Iodine is an essential trace micronutrient for the human body. Both iodine deficiency and iodine excess can cause thyroid-related health problems. Iodine deficiency has been well studied around the world and is mostly under control in many countries. However, iodine excess and its impacts are still under study. Naturally excessive iodine intake results mainly from consumption of seafood containing a high content of iodine or iodine-rich drinking water. The former has been reported in coastal areas of Japan where seaweed is the staple food of local residents( Reference Suzuki, Higuchi and Sawa 1 , Reference Katamine, Mamiya and Sekimoto 2 ). The latter is found in some parts of China where the underground drinking water contains a high concentration of iodine, usually >300 μg/l( Reference Li, Liu and Qu 3 , Reference Zhao, Chen and Maberly 4 ). Hebei is one such province in China. According to a survey conducted in Hebei in 2003–2004, thirty-one counties of six prefectures and 173 townships were identified as having excessive iodine (i.e. iodine content >150 μg/l) in drinking water based on the National Criteria for Classifying High Iodine Regions( Reference Ma, Zhou and Jia 5 ). This excess iodine potentially affects nearly 6 million people and has became a prominent public health issue now that iodine-deficiency disorders are more successfully managed in Hebei Province( Reference Lv, Xie and Zhou 6 ).
Excessive iodine intake can cause iodine overnutrition, goitre and other thyroid disease( Reference Hans 7 ). Endemic goitre induced by excessive dietary iodine has been reported in coastal areas of Japan where iodine intake from seaweed has been reported as >10 000 μg/d( Reference Suzuki, Higuchi and Sawa 1 , Reference Katamine, Mamiya and Sekimoto 2 ). In China, a few studies have revealed that excessive iodine in drinking water, mainly from 300 μg/l to 1300 μg/l, can cause severe iodine overnutrition and high prevalence of endemic goitre in children aged 8–10 years( Reference Li, Liu and Qu 3 , Reference Zhao, Chen and Maberly 4 , Reference Zhao, Wang and Shang 8 ). However, the impact of mildly excessive iodine content in drinking water, in the range of 150–300 μg/l, on the iodine nutrition and goitre status in children aged 8–10 years remains unclear. In the present study, a comprehensive survey of urinary iodine concentration and goitre status in children aged 8–10 years was conducted in thirty townships in Hebei Province of China which had median water iodine between 150 and 300 μg/l. The aim of the study was to clarify children's iodine nutrition and goitre status in these areas in order to identify possible harm caused by excessive iodine intake in this vulnerable age group.
Materials and methods
Sampling method and data collection
Sample selection (children aged 8–10 years)
Schoolchildren aged 8–10 years are commonly used subjects in surveys on iodine excess owing to their easy access and sensitivity to iodine intake( Reference Yv, Zhu and Chen 9 ). A cluster sampling technique, probability proportional to size sampling, in which the probability that a particular sampling unit will be selected in the sample is proportional to the population size of the sampling unit( 10 ), was employed in the present cross-sectional survey.
With township as the sampling unit, first, thirty townships were selected from 111 townships with median water iodine content between 150 and 300 μg/l. One primary school was then randomly selected from each town chosen. Finally forty schoolchildren aged 8–10 years from each school were selected such that a total of 1200 children were selected. Thyroid volume measurement by B-mode ultrasound was conducted for all 1200 children at their schools. Also 360 children from thirty schools (twelve children from each school) were randomly selected for collection of urine samples for measuring urinary iodine concentration. Oral consent for examination of thyroid and urine sample collection was obtained from the headmasters of the investigated schools. The survey was conducted in September of 2009.
Drinking water sample collection
Drinking water samples were collected from households in the thirty villages where the investigated schoolchildren had lived since birth. The population of each of the thirty villages was between 600 and 2000; twenty-four out of the thirty villages had fewer than five wells, the other six villages had more than five wells. The water samples were collected using a systematic sampling method based on their location. The number of drinking water samples depended on the number of water sources. In a village with more than five wells, five households (one in each of the eastern, western, southern, northern and central parts of the village) covered by these wells were randomly chosen to collect water samples. In a village with fewer than five wells, one household for each well was selected. In a village with a centralized water supply (tap water), one household was randomly selected.
Household edible salt sample collection
Edible salt samples were collected at the villages where the investigated schoolchildren were born and lived. A systematic sampling method was used according to their location of east, west, north, south or centre. Two households were randomly selected in each location to collect edible salt to measure iodine content. A total of ten salt samples were collected in each village.
Measurement of thyroid volume by ultrasound
Thyroid volume was measured by using an Aloka SSD-500 echocamera (Aloka, Mure, Japan) equipped with 7·5-MHz linear transducers. The measurement was performed while the child lay on a bed with his/her neck fully exposed. For each thyroid lobe, the maximum perpendicular anteroposterior and mediolateral dimensions were measured on a transverse image of the largest diameter, without including the isthmus. The maximum craniocaudal diameter of each lobe was then measured on a longitudinal image. The thyroid capsule was not included. The ultrasound measurements were done by one experienced examiner who had specialized in thyroid measurement by ultrasound for 5 years.
Thyroid volume was calculated by using the equation of Brunn et al.( Reference Brunn, Block and Ruf 11 ), in which the volume of each lobe (ml) is equal to anteroposterior diameter (cm) × mediolateral diameter (cm) × craniocaudal diameter (cm) × 0·479, and the lobe volumes are summed.
In accordance with the Chinese national criteria for thyroid measurement, goitre was defined by age-specific thyroid volume. The upper limit of thyroid volume for children aged 8, 9 and 10 years was 4·5 ml, 5·0 ml and 6·0 ml, respectively. If the child's thyroid volume exceeded the relevant value, the child was judged as goitrous( 12 ).
Data analysis
Biological sample analysis
The iodine concentration of urine samples was measured by the method of ammonium persulfate oxidation in the provincial laboratory( 13 ). Urinary iodine concentration values from populations are usually not normally distributed. Therefore, the median rather than the mean should be used as the measure of central tendency. Median urinary iodine concentration of a population is a good biochemical indicator for its iodine nutrition. Median urinary iodine concentration of 300 μg/l and above defines a population as having iodine excess( 14 ).
Environmental sample analysis
The iodine content of salt was determined quantitatively by titration in the provincial laboratory. Iodine content was measured by liberating iodine from salt and titrating the iodine with sodium thiosulfate using starch as an external indicator( 15 ). According to the Chinese national standard for iodized salt, edible salt with less than 5 mg iodine/kg is classified as non-iodized salt.
The iodine content in drinking water was determined by the method of arsenic–cerium oxidation–reduction spectrophotometry in the provincial laboratory, in which iodine catalyses the oxidation–reduction between arsenious acid and ammonium cerous sulfate( 16 ). It is stipulated in the Chinese national standard for determination and classification of high water and the endemic areas of iodine excess goitre that drinking water containing more than 150 μg iodine/l is classified as excessive.
Data processing and statistical analysis
Data processing and statistical analyses were performed using the statistical software packages Epi–Info™ 2002 (Centers for Disease Control and Prevention, Atlanta, GA, USA) and SPSS version 13·0 (SPSS Inc., Chicago, IL, USA). Since the distributions of iodine in edible salt, drinking water and children's urine are not normal, the median was employed to describe their central tendency. Differences in children's median urinary iodine concentrations by age, sex and different groups classified on the basis of iodized salt were tested with the Mann–Whitney test.
Results
Iodine content in drinking water and edible salt
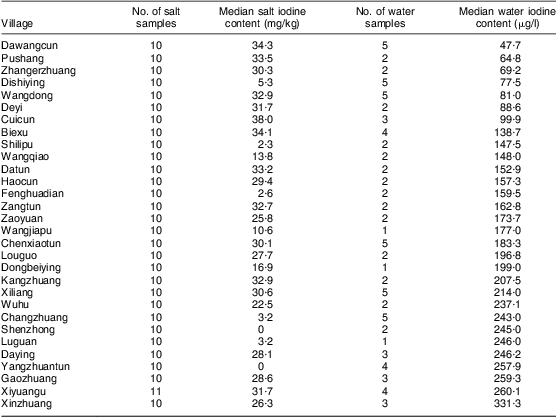
A total of eighty-five drinking water samples were collected from thirty villages and analysed for iodine content. The iodine content ranged from 47·7 μg/l to 945·0 μg/l, with the median iodine content being 166·0 μg/l. Among the thirty villages, the median iodine content in drinking water was >150 μg/l in twenty villages and <150 μg/l in ten villages. Water iodine levels in the thirty villages sampled are listed in Table 1. The number of water samples with iodine content <150 μg/l, 150–300 μg/l and >300 μg/l was thirty-four, forty-seven and four, accounting for 40·0 %, 55·3 % and 4·7 %, respectively.
Table 1 The iodine content in edible salt and drinking water at thirty villages investigated in Hebei Province, China, September 2009
| Village | No. of salt samples | Median salt iodine content (mg/kg) | No. of water samples | Median water iodine content (μg/l) |
| Dawangcun | 10 | 34·3 | 5 | 47·7 |
| Pushang | 10 | 33·5 | 2 | 64·8 |
| Zhangerzhuang | 10 | 30·3 | 2 | 69·2 |
| Dishiying | 10 | 5·3 | 5 | 77·5 |
| Wangdong | 10 | 32·9 | 5 | 81·0 |
| Deyi | 10 | 31·7 | 2 | 88·6 |
| Cuicun | 10 | 38·0 | 3 | 99·9 |
| Biexu | 10 | 34·1 | 4 | 138·7 |
| Shilipu | 10 | 2·3 | 2 | 147·5 |
| Wangqiao | 10 | 13·8 | 2 | 148·0 |
| Datun | 10 | 33·2 | 2 | 152·9 |
| Haocun | 10 | 29·4 | 2 | 157·3 |
| Fenghuadian | 10 | 2·6 | 2 | 159·5 |
| Zangtun | 10 | 32·7 | 2 | 162·8 |
| Zaoyuan | 10 | 25·8 | 2 | 173·7 |
| Wangjiapu | 10 | 10·6 | 1 | 177·0 |
| Chenxiaotun | 10 | 30·1 | 5 | 183·3 |
| Louguo | 10 | 27·7 | 2 | 196·8 |
| Dongbeiying | 10 | 16·9 | 1 | 199·0 |
| Kangzhuang | 10 | 32·9 | 2 | 207·5 |
| Xiliang | 10 | 30·6 | 5 | 214·0 |
| Wuhu | 10 | 22·5 | 2 | 237·1 |
| Changzhuang | 10 | 3·2 | 5 | 243·0 |
| Shenzhong | 10 | 0 | 2 | 245·0 |
| Luguan | 10 | 3·2 | 1 | 246·0 |
| Daying | 10 | 28·1 | 3 | 246·2 |
| Yangzhuantun | 10 | 0 | 4 | 257·9 |
| Gaozhuang | 10 | 28·6 | 3 | 259·3 |
| Xiyuangu | 11 | 31·7 | 4 | 260·1 |
| Xinzhuang | 10 | 26·3 | 3 | 331·3 |
The overall median iodine content of 301 edible salt samples collected at households in the thirty villages was 28·8 mg/kg. In twenty-three villages the median salt iodine was >10 mg/kg, and in seven villages it was <5 mg/kg. The details of salt iodine in the thirty villages are listed in Table 1.
Iodine concentration in urine samples of children aged 8–10 years
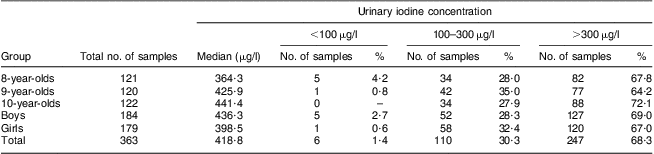
A total of 363 urine samples were collected from children aged 8–10 years and analysed for iodine, with the median urinary iodine concentration found to be 418·8 μg/l. Among the thirty villages investigated, the children's median urinary iodine concentration was >300 μg/l in twenty-three villages and <300 μg/l in seven villages.
Of the 363 urine samples, 184 were from boys and 179 from girls. The median urinary iodine concentration in boys and girls was 436·3 μg/l and 398·5 μg/l, respectively, with no statistically significant difference identified (Mann–Whitney U = 15406·0, P = 0·288). The number of urine samples collected from children aged 8, 9 and 10 years was 121, 120 and 122, and their median iodine concentration was 364·3 μg/l, 425·9 μg/l and 441·4 μg/l, respectively, with no statistically significant difference among them (Mann–Whitney H = 1·563, P = 0·458).
The iodine concentration was >300 μg/l in 248 out of 363 urine samples, accounting for 68·3 % in total. In further classification based on age and sex, the percentage of urine samples with iodine concentration >300 μg/l in each age and sex group varied from 64·2 % to 72·1 %. The median urinary iodine concentration and frequency distribution of urinary iodine concentration of children aged 8–10 years are included in Table 2.
Table 2 Median urinary iodine concentrations and distributions for different age and sex groups of children aged 8–10 years, Hebei Province, China, September 2009
| Urinary iodine concentration | ||||||||
| <100 μg/l | 100–300 μg/l | >300 μg/l | ||||||
| Group | Total no. of samples | Median (μg/l) | No. of samples | % | No. of samples | % | No. of samples | % |
| 8-year-olds | 121 | 364·3 | 5 | 4·2 | 34 | 28·0 | 82 | 67·8 |
| 9-year-olds | 120 | 425·9 | 1 | 0·8 | 42 | 35·0 | 77 | 64·2 |
| 10-year-olds | 122 | 441·4 | 0 | – | 34 | 27·9 | 88 | 72·1 |
| Boys | 184 | 436·3 | 5 | 2·7 | 52 | 28·3 | 127 | 69·0 |
| Girls | 179 | 398·5 | 1 | 0·6 | 58 | 32·4 | 120 | 67·0 |
| Total | 363 | 418·8 | 6 | 1·4 | 110 | 30·3 | 247 | 68·3 |
Impact of iodized salt on children's urinary iodine concentration
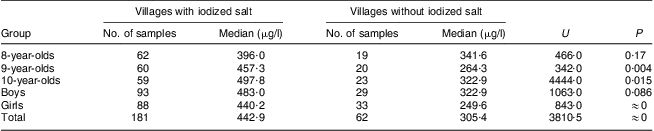
Among the twenty villages with median iodine content in drinking water >150 μg/l, the median salt iodine in fifteen villages was >10 mg/kg. Five villages had salt supplies with median iodine content <5 mg/kg or even 0 mg/kg. The median water iodine content in the fifteen villages with high salt iodine and the five villages with low salt iodine was 214·0 μg/l and 245·7 μg/l, respectively, and the difference was not significant (U = 226·0, P = 0·34). The median urinary iodine concentration of children aged 8–10 years in the fifteen villages with high salt iodine was 442·9 μg/l, while it was 305·4 μg/l in the five villages with low salt iodine, and the difference between them was statistically significant (U = 3810·5, P = 0). Further analysis based on age and sex showed that the median urinary iodine concentrations of children in different age or sex groups in the fifteen villages with high salt iodine were mostly higher than those in the five villages with low salt iodine. In the group of 9-year-olds, the children's median urinary iodine concentration in the fifteen villages with high salt iodine was significantly higher than that in the five villages with low salt iodine, i.e. 457·3 μg/l v. 264·3 μg/l (U = 342·0, P = 0·004). In the group of 10-year-olds, it was 497·8 μg/l v. 322·9 μg/l (U = 4444·0, P = 0·015). Comparing female and male children, it was 440·2 μg/l v. 249·6 μg/l (U = 843·0, P = 0·0). Further results of statistical analyses are provided in Table 3.
Table 3 Comparison of median urinary iodine concentrations in twenty villages with water iodine content >150 μg/l according to the presence of iodized salt in the village and for different age and sex groups of children aged 8–10 years, Hebei Province, China, September 2009
| Villages with iodized salt | Villages without iodized salt | |||||
| Group | No. of samples | Median (μg/l) | No. of samples | Median (μg/l) | U | P |
| 8-year-olds | 62 | 396·0 | 19 | 341·6 | 466·0 | 0·17 |
| 9-year-olds | 60 | 457·3 | 20 | 264·3 | 342·0 | 0·004 |
| 10-year-olds | 59 | 497·8 | 23 | 322·9 | 4444·0 | 0·015 |
| Boys | 93 | 483·0 | 29 | 322·9 | 1063·0 | 0·086 |
| Girls | 88 | 440·2 | 33 | 249·6 | 843·0 | ≈0 |
| Total | 181 | 442·9 | 62 | 305·4 | 3810·5 | ≈0 |
Goitre status of children aged 8–10 years
Among the 1259 children aged 8–10 years examined by ultrasound, 138 were found to have goitre, accounting for approximately 11 % of the group. The goitrous cases detected in the 8, 9 and 10 year age groups numbered fifty-six, forty-eight and thirty-four, respectively, with the goitre rate being 13·1 % (56/426), 11·6 % (48/413) and 8·1 % (34/420), respectively. No statistically significant difference was found among the goitre rates across the different age groups (χ 2 = 5·8, P = 0·06). In terms of gender, seventy-seven and sixty-one goitrous cases were found in boys and girls, with the goitre rate being 12·4 % (77/621) and 9·6 % (61/638). The difference between them was not statistically significant (χ 2 = 2·08, P = 0·15). The goitre details by age and sex are included in Table 4.
Table 4 Comparison of goitre status according to iodine content in drinking water and for different age and sex groups of children aged 8–10 years, Hebei Province, China, September 2009

| Villages with WI < 150 μg/l | Villages with WI > 150 μg/l | Total | |||||||
| Group | No. of children | Goitre cases | % | No. of children | Goitre cases | % | No. of children | Goitre cases | % |
| 8-year-olds | 137 | 17 | 12·4 | 289 | 39 | 13·5 | 426 | 56 | 13·1 |
| 9-year-olds | 140 | 20 | 14·3 | 273 | 28 | 10·3 | 413 | 48 | 11·6 |
| 10-year-olds | 139 | 8 | 5·8 | 281 | 26 | 9·3 | 420 | 34 | 8·1 |
| Boys | 220 | 25 | 11·4 | 401 | 52 | 13·0 | 621 | 77 | 12·4 |
| Girls | 196 | 20 | 10·2 | 442 | 41 | 9·3 | 638 | 61 | 9·6 |
| Total | 416 | 45 | 10·8 | 843 | 93 | 11·0 | 1259 | 138 | 11·0 |
WI, water iodine.
The goitre rate in the twenty villages with median iodine content in drinking water >150 μg/l was 11·0 % (93/843), while it was 10·8 % (45/416) in the ten villages with median iodine content in drinking water <150 μg/l. There was no significant difference between them (χ 2 = 0·01, P = 0·9). The goitre comparisons between villages with different water iodine are included in Table 4. The twenty villages with water iodine >150 μg/l were further broken down into two subgroups based on the status of iodine content in salt. The goitre rate in the group with median salt iodine >10 mg/kg was 12·1 % (71/587) compared with 8·6 % (29/339) in the group with median salt iodine <5 mg/kg. However, the difference was not statistically significant (χ 2 = 2·8, P = 0·094).
Discussion
Based on the results of the present survey, the median urinary iodine concentration of children aged 8–10 years in townships in Hebei Province with mildly excessive iodine (150–300 μg/l) in drinking water was 418·8 μg/l, and 68·3 % (248/363) of children's urine samples had iodine concentration >300 μg/l. These results indicate that the iodine intake of local children is excessive. Similar findings were also reported by other researchers in other parts of China with mildly excessive iodine in drinking water. When median water iodine content varied from 90 to 300 μg/l, children's median urinary iodine concentration fluctuated from 300 to 800 μg/l( Reference Zhao, Wang and Shang 8 , Reference Guo, Qin and Chen 17 – Reference Guo, Qin and Liu 19 ).
Children's median urinary iodine concentration found in the present survey was at the lower end of this range. There are two possible reasons for this. One is that the median water iodine in many of the villages investigated was quite low. The median water iodine content in seven villages, accounting for 23·3 % of the total villages investigated, was <100 μg/l, ranging from 47·7 μg/l to 99·9 μg/l. The other reason is that the supply of iodized salt in some villages, especially those with higher water iodine content, was very poor. The median salt iodine content in six villages was <5 mg/kg, varying from 0 to 3·2 mg/kg, which could provide only <30 μg iodine to the local residents’ daily intake, given their average daily salt consumption was estimated to be 10 g( Reference Xu, Wang and Hu 20 ) and children's daily salt consumption was even less.
Among the twenty villages with median water iodine >150 μg/l, iodized salt is likely to have played some role in children's iodine excess. Children's urinary iodine concentration in the fifteen villages with median salt iodine >10 mg/kg was significantly higher than that in the five villages with median salt iodine <5 mg/kg, implying that iodized salt increased children's iodine excess.
According to the present survey, the goitre rate of children aged 8–10 years in areas with mildly excessive iodine in drinking water was approximately 11 %. This rate is similar to 13·1 % in Shanxi Province found by Jia et al.( Reference Jia, Zhang and Wang 21 ) and 12·7 % in Inner Mongolia found by Zhang et al.( Reference Zhang, Fan and Guo 22 ), but is much higher than 5 % reported by Zhao et al. in Jiangsu Province( Reference Zhao, Wang and Shang 8 ), where the median iodine content in drinking water was also in the range of 150–300 μg/l in those investigated areas. There is no doubt that excessive iodine in drinking water can cause goitre in the general population when it is >300 μg/l, and the goitre is more easily detected because of its remarkable enlargement( Reference Zimmermann, Ito and Hess 23 , Reference Yu, Chen and Tan 24 ). However, when the median water iodine content is <300 μg/l, more study needs to be undertaken to determine whether it can cause epidemic goitre in sensitive populations, like young children aged 8–10 years, given the recognized limitations of the criteria on children's normative thyroid volume.
There is still some disagreement on the normative thyroid volume by ultrasound. WHO recommended a set of age-specific normative values for thyroid volume in 1997( 25 ), which Zimmerman argued were too high. In 2004, Zimmerman et al. proposed much lower normative thyroid volume values based on samples in iodine-sufficient regions in six countries in Asia, Europe, Africa and America( Reference Zimmermann, Hess and Molinari 26 ). However, they also admitted that population-specific references for thyroid volume in countries with long-standing iodine sufficiency may be more accurate than a single international reference. Moreover, normative thyroid volume also closely relates with children's age and body surface area, which is due to genetic differences in their growth and development( Reference Zimmermann, Hess and Molinari 26 ).
The normal thyroid volume criteria employed in the present study were formulated in 1996 and were only age-specific. They may be too old and ignore differences in children's development between the present and over 10 years ago in China. Since children's bodies develop earlier now than in the 1990s, they may have bigger thyroids than their peers in 1990s and in turn be judged incorrectly as goitrous. The goitre rate obtained in the present study could be amplified by these criteria. Consequently, it is difficult to measure the exact prevailing magnitude of children's goitre with the employed criteria. Therefore, the prevalence of goitre in areas with mildly excessive iodine in drinking water, i.e. 150–300 μg/l, needs to be further researched, in particular identifying more accurate criteria of normal thyroid volume in the future.
The impact of iodine content in the range of 150–300 μg/l in drinking water on the iodine nutrition and goitre status in children aged 8–10 years remains unclear. The present study aimed to clarify to what extent mildly excessive iodine in drinking water influences children's iodine nutrition and goitre status. It revealed that the iodine intake of children living in these areas in Hebei Province was excessive, which was exacerbated by consumption of iodized salt. Goitre was identified in these areas, with children's goitre rate being 10·96 %. The study adds to Chinese research about dietary iodine exposure. In some parts of China, Chinese public health officials are facing dual challenges relating to both iodine deficiency and iodine excess. These challenges call for good health risk assessment approaches to identify hazardous iodine levels (either too low or too high) in different geographical settings with differing access to iodized salt and differing water iodine levels in order that prevention and control strategies for iodine deficiency or iodine excess can better be designed to meet the needs of each geographic area.
Acknowledgements
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector. The authors declare no conflict of interest. S.L. was responsible for the study design, data analysis, paper writing and field investigation. J.Z. was in charge of the laboratory detection of iodine. D.X. did the thyroid measurements by ultrasound. S.R. was responsible for the paper revision and English polishing. Z.C., L.J., Y.D. and J.M. took part in the field investigation.






