Inflammatory markers such as C-reactive protein (CRP) and IL-6 are predictors of CVD risk( Reference Luc, Bard and Juhan-Vague 1 ). Indeed, the well-known association between diet and CVD may partly be linked through these inflammatory markers( Reference Fung, McCullough and Newby 2 ). Several epidemiological studies have identified negative associations between inflammatory levels and specific nutrients such as polyphenols( Reference Cassidy, Rogers and Peterson 3 , Reference Pounis, Bonaccio and Di Castelnuovo 4 ), PUFA( Reference Muka, Kiefte-de Jong and Hofman 5 ), histidine( Reference Li, Li and Qi 6 ) and branched-chain amino acids( Reference Li, Li and Liu 7 ).
Besides specific nutrients, dietary scores based on the consumption of specific food items have also been linked with inflammatory levels. For example, hypothesis-oriented scores such as the Mediterranean diet score( Reference Trichopoulou, Costacou and Bamia 8 ) have been linked with lower platelet and white blood cell counts( Reference Bonaccio, Di Castelnuovo and De Curtis 9 ). Similarly, the Southern European Atlantic Diet( Reference Guallar-Castillón, Oliveira and Lopes 10 ) and the Baltic Sea diet( Reference Kanerva, Kaartinen and Rissanen 11 ) have been associated with lower levels of CRP, while the Alternative Healthy Eating Index (AHEI) of McCullough et al.( Reference McCullough, Feskanich and Stampfer 12 ) was negatively associated with IL-6 levels( Reference Fung, McCullough and Newby 2 , Reference Akbaraly, Shipley and Ferrie 13 ).
Dietary patterns obtained through principal component analysis have also been shown to be associated with inflammatory levels. For example, ‘prudent’( Reference Wood, Strachan and Thies 14 ) or ‘health-aware’( Reference Corley, Kyle and Starr 15 ) patterns were negatively associated with CRP levels. Ozawa et al. used reduced rank regression analysis and identified a ‘dietary inflammatory pattern’ rich in red and processed meat, peas, legumes and fried food, and low in whole grains, which was positively associated with IL-6 levels and other inflammatory markers( Reference Ozawa, Shipley and Kivimaki 16 ). However, most studies assessed only a limited number of nutrients or foods, or even focused on a single dietary score or pattern, while studies comparing simultaneously the associations between different dietary factors and inflammatory markers are scarce( Reference Bonaccio, Di Castelnuovo and De Curtis 9 , Reference Corley, Kyle and Starr 15 , Reference Nettleton, Matijevic and Follis 17 , Reference Oliveira, Rodriguez-Artalejo and Lopes 18 ).
Hence, the present study aimed at assessing the associations between a wide range of dietary factors (macro- and micronutrients, single foods, dietary patterns and scores) and several inflammatory markers. Our hypothesis was that dietary patterns would be more associated with inflammatory markers than individual foods or nutrients.
Participants and methods
Participants
The CoLaus study is a population-based study assessing the clinical, biological and genetic determinants of CVD in the city of Lausanne, Switzerland( Reference Firmann, Mayor and Vidal 19 ). The sampling procedure of the CoLaus study was as follows: the source population was defined as all individuals aged between 35 and 75 years registered in the population register of the city of Lausanne. The register includes all individuals living in this city for more than 90d. A simple, non-stratified random sample of 19 830 individuals (corresponding to 35 % of the source population) was drawn and the selected individuals were invited to participate by letter. If no answer was obtained, a second letter was sent and if no answer was obtained, the individuals were contacted by telephone. The following inclusion criteria were applied: (i) written informed consent; and (ii) willingness to take part in the examination and to provide blood samples.
Recruitment of the baseline sample began in June 2003 and ended in May 2006, enrolling 6733 participants who underwent an interview, a physical examination and a blood analysis. The first follow-up was performed between April 2009 and September 2012, 5·6 years on average after collection of the baseline data, and included 5064 of the initial participants. The information collected in the first follow-up was similar to that collected in the baseline examination except that in the first follow-up information regarding food consumption and detailed physical activity was also collected. Hence, for the present study, only data from the first follow-up examination were considered, as dietary intake assessment was first introduced then.
Blood samples
Venous blood samples (50 ml) were drawn in the fasting state. High-sensitive CRP (hs-CRP) was assessed by immunoassay and latex HS (IMMULITE 1000–High; Diagnostic Products Corporation, Los Angeles, CA, USA) with maximum intra- and inter-batch CV of 1·3 and 4·6 %, respectively. Serum samples were kept at −80°C before assessment of IL-6 and TNF-α and sent on dry ice to the laboratory. Levels of these cytokines were measured using a multiplexed particle-based flow cytometric cytokine assay( Reference Vignali 20 ). Milliplex kits were purchased from Millipore (Zug, Switzerland). The procedures closely followed the manufacturer’s instructions. The analysis was conducted using a conventional flow cytometer (FC500 MPL; Beckman Coulter, Nyon, Switzerland). Lower limits of detection for IL-6 and TNF-α were 0·2pg/ml. A good agreement between signal and cytokine was found within the assay range (R 2≥0·99). Intra- and inter-assay CV were respectively 16·9 and 16·1 % for IL-6 and 12·5 and 13·5 % for TNF-α. For quality control, repeated measurements were conducted in eighty participants randomly drawn from the initial sample. Spearman rank correlations between duplicate measurements were 0·961 and 0·891 for IL-6 and TNF-α, respectively (all P<0·001). Lin’s correlation coefficients were 0·971 and 0·945 and intra-class correlation coefficients were 0·972 and 0·946 for IL-6 and TNF-α, respectively (all P<0·001), indicating a good reproducibility.
Dietary intake
Dietary intake was assessed using a validated self-administered, semi quantitative FFQ which also included portion size( Reference Bernstein, Huot and Morabia 21 ). This FFQ has been validated against 24 h recalls among 626 volunteers from the Geneva population( Reference Bernstein, Huot and Morabia 21 – Reference Beer-Borst, Costanza and Pechère-Bertschi 23 ). The FFQ assesses the dietary intake of the previous 4 weeks and consists of ninety-seven different food items accounting for more than 90 % of the intake of energy, proteins, fat, carbohydrates, alcohol, cholesterol, vitamin D and retinol, and 85 % of fibre, carotene and Fe. For each item, seven consumption frequencies were provided: (i) ‘less than once during the last 4 weeks’; (ii) ‘once per month’; (iii) ‘2–3 times per month’; (iv) ‘1–2 times per week’; (v) ‘3–4 times per week’; (vi) ‘once per day’; and (vii) ‘2 or more times per day’. Participants indicated the average serving size (smaller, equal or larger) compared with a reference size. Daily consumption of the different food items was computed based on frequency and portion size and expressed in millilitres (for drinks) or grams (for other foods). Although the maximum frequency was ‘2 or more times per day’, there were six items assessing fruit intake and eight items assessing vegetable intake. Hence, in the unlikely case a participant would eat fruits or vegetables pertaining to a single item (for example, only berries), the multiplicity of the items related to fruits and vegetables would allow an adequate estimation of their intake. Conversion into nutrients was performed based on the French CIQUAL food composition table( 24 ) taking account of portion size.
Three hypothesis-oriented dietary scores were computed, two based on the Mediterranean diet, the third on a modification of the AHEI. The first Mediterranean dietary score (hereby designated as ‘Mediterranean score 1’) was derived from Trichopoulou et al.( Reference Trichopoulou, Costacou and Bamia 8 ); the score ranges between 0 and 8. The second Mediterranean dietary score (hereby designated as ‘Mediterranean score 2’) is adapted to the Swiss population and was computed according to Vormund et al.( Reference Vormund, Braun and Rohrmann 25 ). Contrary to the score from Trichopoulou et al., dairy products are considered as beneficial. The score thus ranges between 0 and 9. The AHEI was adapted from McCullough et al.( Reference McCullough, Feskanich and Stampfer 12 ). In our study, the amount of trans fat could not be assessed, and we considered all participants taking multivitamins as taking them for a duration ≥5 years. Thus, the modified AHEI score ranged between 2·5 and 77·5, instead of 2·5 and 87·5 for the original AHEI score( Reference McCullough, Feskanich and Stampfer 12 ). For all three scores, higher values represented a healthier diet.
A restricted version of the Dietary Inflammatory Index (DII) was computed using available data and the corresponding overall inflammatory effect scores( Reference Shivappa, Steck and Hurley 26 ). The higher the index, the higher its inflammatory capacity. The procedures to compute the three hypothesis-oriented dietary scores and the DII are summarized in the online supplementary material, Supplemental Table 1.
Dietary patterns were derived using principal component analysis based on food consumption frequencies. Three dietary patterns were identified: ‘Meat & fries’, ‘Fruits & vegetables’ and ‘Fatty & sugary’. Detailed description of assessment and characteristics of the dietary patterns is provided elsewhere( Reference Marques-Vidal, Waeber and Vollenweider 27 ).
Other covariates
Sociodemographic and lifestyle data were collected by self-administered questionnaires. Educational level was categorized as low (primary), middle (apprenticeship or secondary school) and high (university). Smoking status was categorized as never, former (irrespective of the time since quitting) and current (irrespective of the amount smoked). Physical activity was assessed by a questionnaire validated in the population of Geneva( Reference Bernstein, Sloutskis and Kumanyika 28 ). This self-reported questionnaire assesses the type and duration of seventy kinds of (non-)professional activities and sports during the previous week. Sedentary status was defined as spending more than 90 % of the daily energy in activities below moderate and high intensity (defined as requiring at least 4×BMR)( Reference Guessous, Gaspoz and Theler 29 ) and categorized as a dichotomous variable (yes/no). BMR multiples are close to metabolic equivalent of task (MET) multiples, although MET multiples do not take participant sex, age or height into account.
Body weight and height were measured with participants standing without shoes in light indoor clothing. Weight was measured in kilograms to the nearest 0·1 kg using a Seca™ scale and height was measured to the nearest 5 mm using a Seca™ height gauge (Seca, Hamburg, Germany). BMI was defined as weight/height2 and categorized as normal (BMI<25·0 kg/m2); overweight (25·0≤BMI<30·0 kg/m2) and obese (BMI≥30·0 kg/m2). Due to small numbers (n 72), underweight participants (BMI<18·5 kg/m2) were included in the ‘normal’ category.
Exclusion criteria
Participants with the following characteristics were excluded: (i) no dietary data; (ii) no socio-clinical data; (iii) no inflammatory data; (iv) anti-inflammatory drugs; (v) inflammation (CRP>20 mg/l); and (vi) total energy intake of <3556 or >18 828 kJ/d (<850 or >4500 kcal/d).
Statistical analysis
Statistical analysis was performed using the statistical software package Stata version 15.1 for Windows. Participants’ characteristics were expressed as number and percentage for categorical variables, or as mean and standard deviation for continuous variables.
Bivariate associations were assessed using Spearman non-parametric rank correlation. Dietary markers significantly associated on bivariate analysis with inflammatory markers were further explored using multivariable analysis. Multivariable analysis was performed using linear regression adjusting for age (continuous), BMI (continuous), gender, smoking (never, former, current), educational level (high, middle, low), sedentary status (yes/no), diabetes (yes/no) and total energy intake (continuous). Results were expressed as standardized linear regression coefficients, which can be interpreted as multivariable-adjusted correlations. For multivariable analyses, inflammatory markers were log-transformed.
The importance of dietary scores and patterns relative to single foods was further addressed by entering simultaneously in each model one dietary pattern and the foods significantly associated with inflammatory markers. Given the number of associations tested, statistical significance was considered for a two-sided test with P<0·01 to reduce the false positive detection rate.
Results
Characteristics of the participants
Of the initial 5064 participants, 1037 (20·5 %) were excluded. The reasons for exclusion are indicated in Fig. 1 and the characteristics of excluded and included participants are summarized in the online supplementary material, Supplemental Table 2. Excluded participants were older, had a higher BMI, a lower education, and were prone to smoke, be sedentary and have diabetes.
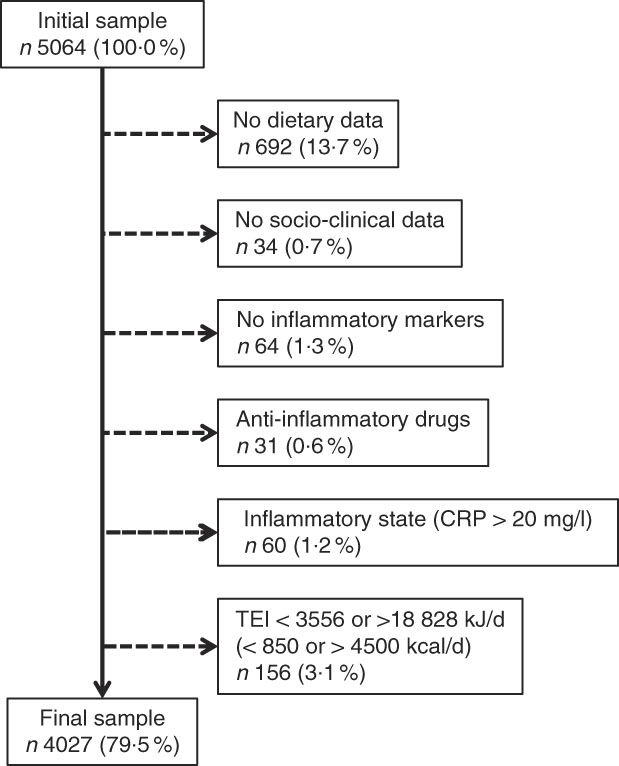
Fig. 1 Selection of participants for the present study (CRP, C-reactive protein; TEI, total energy intake)
Associations of individual nutrients with inflammatory markers
On bivariate analysis, PUFA was positively associated with IL-6, and carotene was negatively associated with CRP and leucocyte count (Table 1). However, these relationships were no longer significant when adjusting for confounders (Table 2).
Table 1 Bivariate associations between inflammatory and dietary markers; CoLaus study, Lausanne, Switzerland, 2009–2012

CRP, C-reactive protein; AHEI, Alternative Healthy Eating Index; DII, Dietary Inflammatory Index.
Data from 4027 participants. Results are expressed as Spearman correlations.
* Significant correlation: P<0·01.
† Mediterranean diet score of Trichopoulou et al.( Reference Trichopoulou, Costacou and Bamia 8 ).
‡ Mediterranean diet score of Vormund et al.( Reference Vormund, Braun and Rohrmann 25 ).
Table 2 Multivariable associations between selected inflammatory and dietary markers; CoLaus study, Lausanne, Switzerland, 2009–2012

CRP, C-reactive protein; AHEI, Alternative Healthy Eating Index; DII, Dietary Inflammatory Index; –, not assessed.
Data from 4027 participants. Results are expressed as standardized regression coefficients adjusted for age (continuous), BMI (continuous), gender, smoking (never, former, current), educational level (high, middle, low), sedentary status (yes/no), diabetes (yes/no) and total energy intake (continuous).
* Significant association: P<0·01.
† Mediterranean diet score of Trichopoulou et al.( Reference Trichopoulou, Costacou and Bamia 8 ).
‡ Mediterranean diet score of Vormund et al.( Reference Vormund, Braun and Rohrmann 25 ).
Associations of single foods with inflammatory markers
On bivariate analysis, fruits, carrots and tofu were negatively associated with CRP and leucocyte count; green salad, bananas, apples and kiwis were negatively associated with leucocyte count, while berries showed a negative association with IL-6 levels (Table 1). After multivariate adjustment, only the negative association between fruits and CRP retained statistical significance (Table 2).
Associations of dietary patterns and scores with inflammatory markers
On bivariate analysis, the ‘Meat & fries’ pattern was positively associated and the ‘Fruits & vegetables’ pattern was negatively associated with CRP levels and leucocyte count (Table 1). After multivariable adjustment, only the negative association between the ‘Fruits & vegetables’ pattern and CRP levels remained (Table 2).
On bivariate analysis, both Mediterranean scores and the AHEI were negatively associated with CRP levels; the AHEI was also negatively associated with leucocyte count (Table 1). After multivariable adjustment, only the negative associations of Mediterranean score 1 and the AHEI with CRP levels remained (Table 2). The DII was positively associated with CRP levels and leucocyte count on bivariate analysis (Table 1), but those associations were no longer significant after multivariable adjustment (Table 2).
When entered simultaneously with fruit intake, the ‘Fruits & vegetables’ pattern, Mediterranean score 1 and the AHEI tended to remain significantly and negatively associated with CRP levels, while the association with fruit intake was no longer significant (see online supplementary material, Supplemental Table 3).
Discussion
The present study is one of the few that compared the associations between different dietary factors and inflammatory markers in the general population. Our results show that (un)healthy dietary behaviours are associated with inflammatory markers, while individual nutrients or foods are not.
Associations of individual nutrients with inflammatory markers
Only a limited number of macro- and micronutrients were associated with inflammatory markers on bivariate analysis, and no significant association remained after adjustment for confounders. These findings are in agreement with a cross-sectional Scottish study( Reference Corley, Kyle and Starr 15 ), which also failed to find any significant association between several micronutrients (flavonoids and antioxidants) and inflammatory markers. Conversely, they do not replicate a literature review where large numbers of foods and nutrients were found to be associated (either positively or negatively) with inflammatory markers( Reference Shivappa, Steck and Hurley 26 ). It would be important to assess which of all these foods and nutrients are significantly and independently associated with inflammatory markers, via multivariable analyses.
Associations of single foods with inflammatory markers
Fruit intake was negatively associated with CRP levels, but not with IL-6, TNF-α or leucocyte count. The association with CRP remained after multivariable analysis, a finding in agreement with two cross-sectional studies conducted in Portugal( Reference Oliveira, Rodriguez-Artalejo and Lopes 18 ) and Iran( Reference Esmaillzadeh, Kimiagar and Mehrabi 30 ). A possible explanation is the high polyphenol content of fruits( Reference Pérez-Jiménez, Neveu and Vos 31 ), which has been linked with a decrease in inflammation levels( Reference Cassidy, Rogers and Peterson 3 , Reference Pounis, Bonaccio and Di Castelnuovo 4 ). Our results thus suggest that an adequate consumption of fruit could decrease inflammatory levels.
Associations of dietary patterns and scores with inflammatory markers
The Mediterranean diet scores were negatively associated with CRP levels, and this association persisted for Mediterranean score 1 after multivariable adjustment. These findings agree with previous cross-sectional studies( Reference Bonaccio, Di Castelnuovo and De Curtis 9 , Reference Chrysohoou, Panagiotakos and Pitsavos 32 ), suggesting that the beneficial effect of the Mediterranean diet on CVD might partly be linked to a decreased inflammatory status.
The AHEI was negatively associated with CRP levels. This finding concurs with some prospective and cross-sectional studies where the AHEI was inversely associated with CRP( Reference Fargnoli, Fung and Olenczuk 33 ) or IL-6 levels( Reference Fung, McCullough and Newby 2 , Reference Akbaraly, Shipley and Ferrie 13 ), but not with other cross-sectional or case–control studies which failed to find any association( Reference Huang, Tobias and Hruby 34 , Reference Neelakantan, Naidoo and Koh 35 ). A possible explanation for the lack of association in the latter studies is their relatively small sample size (<1000 participants), which might have reduced statistical power.
Of the three dietary patterns obtained, only the ‘Fruits & vegetables’ retained its negative association with CRP after multivariable adjustment. These findings are partly in agreement with other studies, which also assessed dietary patterns using principal component analysis. Indeed, both the ‘health-aware’ pattern from the Lothian Birth Cohort cross-sectional study( Reference Corley, Kyle and Starr 15 ) and the ‘prudent’ pattern from the Aberdeen Prospective Osteoporosis Screening Study cohort( Reference Wood, Strachan and Thies 14 ), which scored high in fruits and vegetables, were negatively associated with CRP levels.
Interestingly, the ‘Fruits & vegetables’ pattern, Mediterranean score 1 and the AHEI showed stronger negative associations with CRP levels than single fruit intake, indicating that the associations were due to not only a higher fruit intake, but also that other food items related to the pattern/score might intervene. Overall, our results suggest that a diet rich in fruits (but not only) is associated with lower inflammatory levels.
No associations were found between the DII and inflammatory markers after multivariable adjustment. A likely explanation is that the DII computed in the present study was based on only fourteen of the forty-five items that constitute the original DII (see online supplementary material, Supplemental Table 1)( Reference Shivappa, Steck and Hurley 26 ). This might have led to inadequate DII values and thus biased estimates. Indeed, most items not included in the computation had a negative score (Supplemental Table 1). Future studies will have to assess the validity of DII computed using a limited data set.
Importance for clinical practice
Many studies have focused on the associations between single nutrients or foods and inflammatory markers. Still, increasing or decreasing the consumption of specific nutrients or of selected foods might be difficult to achieve in general practice. Our results indicate that dietary recommendations focused on the consumption of several food groups are more important than recommendations focused on specific foods or nutrients( Reference Piepoli, Hoes and Agewall 36 ). Hence, in clinical practice, generic recommendations could be provided, instead of focusing on specific foods or nutrients, which are difficult to identify and to integrate in a normal diet. This would facilitate dietary counselling by general practitioners, whose nutritional knowledge is usually low( Reference Han, Auer and Cornuz 37 ).
Similarly, from a public health perspective, simple messages aimed at a healthier eating and increased consumption of fruits and vegetables( 38 ) could be delivered. The impact of such measures in the general population could then be monitored by any of the scores (AHEI, Mediterranean or ‘Fruits & vegetables’) rather than by complex nutrient assessment.
Strengths and limitations
Our study has several strengths. First, it is one of the very few simultaneously comparing the associations between different dietary factors and inflammatory markers( Reference Corley, Kyle and Starr 15 ). Second, due to the population-based setting, our results can be transposed to other populations and practical recommendations can be used in public health and clinical practice.
The study also has several limitations. First, and as already indicated, no information regarding polyphenols was available. Hence, it was not possible to confirm previous findings( Reference Cassidy, Rogers and Peterson 3 , Reference Pounis, Bonaccio and Di Castelnuovo 4 ). Future studies should rely either on an extensive food composition database or on the direct measurement of polyphenols in serum or urine. Second, Mediterranean score 1 is based on a Greek population’s food consumption, and the scores obtained cannot be directly transposed to a Swiss population. Third, the cross-sectional setting of the study does not allow establishing a temporal relationship between dietary intake and inflammatory markers. Still, they are in line with the results of randomized controlled trials showing that an increased consumption of flavonoid-rich fruits and vegetables( Reference Macready, George and Chong 39 ) or whole-grain wheat( Reference Vitaglione, Mennella and Ferracane 40 ) decreases inflammatory markers. Finally, the FFQ assessed dietary intake for the last 4 weeks and not for the whole year. Still, it has been shown that FFQ assessing short periods do not differ from FFQ assessing long periods( Reference Freedman, Commins and Moler 41 ), while minimizing recall bias.
Conclusion
Our results show that healthy dietary behaviours, but not individual foods, are negatively associated with inflammatory markers in the general population.
Acknowledgements
Financial support: The CoLaus study was and is supported by research grants from GlaxoSmithKline, the Faculty of Biology and Medicine of Lausanne, and the Swiss National Science Foundation (grants numbers 33CSCO-122661, 33CS30-139468 and 33CS30-148401). The funding sources had no involvement in the study design, conduct of the study, data collection, analysis of samples or data, interpretation of findings, writing of the report, or decision to submit the article for publication. Conflict of interest: The authors report no conflict of interest. Authorship: E.P. performed part of the statistical analyses and wrote most of the article; P.M.-V. collected data, performed most of the statistical analysis and wrote part of the article; P.V. and I.G. revised the article for important intellectual content. P.M.-V. had full access to the data and is the guarantor of the study. Ethics of human subject participation: The institutional Ethics Committee of the University of Lausanne, which afterwards became the Ethics Commission of Canton Vaud (www.cer-vd.ch), approved the baseline CoLaus study (reference Reference Ozawa, Shipley and Kivimaki16/03, decisions of 13 January and 10 February 2003); the approval was renewed for the first (reference 33/09, decision of 23 February 2009) and the second (reference 26/14, decision of 11 March 2014) follow-ups. The full decisions of the CER-VD can be obtained from the authors upon request. The study was performed in agreement with the Declaration of Helsinki and its former amendments, and in accordance with the applicable Swiss legislation. All participants gave their signed informed consent before entering the study.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980018002355





