive associations between long-term antibiotic use during early life (defined by the UK Biobank as child or teenager) and anxiety and depression. Lastly, there are also studies showing that antibiotic use can induce hypomania or mania, also known as antibiomania (Abouesh, Stone, & Hobbs, Reference Abouesh, Stone and Hobbs2002). In a review of 47 published cases, clarithromycin, a broad spectrum antibiotic, was related to (hypo)mania in 16 out of the 47 cases (Lambrichts, Van Oudenhove, & Sienaert, Reference Lambrichts, Van Oudenhove and Sienaert2017). One explanation could be that the administration of antibiotics can result in changes in the microbiome which could in turn increases the risk of (hypo)mania, for example by lowering the bacterial production of gamma-amino-butyric acid (Dickerson, Severance, & Yolken, Reference Dickerson, Severance and Yolken2017).
Gastrointestinal permeability in patients with severe psychiatric disorders
Gastrointestinal barrier
The gastrointestinal barrier is a dynamic, multilayer system which consists of a physical barrier and a biochemical barrier. The main components of the physical barrier are the epithelial cells sealed by tight junctions and the gut mucosa. The biochemical barrier consists of the gut microbiota and the mucosal immune system. Its main function is to keep pathogens out of the host's internal milieu, while at the same time facilitate the absorption of nutrients, water and electrolytes (Bischoff et al., Reference Bischoff, Barbara, Buurman, Ockhuizen, Schulzke, Serino and Wells2014). For the homoeostasis of the organism, the permeability of the gastrointestinal barrier needs to be maintained within a narrow equilibrium.
Diet and lifestyle factors such as energy-dense food and alcohol can disturb gut permeability and lead to translocation of luminal components and reactivity of the intestinal immune system (Bischoff et al., Reference Bischoff, Barbara, Buurman, Ockhuizen, Schulzke, Serino and Wells2014). Increased intestinal permeability can also be a result of changes in gut microbiota, infections or reduced perfusion of the gut (Bischoff et al., Reference Bischoff, Barbara, Buurman, Ockhuizen, Schulzke, Serino and Wells2014).
Gastrointestinal permeability and brain structure and function
Gastrointestinal permeability and brain structure and function are governed by a bidirectional interaction. On one hand, pre-clinical studies (Keita, Söderholm, & Ericson, Reference Keita, Söderholm and Ericson2010; Kiliaan et al., Reference Kiliaan, Saunders, Bijlsma, Cecilia Berin, Taminiau, Groot and Perdue1998; Söderholm et al., Reference Söderholm, Yang, Ceponis, Vohra, Riddell, Sherman and Perdue2002; Vicario et al., Reference Vicario, Guilarte, Alonso, Yang, Martínez, Ramos and Santos2010) and studies in healthy volunteers (Vanuytsel et al., Reference Vanuytsel, Van Wanrooy, Vanheel, Vanormelingen, Verschueren, Houben and Tack2014) suggest a causal effect of psychosocial stress on gut permeability, likely through corticotropin-releasing hormone-mediated mast cell activation and decreased blood flow to the gut during stressful periods. On the other hand, it is hypothesized that increased gut permeability and abnormal influx of food- and bacteria-derived antigens drives systemic low-grade immune dysregulation, which in turn affects brain structure and function (Genedi et al., Reference Genedi, Janmaat, Haarman and Sommer2019). Lastly, psychiatric comorbidity is common in diseases with known structural and functional abnormalities of the gastrointestinal barrier, such as Crohn's disease and colitis ulcerosa (Bennett, Tennant, Piesse, Badcock, & Kellow, Reference Bennett, Tennant, Piesse, Badcock and Kellow1998; Faresjö et al., Reference Faresjö, Grodzinsky, Johansson, Wallander, Timpka and Åkerlind2007; Nicholl et al., Reference Nicholl, Halder, Macfarlane, Thompson, O'Brien, Musleh and McBeth2008). It is therefore hypothesized that abnormal intestinal permeability is involved both as an effect and as a cause of severe psychiatric disorder, yet, this is still a research area in its infancy.
Evidence of gastrointestinal barrier dysfunction in MDD, BD and SSD
In MDD, BD and SSD, gastrointestinal permeability has been assessed with markers of structural barrier integrity and paracellular permeability such as zonulin and intestinal-type fatty acid-binding protein (I-FABP) (Table 1). In a study in MDD or anxiety disorder, authors reported significantly higher levels of zonulin and I-FABP in patients compared to controls and this was associated with gut dysbiosis (Stevens et al., Reference Stevens, Goel, Seungbum, Richards, Holbert, Pepine and Genomics2018). Alvarez-Mon et al. (Reference Alvarez-Mon, Gómez, Orozco, Lahera, Sosa, Diaz and Alvarez-Mon2019) also reported significantly higher levels of I-FABP in MDD patients compared to controls but no significant difference was observed for zonulin. In another study, patients of different psychiatric diagnoses who had recently attempted suicide had higher levels of I-FABP but lower levels of zonulin compared to both MDD patients without a history of suicide attempt and healthy controls (Ohlsson et al., Reference Ohlsson, Gustafsson, Lavant, Suneson, Brundin, Westrin and Lindqvist2019). In BD, patients in full remission showed higher zonulin and claudin-5 compared to controls, while there were no differences between active manic episode and remission (Kılıç, Işık, Demirdaş, Doğuç, & Bozkurt, Reference Kılıç, Işık, Demirdaş, Doğuç and Bozkurt2020). Interestingly, this observation might suggest that zonulin and claudin-5 are ‘trait’ rather than ‘state’ markers of BD. However, the small sample size of this study precludes firm conclusions, before the findings are confirmed in larger populations. Moreover, there is a certain level of uncertainty surrounding the measurement of zonulin with commercially available ELISA kits. The results should be interpreted with caution until an in depth understanding of the biomarker is acquired (Massier, Chakaroun, Kovacs, & Heiker, Reference Massier, Chakaroun, Kovacs and Heiker2021). In schizophrenia, Maes, Kanchanatawan, Sirivichayakul, and Carvalho (Reference Maes, Kanchanatawan, Sirivichayakul and Carvalho2019a) and Maes, Sirivichayakul, Kanchanatawan, and Vodjani (Reference Maes, Sirivichayakul, Kanchanatawan and Vodjani2019b) showed that immunoglobulin M (IgM) responses to zonulin were higher in patients compared to controls, while IgM response to occludin was significantly associated with deficit schizophrenia. Authors also reported an increased ratio of IgM towards components of the paracellular route (zonulin + occludin)/components of the transcellular route (talin + actin + vinculin) in deficit v. non-deficit schizophrenia and in schizophrenia v. controls. This ratio was significantly associated with increased IgA responses to Gram-negative bacteria. Schizophrenia patients with deficit syndrome (i.e. severe negative and cognitive symptoms) can be expected to have unhealthy dietary habits, which could explain the association with IgM response to occludin. Barber et al. (Reference Barber, Sturgeon, Fasano, Gascella, Eaton, McMahon and Kelly2019) showed that 42.9% of the patients had higher levels of zonulin than the cut-off for elevated levels (>2.33 mg/dl).
Table 1. Main findings of studies assessing gut permeability in MDD, BD and SSD
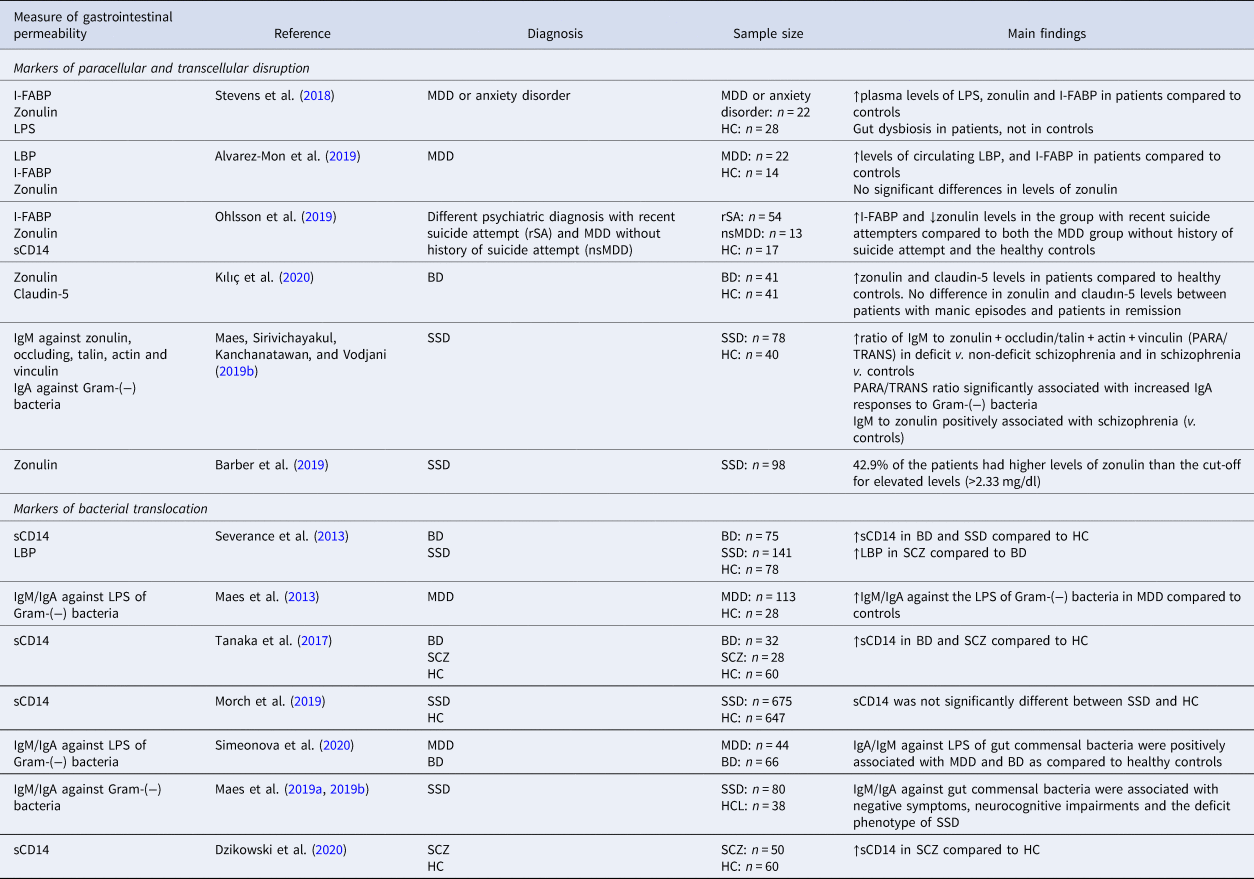
sCD14, soluble cluster of differentiation 14; LBP, lipopolysaccharide (LPS) binding protein; I-FABP, fatty acid-binding protein.
Markers of bacteria translocation can also reflect abnormal gastrointestinal permeability. LPS and LBP were up-regulated in MDD or anxiety disorder (Alvarez-Mon et al., Reference Alvarez-Mon, Gómez, Orozco, Lahera, Sosa, Diaz and Alvarez-Mon2019; Stevens et al., Reference Stevens, Goel, Seungbum, Richards, Holbert, Pepine and Genomics2018). In BD, sCD14, a co-receptor for the LBP, used as a marker for bacterial translocation was significantly higher in patients compared to controls (Severance et al., Reference Severance, Gressitt, Stallings, Origoni, Khushalani, Leweke and Yolken2013). In SSD, sCD14 was significantly higher in multiple studies (Dzikowski et al., Reference Dzikowski, Juchnowicz, Dzikowska, Rog, Próchnicki, Kozioł and Karakula-Juchnowicz2020; Severance et al., Reference Severance, Gressitt, Stallings, Origoni, Khushalani, Leweke and Yolken2013; Tanaka et al., Reference Tanaka, Matsuda, Hayes, Yang, Rodriguez, Severance and Eaton2017; Weber et al., Reference Weber, Gressitt, Cowan, Niebuhr, Yolken and Severance2018) but not all (Morch et al., Reference Morch, Dieset, Færden, Reponen, Hope, Hoseth and Andreassen2019). In regard to antibodies against gut commensal bacteria, in MDD, Maes et al. (Reference Maes, Kubera, Leunis, Berk, Geffard and Bosmans2013) showed increased IgM/IgA against Gram-negative bacteria. More recently, Simeonova et al. (Reference Simeonova, Stoyanov, Leunis, Carvalho, Kubera, Murdjeva and Maes2020) showed increased immune responses to Gram-negative bacteria in both MDD and BD, especially in the presence of melancholia, compared to healthy controls. Interestingly, IgA/IgM response profiles differ among the two diagnosis, as well as between BD I and II subtypes and patients with melancholia. This might reflect a common underlying disruption of the gut barrier across diagnoses, accompanied however by distinct microbiome profiles and immune susceptibilities, though this remains to be confirmed in future studies. In SSD IgM/IgA against gut commensal bacteria were associated with negative symptoms, neurocognitive impairments and the deficit phenotype of SSD (Maes et al., Reference Maes, Sirivichayakul, Kanchanatawan and Vodjani2019b).
GM in three major psychiatric disorders
Alpha and beta diversity
α-Diversity is the mean species diversity in sites or habitats at a local scale (Whittaker, Reference Whittaker1972). α-Diversity is often measured by the Fisher, Ace, Chao, Simpson and/or Shannon indices. β-Diversity shows differentiation among habitats (Whittaker, Reference Whittaker1972). Multiple studies analysed diversity metrics; the findings however were mixed.
For MDD studies (Table 2), α-diversity was not different from controls in three studies (Chung et al., Reference Chung, Chen, Chou, Chen, Lee, Chuang and Kuo2019; Zheng et al., Reference Zheng, Zeng, Zhou, Liu, Fang, Xu and Fan2016, Reference Zheng, Yang, Li, Wu, Liang, Yin and Wang2020). Two studies found lower α-diversity in MDD (Huang et al., Reference Huang, Shi, Li, Shen, Shi, Wang and Liang2018; Liu et al., Reference Liu, Zhang, Wang, Wang, Zhang, Jiang and Duan2016). Four studies found inconsistent findings across indices (Jiang et al., Reference Jiang, Ling, Zhang, Mao, Ma, Yin and Ruan2015; Kelly et al., Reference Kelly, Borre, O’ Brien, Patterson, El Aidy, Deane and Dinan2016; Lai et al., Reference Lai, Deng, Xu, Zhao, Xu, Liu and Rong2021; Rong et al., Reference Rong, Xie, Zhao, Lai, Wang, Xu and Liu2019). β-Diversity was significantly different between MDD and controls in four studies (Huang et al., Reference Huang, Shi, Li, Shen, Shi, Wang and Liang2018; Kelly et al., Reference Kelly, Borre, O’ Brien, Patterson, El Aidy, Deane and Dinan2016; Lai et al., Reference Lai, Deng, Xu, Zhao, Xu, Liu and Rong2021; Zheng et al., Reference Zheng, Zeng, Zhou, Liu, Fang, Xu and Fan2016) and not different in three studies (Chung et al., Reference Chung, Chen, Chou, Chen, Lee, Chuang and Kuo2019; Jiang et al., Reference Jiang, Ling, Zhang, Mao, Ma, Yin and Ruan2015; Rong et al., Reference Rong, Xie, Zhao, Lai, Wang, Xu and Liu2019).
Table 2. Studies of the GM in major depressive disorder
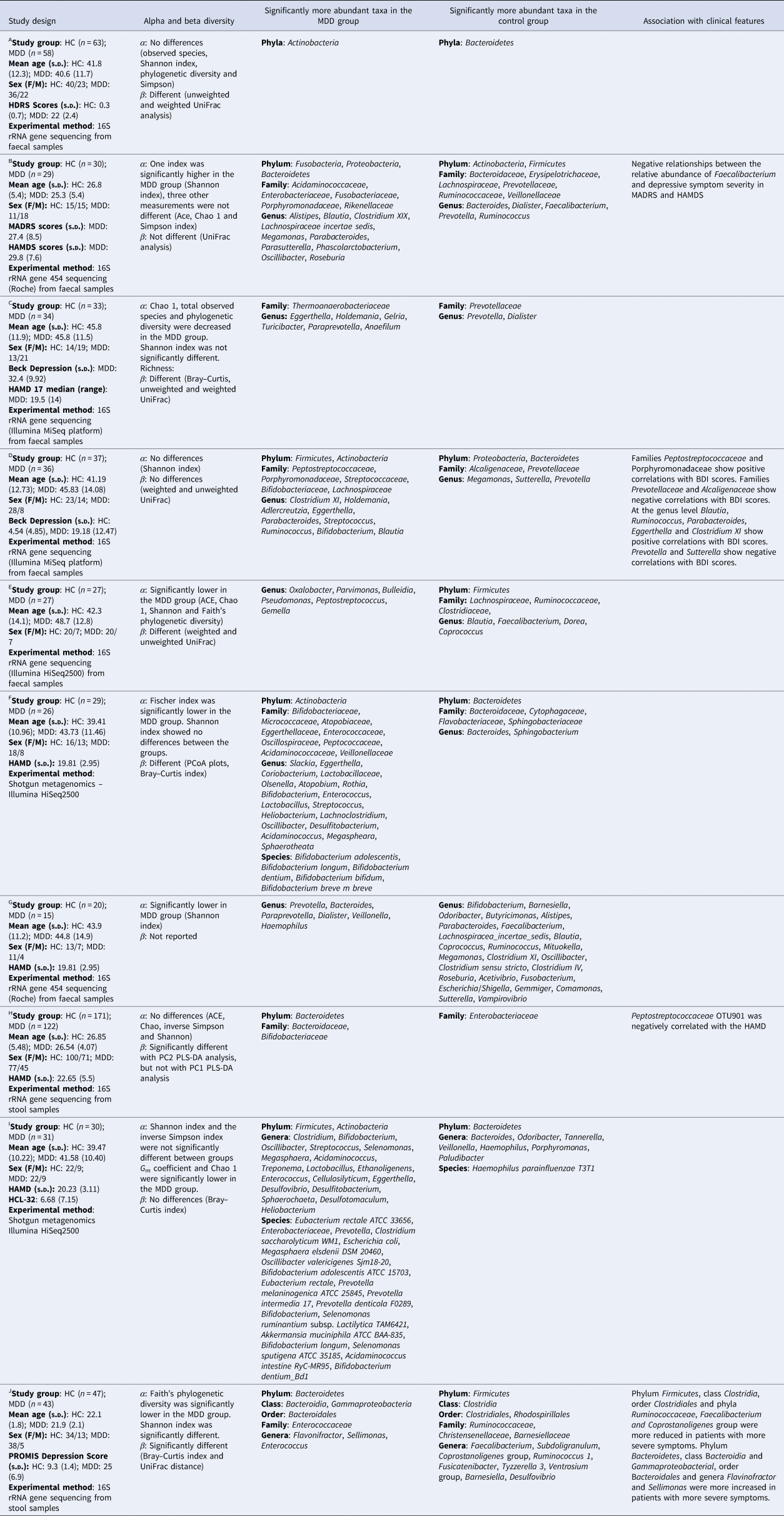
HC, healthy controls; MDD, major depressive disorder; PCoA, principal coordinates analysis; PLS-DA, partial least-squares discriminant analysis; HDRS, Hamilton Depression Rating Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; HAMDS, Hamilton's Depression Scale; PROMIS Depression Score: Patient-Reported Outcomes Measurement Information System Depression Score; OTU, operational taxonomic unit.
AZheng et al. (Reference Zheng, Zeng, Zhou, Liu, Fang, Xu and Fan2016); BJiang et al. (Reference Jiang, Ling, Zhang, Mao, Ma, Yin and Ruan2015); cKelly et al. (Reference Kelly, Borre, O’ Brien, Patterson, El Aidy, Deane and Dinan2016); DChung et al. (Reference Chung, Chen, Chou, Chen, Lee, Chuang and Kuo2019); EHuang et al. (Reference Huang, Shi, Li, Shen, Shi, Wang and Liang2018); FLai et al. (Reference Lai, Deng, Xu, Zhao, Xu, Liu and Rong2021); GLiu et al. (Reference Liu, Zhang, Wang, Wang, Zhang, Jiang and Duan2016); HZheng et al. (Reference Zheng, Yang, Li, Wu, Liang, Yin and Wang2020); IRong et al. (Reference Rong, Xie, Zhao, Lai, Wang, Xu and Liu2019); JLiu et al. (Reference Liu, Rowan-Nash, Sheehan, Walsh, Sanzari, Korry and Belenky2020).
For α-diversity in the BD studies (Table 3), three had inconsistent findings (Hu et al., Reference Hu, Li, Huang, Lai, Li, Sublette and Xu2019; Painold et al., Reference Painold, Mörkl, Kashofer, Halwachs, Dalkner, Bengesser and Reininghaus2018; Rong et al., Reference Rong, Xie, Zhao, Lai, Wang, Xu and Liu2019) and one found no differences (Zheng et al., Reference Zheng, Yang, Li, Wu, Liang, Yin and Wang2020). β-Diversity was different in two studies (Hu et al., Reference Hu, Li, Huang, Lai, Li, Sublette and Xu2019; Zheng et al., Reference Zheng, Yang, Li, Wu, Liang, Yin and Wang2020) and two found no differences (Painold et al., Reference Painold, Mörkl, Kashofer, Halwachs, Dalkner, Bengesser and Reininghaus2018; Rong et al., Reference Rong, Xie, Zhao, Lai, Wang, Xu and Liu2019).
Table 3. Studies of the GM in individuals with BD
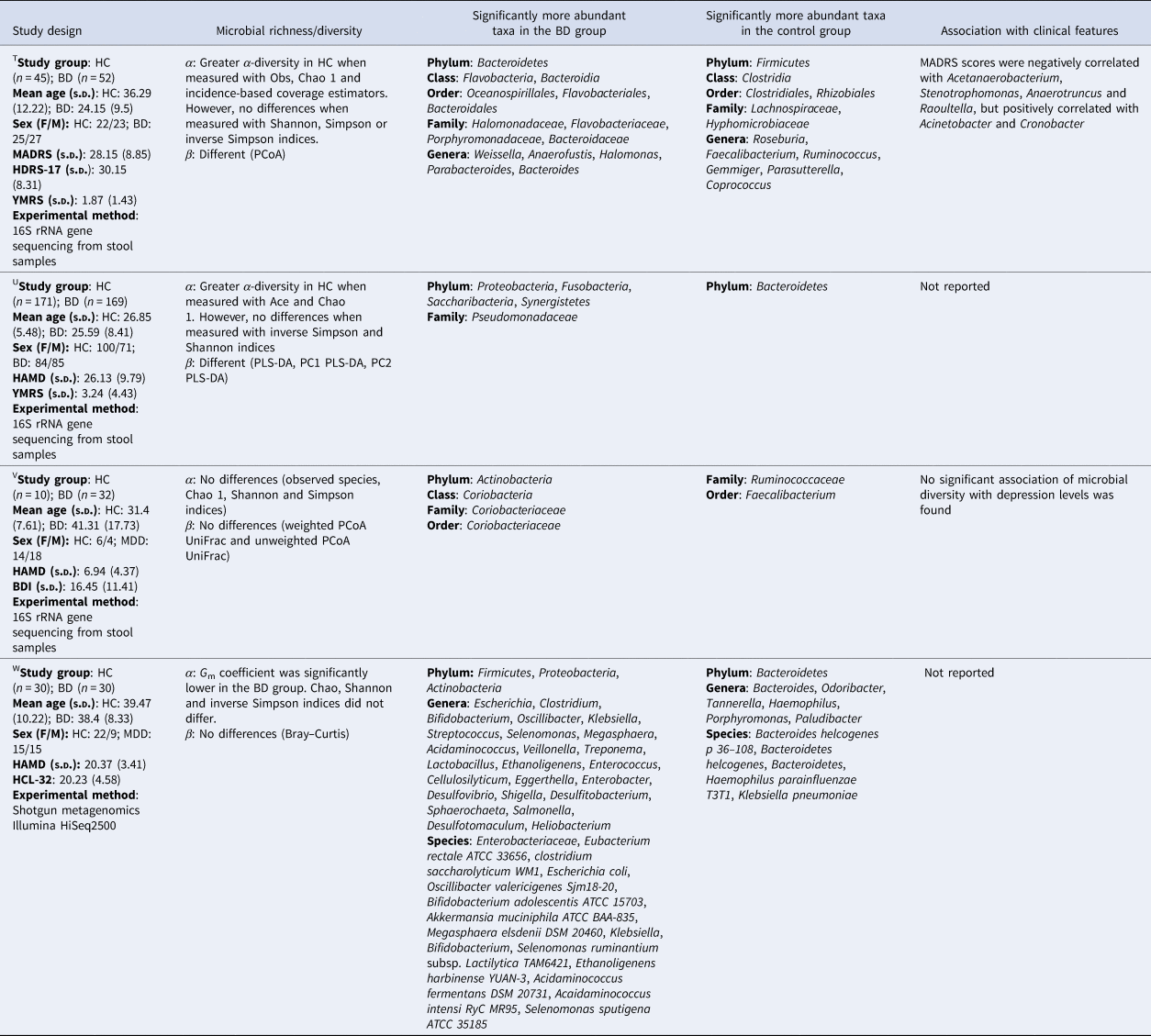
HC, healthy controls; BD, bipolar disorder; PLS-DA, partial least-squares discriminant analysis; PCoA, principal coordinates analysis; HCL-32, Hypomania Check List-32; HAMD, Hamilton's Depression Scale; BDI, Beck Depression Inventory; MADRS, Montgomery–Åsberg Depression Rating Scale; HDRS-17, 17-item Hamilton Depression Rating Scale.
THu et al. (Reference Hu, Li, Huang, Lai, Li, Sublette and Xu2019); UZheng et al. (Reference Zheng, Yang, Li, Wu, Liang, Yin and Wang2020); VPainold et al. (Reference Painold, Mörkl, Kashofer, Halwachs, Dalkner, Bengesser and Reininghaus2018); WRong et al. (Reference Rong, Xie, Zhao, Lai, Wang, Xu and Liu2019).
In SSD (Table 4), three studies reported lower α-diversity in the SSD groups compared to healthy controls (Ma et al., Reference Ma, Asif, Dai, He, Zheng, Wang and Chen2020; Xu et al., Reference Xu, Wu, Liang, He, Gu, Li and Wang2020; Zheng et al., Reference Zheng, Zeng, Liu, Chen, Pan, Han and Xie2019), four reported no differences (He et al., Reference He, Kosciolek, Tang, Zhou, Li, Ma and Chen2018; Li et al., Reference Li, Zhuo, Huang, Huang, Zhou, Xiong and Wu2020; Nguyen et al., Reference Nguyen, Kosciolek, Maldonado, Daly, Martin, McDonald and Jeste2018; Shen et al., Reference Shen, Xu, Li, Huang, Yuan, Wang and Liang2018), two did not report on α-diversity (Schwarz et al., Reference Schwarz, Maukonen, Hyytiäinen, Kieseppä, Orešič, Sabunciyan and Suvisaari2018; Yuan et al., Reference Yuan, Zhang, Wang, Liu, Li, Kumar and Song2018) and one reported higher α-diversity in the SSD group (Zhu et al., Reference Zhu, Ju, Wang, Wang, Guo, Ma and Ma2020b). β-Diversity was different between groups in five studies (He et al., Reference He, Kosciolek, Tang, Zhou, Li, Ma and Chen2018; Nguyen et al., Reference Nguyen, Hathaway, Kosciolek, Knight and Jeste2021; Shen et al., Reference Shen, Xu, Li, Huang, Yuan, Wang and Liang2018; Xu et al., Reference Xu, Wu, Liang, He, Gu, Li and Wang2020; Zheng et al., Reference Zheng, Zeng, Liu, Chen, Pan, Han and Xie2019), from those five, two studies reported tighter clustering in the healthy control group (Nguyen et al., Reference Nguyen, Kosciolek, Maldonado, Daly, Martin, McDonald and Jeste2018; Shen et al., Reference Shen, Xu, Li, Huang, Yuan, Wang and Liang2018).
Table 4. Studies of the GM in schizophrenia-spectrum disorder
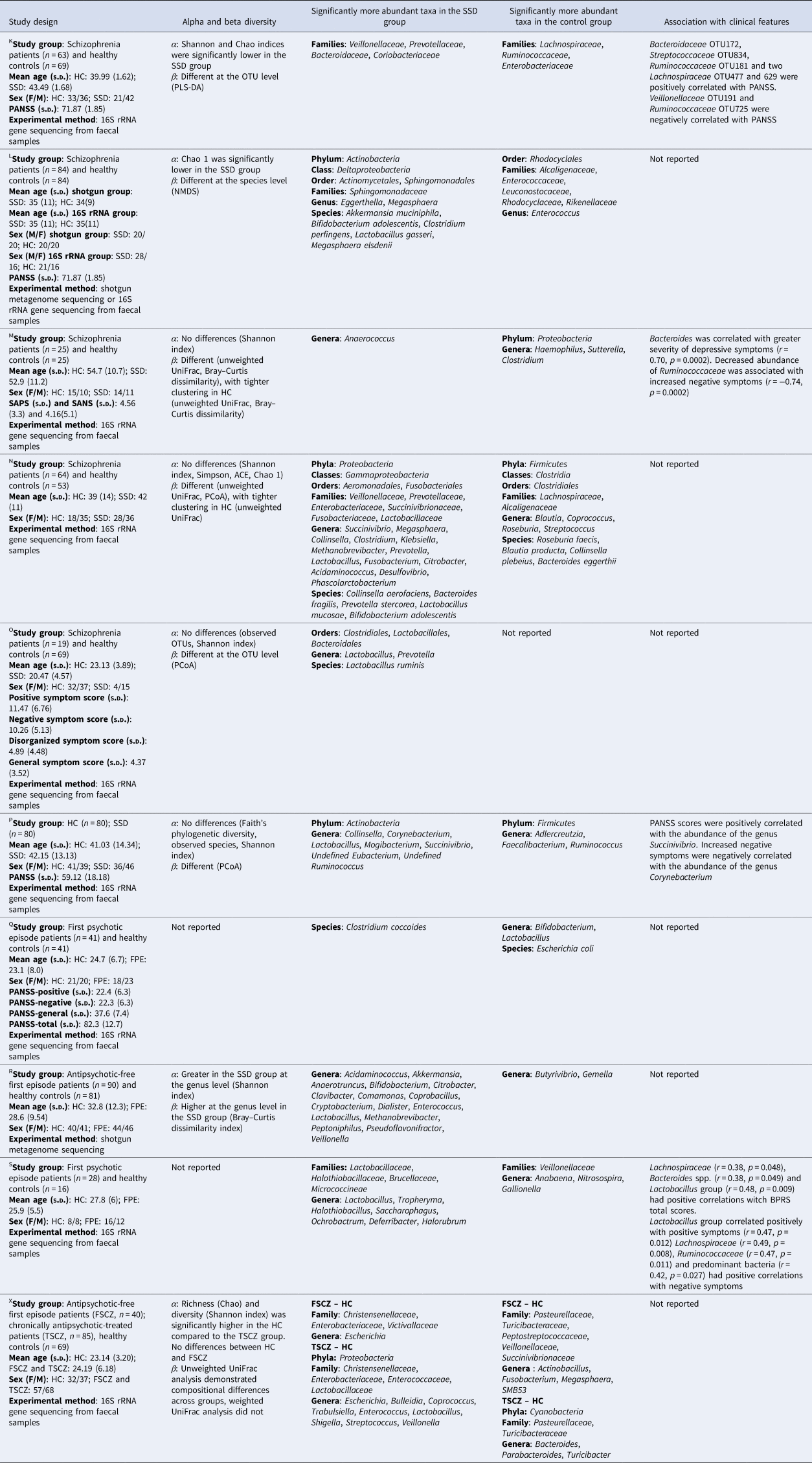
HC, healthy controls; FPE, first psychotic episode patients; SSD, schizophrenia-spectrum disorder patients; FSCZ, antipsychotic-free first episode patients; TSCZ, chronically antipsychotic-treated patients; OTU, operational taxonomic unit; PLS-DA, partial least-squares discriminant analysis; NMDS, non-metric multidimensional scaling; PCoA, principal coordinates analysis; BPRS, Brief Psychiatric Rating Scale; PANSS, Positive and Negative Syndrome Score.
KZheng et al. (Reference Zheng, Zeng, Liu, Chen, Pan, Han and Xie2019); LXu et al. (Reference Xu, Wu, Liang, He, Gu, Li and Wang2020); MNguyen et al. (Reference Nguyen, Hathaway, Kosciolek, Knight and Jeste2021); NShen et al. (Reference Shen, Xu, Li, Huang, Yuan, Wang and Liang2018); OHe et al. (Reference He, Kosciolek, Tang, Zhou, Li, Ma and Chen2018); PLi et al. (Reference Li, Zhuo, Huang, Huang, Zhou, Xiong and Wu2020); QYuan et al. (Reference Yuan, Zhang, Wang, Liu, Li, Kumar and Song2018); RZhu et al. (Reference Zhu, Ju, Wang, Wang, Guo, Ma and Ma2020b); SSchwarz et al. (Reference Schwarz, Maukonen, Hyytiäinen, Kieseppä, Orešič, Sabunciyan and Suvisaari2018); XMa et al. (Reference Ma, Asif, Dai, He, Zheng, Wang and Chen2020).
In the present review more studies reported inconsistent findings (n = 7) or no differences (n = 8) than studies who reported lower α-diversity in the psychiatric disorders (n = 5). These results are in line with other studies (Sanada et al., Reference Sanada, Nakajima, Kurokawa, Barceló-Soler, Ikuse, Hirata and Kishimoto2020; Simpson et al., Reference Simpson, Diaz-Arteche, Eliby, Schwartz, Simmons and Cowan2021), suggesting that host–microbe interactions are more complex than can be modelled by α-diversity.
Findings at different taxonomic levels
A large number of bacterial taxa were significantly different in their relative abundance between control and psychiatric groups [MDD groups (Table 2), BD groups (Table 3) and SSD groups (Table 4)]. Interestingly, multiple bacterial taxa abundances were similar between psychiatric disorders (Fig. 2). All investigations reported taxonomic differences between the neuropsychiatric disorders and healthy control groups; in the next paragraphs the most important differences, similarities and findings are discussed.
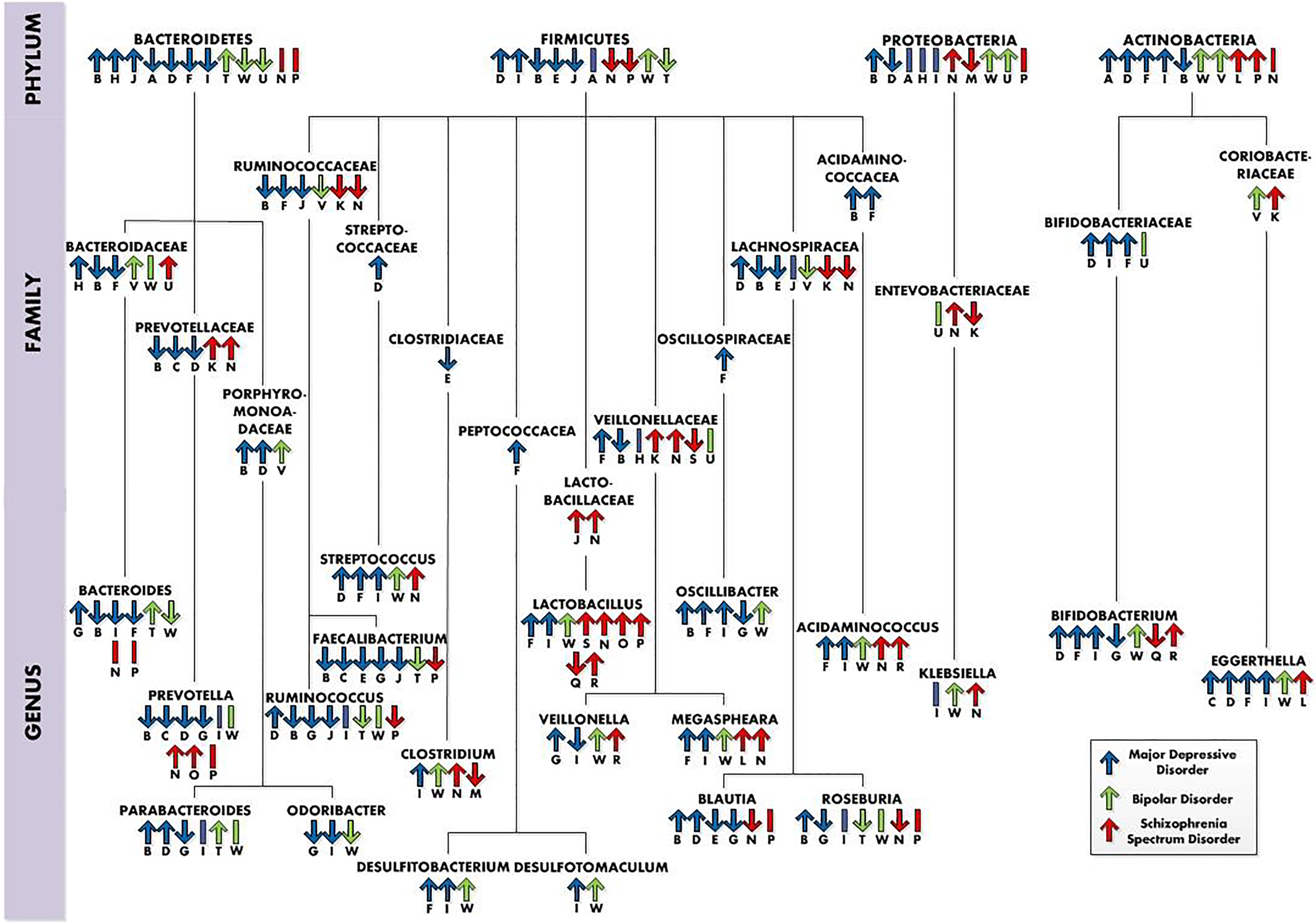
Fig. 2. Taxonomic differences in neuropsychiatric disorders (at the phylum, family and genus levels), whereby ‘↑’ = higher relative abundance in the neuropsychiatric disorder group, ‘↓’ = lower relative abundance in the neuropsychiatric group and ‘I’ = no differences in abundance. The letters below the arrows refer to the studies the information was retrieved from and also can be connected to the letters in the tables. Studies: AZheng et al. (Reference Zheng, Zeng, Zhou, Liu, Fang, Xu and Fan2016); BJiang et al. (Reference Jiang, Ling, Zhang, Mao, Ma, Yin and Ruan2015); CKelly et al. (Reference Kelly, Borre, O’ Brien, Patterson, El Aidy, Deane and Dinan2016); DChung et al. (Reference Chung, Chen, Chou, Chen, Lee, Chuang and Kuo2019); EHuang et al. (Reference Huang, Shi, Li, Shen, Shi, Wang and Liang2018); FLai et al. (Reference Lai, Deng, Xu, Zhao, Xu, Liu and Rong2021); GLiu et al. (Reference Liu, Zhang, Wang, Wang, Zhang, Jiang and Duan2016); HZheng et al. (Reference Zheng, Yang, Li, Wu, Liang, Yin and Wang2020); IRong et al. (Reference Rong, Xie, Zhao, Lai, Wang, Xu and Liu2019); JLiu et al. (Reference Liu, Rowan-Nash, Sheehan, Walsh, Sanzari, Korry and Belenky2020); KZheng et al. (Reference Zheng, Zeng, Liu, Chen, Pan, Han and Xie2019); LXu et al. (Reference Xu, Wu, Liang, He, Gu, Li and Wang2020); MNguyen et al. (Reference Nguyen, Kosciolek, Maldonado, Daly, Martin, McDonald and Jeste2018); NShen et al. (Reference Shen, Xu, Li, Huang, Yuan, Wang and Liang2018); OHe et al. (Reference He, Kosciolek, Tang, Zhou, Li, Ma and Chen2018); PLi et al. (Reference Li, Zhuo, Huang, Huang, Zhou, Xiong and Wu2020); QRong et al. (Reference Rong, Xie, Zhao, Lai, Wang, Xu and Liu2019); RPainold et al. (Reference Painold, Mörkl, Kashofer, Halwachs, Dalkner, Bengesser and Reininghaus2018); SSchwarz et al. (Reference Schwarz, Maukonen, Hyytiäinen, Kieseppä, Orešič, Sabunciyan and Suvisaari2018); THu et al. (Reference Hu, Li, Huang, Lai, Li, Sublette and Xu2019); UZheng et al. (Reference Zheng, Yang, Li, Wu, Liang, Yin and Wang2020); VPainold et al. (Reference Painold, Mörkl, Kashofer, Halwachs, Dalkner, Bengesser and Reininghaus2018); WRong et al. (Reference Rong, Xie, Zhao, Lai, Wang, Xu and Liu2019).
Firmicutes
The most abundant phylum in the human gut, Firmicutes, showed multiple inconsistencies at the phylum, family and genus levels (Fig. 2). At the genus level, divergent findings were reported for Ruminococcus, which was higher in one MDD study (Chung et al., Reference Chung, Chen, Chou, Chen, Lee, Chuang and Kuo2019), but lower in three other MDD studies (Jiang et al., Reference Jiang, Ling, Zhang, Mao, Ma, Yin and Ruan2015; Liu et al., Reference Liu, Rowan-Nash, Sheehan, Walsh, Sanzari, Korry and Belenky2020, Reference Liu, Zhang, Wang, Wang, Zhang, Jiang and Duan2016), one BD study (Hu et al., Reference Hu, Li, Huang, Lai, Li, Sublette and Xu2019) and one SSD study (Li et al., Reference Li, Zhuo, Huang, Huang, Zhou, Xiong and Wu2020). In line with these results, inconsistent findings were also reported in relation to clinical features. One study reported that decreased relative abundance of Ruminococcaceae was associated with an increase in negative symptoms (Nguyen et al., Reference Nguyen, Kosciolek, Maldonado, Daly, Martin, McDonald and Jeste2018). Another study reported Ruminococcaceae OTU725 to be negatively correlated with symptom severity in SSD (Zheng et al., Reference Zheng, Zeng, Liu, Chen, Pan, Han and Xie2019). In contrast, one study found a positive correlation of Ruminococcaceae with negative symptoms (Schwarz et al., Reference Schwarz, Maukonen, Hyytiäinen, Kieseppä, Orešič, Sabunciyan and Suvisaari2018). One MDD study found a positive correlation between Ruminococcus and symptom severity scores (Chung et al., Reference Chung, Chen, Chou, Chen, Lee, Chuang and Kuo2019). Another MDD study found a negative association of Ruminococcaceae with symptom severity (Liu et al., Reference Liu, Rowan-Nash, Sheehan, Walsh, Sanzari, Korry and Belenky2020).
Moreover, at the genus level Faecalibacterium were decreased in five MDD studies (Huang et al., Reference Huang, Shi, Li, Shen, Shi, Wang and Liang2018; Jiang et al., Reference Jiang, Ling, Zhang, Mao, Ma, Yin and Ruan2015; Kelly et al., Reference Kelly, Borre, O’ Brien, Patterson, El Aidy, Deane and Dinan2016; Liu et al., Reference Liu, Rowan-Nash, Sheehan, Walsh, Sanzari, Korry and Belenky2020, Reference Liu, Zhang, Wang, Wang, Zhang, Jiang and Duan2016), one BD study (Hu et al., Reference Hu, Li, Huang, Lai, Li, Sublette and Xu2019) and one SSD study (Li et al., Reference Li, Zhuo, Huang, Huang, Zhou, Xiong and Wu2020). In line with these results Jiang et al. (Reference Jiang, Ling, Zhang, Mao, Ma, Yin and Ruan2015) found a negative correlation between Faecalibacterium and depressive symptom severity. Furthermore, Liu et al. (Reference Liu, Rowan-Nash, Sheehan, Walsh, Sanzari, Korry and Belenky2020) observed reduced Faecalibacterium in MDD patients with more severe symptoms. Moreover, the recently published systematic review and meta-analysis of Nikolova et al. (Reference Nikolova, Hall, Hall, Cleare, Stone and Young2021) observed depleted levels of Faecalibacterium in BD, MDD and schizophrenia as well. Faecalibacterium, especially species Faecalibacterium prausnitzii, is usually considered a ‘good’ gut bacterium and is associated with positive healthy outcomes, and its depletion with negative healthy outcomes (Ferreira-Halder, de Faria, & Andrade, Reference Ferreira-Halder, de Faria and Andrade2017; Gacesa et al., Reference Gacesa, Kurilshikov, Vich Vila, Sinha, Klaassen, Bolte and Weersma2022). Furthermore, F. prausnitzii was negatively associated with MDD (Gacesa et al., Reference Gacesa, Kurilshikov, Vich Vila, Sinha, Klaassen, Bolte and Weersma2022).
The genus Streptococcus was reported to be higher in three MDD studies (Chung et al., Reference Chung, Chen, Chou, Chen, Lee, Chuang and Kuo2019; Lai et al., Reference Lai, Deng, Xu, Zhao, Xu, Liu and Rong2021; Rong et al., Reference Rong, Xie, Zhao, Lai, Wang, Xu and Liu2019), one BD study (Rong et al., Reference Rong, Xie, Zhao, Lai, Wang, Xu and Liu2019) and one SSD study (Shen et al., Reference Shen, Xu, Li, Huang, Yuan, Wang and Liang2018). In line with these results, Zheng et al. (Reference Zheng, Zeng, Liu, Chen, Pan, Han and Xie2019) reported a positive correlation between Streptococcaceae OTU834 and symptoms severity. At the family level, the relative abundance of Lactobacillaceae was reported to be higher in two SSD studies (Schwarz et al., Reference Schwarz, Maukonen, Hyytiäinen, Kieseppä, Orešič, Sabunciyan and Suvisaari2018; Shen et al., Reference Shen, Xu, Li, Huang, Yuan, Wang and Liang2018). Consistently, at the genus level the relative abundance of Lactobacillus was significantly higher in two MDD studies (Lai et al., Reference Lai, Deng, Xu, Zhao, Xu, Liu and Rong2021; Rong et al., Reference Rong, Xie, Zhao, Lai, Wang, Xu and Liu2019), in one BD study (Rong et al., Reference Rong, Xie, Zhao, Lai, Wang, Xu and Liu2019) and in four SSD studies (He et al., Reference He, Kosciolek, Tang, Zhou, Li, Ma and Chen2018; Li et al., Reference Li, Zhuo, Huang, Huang, Zhou, Xiong and Wu2020; Schwarz et al., Reference Schwarz, Maukonen, Hyytiäinen, Kieseppä, Orešič, Sabunciyan and Suvisaari2018; Shen et al., Reference Shen, Xu, Li, Huang, Yuan, Wang and Liang2018). In line with these results, general symptom severity and positive symptom severity were positively correlated with Lactobacillus (Schwarz et al., Reference Schwarz, Maukonen, Hyytiäinen, Kieseppä, Orešič, Sabunciyan and Suvisaari2018). These are interesting results since specific strains of Lactobacillus are commonly used in probiotics (Simpson et al., Reference Simpson, Diaz-Arteche, Eliby, Schwartz, Simmons and Cowan2021). However, other studies have observed increased Lactobacillus as well in other disorders, like inflammatory bowel disease, indicating specific strains may have inflammatory potential (Wang et al., Reference Wang, Chen, Zhou, Wang, Song, Huang and Xia2014). Rocha-Ramírez et al. (Reference Rocha-Ramírez, Pérez-Solano, Castañón-Alonso, Moreno Guerrero, Ramírez Pacheco, García Garibay and Eslava2017) found that several Lactobacillus species increased proinflammatory cytokines such as interleukin-8 (IL-8), tumour necrosis factor-α, IL-12p70 and IL-6. Moreover, Zhu et al. (Reference Zhu, Ju, Wang, Wang, Guo, Ma and Ma2020b) reported increases of subspecies of Lactobacillus not typically present in the healthy gut in schizophrenia patients. It remains to be determined which species of Lactobacillus genus are increased in psychiatric disorders.
Actinobacteria
At the family level in the phylum Actinobacteria, Coriobacteriaceae were relatively more abundant in one BD study (Painold et al., Reference Painold, Mörkl, Kashofer, Halwachs, Dalkner, Bengesser and Reininghaus2018) and one SSD study (Zheng et al., Reference Zheng, Zeng, Liu, Chen, Pan, Han and Xie2019). From Coriobacteriaceae, the abundance of Eggerthella was relatively higher in four MDD studies (Chung et al., Reference Chung, Chen, Chou, Chen, Lee, Chuang and Kuo2019; Kelly et al., Reference Kelly, Borre, O’ Brien, Patterson, El Aidy, Deane and Dinan2016; Lai et al., Reference Lai, Deng, Xu, Zhao, Xu, Liu and Rong2021; Rong et al., Reference Rong, Xie, Zhao, Lai, Wang, Xu and Liu2019), in one BD study (Rong et al., Reference Rong, Xie, Zhao, Lai, Wang, Xu and Liu2019) and one SSD study (Xu et al., Reference Xu, Wu, Liang, He, Gu, Li and Wang2020). In line with these results, Eggerthella correlated positively with depression, anxiety and stress scores (Chung et al., Reference Chung, Chen, Chou, Chen, Lee, Chuang and Kuo2019). Gacesa et al. (Reference Gacesa, Kurilshikov, Vich Vila, Sinha, Klaassen, Bolte and Weersma2022) found a positive association of the species Eggerthella lenta with BD. Moreover, Rekdal, Bess, Bisanz, Turnbaugh, and Balskus (Reference Rekdal, Bess, Bisanz, Turnbaugh and Balskus2019) suggest that E. lenta is capable of selectively removing the para-hydroxyl group of dopamine, thereby decreasing dopamine levels. This suggestion of Rekdal et al. (Reference Rekdal, Bess, Bisanz, Turnbaugh and Balskus2019) could explain the positive correlation found between Eggerthella and depression scores. Moreover, the BD patients in the study of Rong et al. (Reference Rong, Xie, Zhao, Lai, Wang, Xu and Liu2019) were at the time of the study in a major depressive episode. Eggerthella could be related to depression in multiple disorders and could therefore be a target for depression. Unfortunately, Eggerthella and E. lenta, previously known as Eubacterium lentum, have been underrecognized due to historical difficulties with phenotypic identification, therefore not much information is available about the bacterial species.
Only a few studies have investigated microbiome difference among subtypes of psychiatric disorders. Hu et al. (Reference Hu, Li, Huang, Lai, Li, Sublette and Xu2019) compared the GM of BD type 1 to BD type 2 patients with each other. They observed relatively higher relative abundance of the families Streptococcaceae and Erysipelotrichaceae, genera Streptococcus, Bacilli and Veillonella and lower relative abundance of the genus Ruminococcus in the BD type 1 group compared to the BD type 2 group. Zheng et al. (Reference Zheng, Yang, Li, Wu, Liang, Yin and Wang2020) compared unipolar depression to bipolar depression and found Bacteroidaceae and Veilonellaceae to be higher and Enterobacteriaceae and Pseudomonadaceae lower in MDD v. BD. Studying the GM of subtypes of psychiatric disorders has the potential to be of great value in understanding differences between these subtypes.
Inconsistencies across studies may be attributable partly to the heterogeneity in sample characteristics across studies. Most studies used small sample sizes; only one study used a sample size of more than 100 patients (Zheng et al., Reference Zheng, Yang, Li, Wu, Liang, Yin and Wang2020). In addition, dietary habits may change among sites of study and socio-economic class of the participants. Then, psychiatric medication probably affects the microbiome. Methods used for obtaining the taxonomic profiles were not consistent. Moreover, statistical analysis used to compare GM composition between groups was quite heterogeneous across studies. Microbiota composition differences between groups were analysed by using several statistical methods, namely, analysis of composition of microbiomes, linear discriminant analysis effect size, Wilcoxon rank-sum test, Mann–Whitney U test, Kruskal–Wallis test and Welch's t test.
The most consistent findings across studies were higher relative abundances of the genera Streptococcus, Lactobacillus and Eggerthella and lower relative abundances of the genus Faecalibacterium in the neuropsychiatric disorders (Fig. 2), perhaps most interesting in the relationship between the psychiatric disorders and lower levels of Faecalibacterium. F. prausnitzii has been shown to have anti-inflammatory effects, to produce the SCFA butyrate and has been associated with improving the intestinal barrier by increasing levels of tight junction proteins occludin and E-cadherin (Carlsson et al., Reference Carlsson, Yakymenko, Olivier, Håkansson, Postma, Keita and Söderholm2013; He, Zhao, & Li, Reference He, Zhao and Li2021; Laval et al., Reference Laval, Martin, Natividad, Chain, Miquel, Desclée de Maredsous and Langella2015; Martín et al., Reference Martín, Miquel, Chain, Natividad, Jury, Lu and Bermúdez-Humarán2015). Next to that, F. prausnitzii has been associated with smoking, which is in line with the fact that the percentage of smokers is higher in psychiatric disorders (Gacesa et al., Reference Gacesa, Kurilshikov, Vich Vila, Sinha, Klaassen, Bolte and Weersma2022; Lê Cook et al., Reference Lê Cook, Wayne, Kafali, Liu, Shu and Flores2014). Multiple of the findings, like higher relative abundances of Actinobacteria and lower abundancy of Prevotella in MDD have been associated unhealthy dietary patterns. Higher relative abundances of Actinobacteria have been associated with high-fat and animal protein diet (Fritsch et al., Reference Fritsch, Garces, Quintero, Pignac-Kobinger, Santander, Fernández and Abreu2021). Low carbohydrate intake has been associated with reduced Prevotellaceae (Kang et al., Reference Kang, Park, Ilhan, Wallstrom, LaBaer, Adams and Krajmalnik-Brown2013). However, the majority of the studies did not control for diet, making it difficult to relate the findings to possible unhealthy diet patterns in the disorders.
Treatments targeting the GM
Understanding how our GM can be targeted could lead to the development of microbiota-based therapies.
Probiotics
Probiotics are defined as live organisms that exert a health benefit when ingested in an adequate amount. Probiotics contain living beneficial bacteria, traditionally from genera Lactobacilli and Bifidobacteria.
In MDD, Kazemi et al. (Reference Kazemi, Noorbala, Azam, Eskandari and Djafarian2019) conducted an 8 week randomized, double-blind, placebo-controlled study, in which 110 depressed patients were randomly assigned to receive probiotic (L. helveticus and B. longum), prebiotic (galactooligosaccharide) or placebo treatment. Probiotic supplementation resulted in a significant decrease in symptom severity compared to both the prebiotic and the placebo groups. Additionally, the serum kynurenine/tryptophan ratio was significantly decreased in the probiotic group compared to the placebo group. Another randomized, double-blind, placebo-controlled 8 weeks probiotics study by Akkasheh et al. (Reference Akkasheh, Kashani-Poor, Tajabadi-Ebrahimi, Jafari, Akbari, Taghizadeh and Esmaillzadeh2016) studied a probiotic (Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum) in 40 MDD patients. As a result symptom severity decreased significantly in the probiotic group. In addition, serum insulin levels, insulin resistance and serum high-sensitivity C-reactive protein concentrations were decreased.
In BD, Dickerson et al. (Reference Dickerson, Adamos, Katsafanas, Khushalani, Origoni, Savage and Yolken2018) found that the adjunctive probiotic treatment (Lactobacillus rhamnosus strain GG and Bifidobacterium animalis subsp. lactis strain Bb12) for 24 weeks prevented rehospitalization in patients with acute mania (n = 66). Probiotic treatment also resulted in fewer days of hospitalization. Another study (n = 38) found no effects of probiotics compared to placebo on symptom severity of both depression and mania (Shahrbabaki et al., Reference Shahrbabaki, Sabouri, Sabahi, Barfeh, Divsalar, Esmailzadeh and Ahmadi2020). In another longitudinal cohort study 20 euthymic individuals with BD received a probiotic called ‘OMNi-BiOTiC® Stress Repair’ (B. bifidum W23, B. lactis W51, B. lactis W52, L. acidophilus W22, L. casei W56, Lactobacillus paracasei W20, L. plantarum W62, L. salivarius W24, L. lactis W19) over a time period of 3 months (Reininghaus et al., Reference Reininghaus, Wetzlmair, Fellendorf, Platzer, Queissner, Birner and Dalkner2020). Cognition concerning attention and psychomotor processing speed was improved as well as executive functioning.
Three studies investigated the effect of probiotics in SSD patients. One studied the combination three bacteria (L. acidophilus, B. bifidum, L. reuteri and L. fermentum) and vitamin D in a randomized placebo-controlled trial (n = 60) (Ghaderi et al., Reference Ghaderi, Banafshe, Mirhosseini, Moradi, Karimi, Mehrzad and Asemi2019). The combination was associated with significant improvement in symptom severity and had beneficial effects on metabolic profiles in reducing fasting plasma glucose levels, insulin concentrations, triglycerides and total cholesterol levels. Another study investigated a probiotic (L. acidophilus, B. lactis, B. bifidum and B. longum) and selenium co-supplementation in a randomized placebo-controlled trial with 60 people with chronic schizophrenia (Jamilian & Ghaderi, Reference Jamilian and Ghaderi2021). The probiotic and selenium co-supplementation resulted in a significant improvement in symptom severity compared to the placebo group. However, in both studies it is uncertain which component of the intervention was responsible for the changes. Only one study studied the effect of a probiotic alone (Dickerson et al., Reference Dickerson, Stallings, Origoni, Katsafanas, Savage, Schweinfurth and Yolken2014). Their 65 participants were subjected to a double-blind adjunctive probiotic (L. rhamnosus strain GG and B. animalis subsp. lactis strain Bb12) or placebo therapy for 14 weeks. No significant differences were found in symptom severity between probiotic and placebo supplementation.
Prebiotics
Prebiotics are non-digestible fibres that are selectively metabolized by the intestinal microbiome. Prebiotics mostly include fructans and glucans. So far, only one study has studied the effects of prebiotics in SSD, namely, by using the purified prebiotic Bimuno™ galactooligosaccharides (B-GOS®) formulation. Thirty-nine non-hospitalized participants with non-affective psychosis received either B-GOS® or a placebo for 24 weeks. Participants performed the Brief Assessment of Cognition in Schizophrenia (BACS, Keefe et al., Reference Keefe, Goldberg, Harvey, Gold, Poe and Coughenour2004) and the B-GOS® group had significantly improved global cognitive performance, compared to the placebo group (Kao et al., Reference Kao, Safarikova, Marquardt, Mullins, Lennox and Burnet2019). While interesting, this study requires replication in larger studies.
Faecal microbiota transplantation
Faecal transplants from depressed patients to germ-free rats show interesting results. Kelly et al. (Reference Kelly, Borre, O’ Brien, Patterson, El Aidy, Deane and Dinan2016) showed that FMT from depressed patients to microbiota-depleted rats can induce behavioural and physiological features characteristic of MDD in the receiving animals, including anhedonia and anxiety-like behaviour, as well as an increased plasma kynurenine/tryptophan ratio. In two studies, FMT from schizophrenia patients to germ-free mice led to hyperactivity and reduced anxiety in the open-field test (Zheng et al., Reference Zheng, Zeng, Liu, Chen, Pan, Han and Xie2019; Zhu et al., Reference Zhu, Guo, Wang, Ju, Wang, Ma and Ma2020a). Zhu et al. (Reference Zhu, Guo, Wang, Ju, Wang, Ma and Ma2020a) also found that mice that received FMT from schizophrenia patients displayed impaired spatial learning and memory. Remarkable, however, was the heightened amount of social interaction these mice had compared to the control mice. In human, FMT has shown initial positive effects on autism spectrum disorder patients (Kang et al., Reference Kang, Adams, Coleman, Pollard, Maldonado, McDonough-Means and Krajmalnik-Brown2019, Reference Kang, Adams, Gregory, Borody, Chittick, Fasano and Krajmalnik-Brown2017).
The most traditional and widely used probiotics (consisting of Bifidobacterium spp. and Lactobacillus spp.) are safe, yet they are not disease-specific. Although different Lactobacillus and Bifidobacterium strains were used in the probiotic studies mentioned above, most of the GM studies in the psychiatric disorders already showed heightened Lactobacillus and Bifidobacterium levels compared to their control groups. Rather, F. prausnitzii could be a promising target for therapeutic purpose in psychiatric patients (Verhoog et al., Reference Verhoog, Taneri, Díaz, Marques-Vidal, Troup, Bally and Muka2019). Research in rats showed preventive and therapeutic effects of F. prausnitzii on chronic unpredictable mild stress-induced depression-like and anxiety-like behaviour (Hao, Wang, Guo, & Liu, Reference Hao, Wang, Guo and Liu2019). Supplementation of prebiotics consisting of inulin-oligofructose or inulin-type fructans and fructo-oligosaccharides have been demonstrated to increase F. prausnitzii levels (Dewulf et al., Reference Dewulf, Cani, Claus, Fuentes, Puylaert, Neyrinck and Delzenne2013; Hustoft et al., Reference Hustoft, Hausken, Ystad, Valeur, Brokstad, Hatlebakk and Lied2017; Ramirez-Fariaz et al., Reference Ramirez-Fariaz, Slezak, Fuller, Duncan, Holtrop and Louis2009), although this is not a consistent finding (Majid, Cole, Emery, & Whelan, Reference Majid, Cole, Emery and Whelan2014).
Conclusions
The GM revolution has opened new frontiers for examining the relation between the brain and the GM in the context of understanding and treating/preventing psychiatric disorders. This paper provides a detailed overview of current findings regarding alterations of the GM in MDD, BD and SSD patients. All the reviewed studies reported alterations of the GM in the psychiatric disorders. Alterations may partly be caused by medication use, and other lifestyle factors like smoking, diet and alcohol use. Diversity metrics and microbial relative abundance reported to be anomalous across articles varied. The most consistent findings across studies were higher relative abundances of the genera Streptococcus, Lactobacillus and Eggerthella and lower relative abundance of the anti-inflammatory butyrate-producing genus Faecalibacterium in the psychiatric disorders. All three increased genera were reported to be associated with higher symptom severity. The similarities found between the disorders in the relative abundances and the associations of certain genera and symptoms suggest overlap in MDD, BD and SSD. So far, the results of probiotics trials have been highly discrepant, though few have shown promising results. Findings on prebiotics and FMT are too limited to draw definitive conclusions. There is a need of expanding our knowledge on bacterial species in bigger populations with psychiatric disorders, including the influence of medication and dietary habits. Information about differences in specific bacterial strains could lead to the use of disease-specific pro/prebiotics. In the end, study of the GM could lead to a new strategy of treating psychiatric disorders.
Financial support
The research was supported by grants from the Stanley Medical Research Institute (grant number 18T-004) and ZonMW (Netherlands Organisation for Health Research and Development; grant number 636320010).
Conflict of interest
None.









