Childhood is a period of rapid neural and cognitive development(Reference Bourre1,Reference Lenroot and Giedd2) that involves the myelination of various brain areas(Reference Thatcher3,Reference Bryan, Osendarp and Hughes4) and the acquisition of higher-order cognitive abilities to regulate goal-directed activity, emotions and decision-making(Reference Atallah, Frank and O’Reilly5,Reference Diamond6) . A sufficient quantity and quality of various nutrients, particularly carbohydrates and fatty acids (FAs), are vital for optimal cognitive development in children(Reference Isaacs and Oates7). The modern era has introduced a poor diet quality, including a low intake of fibre and a high intake of refined sugars and SFAs, among children(Reference Danaei, Finucane and Lu8). A poor diet quality may impair neural and cognitive development in children(Reference Nyaradi, Li and Hickling9,Reference Haapala, Lintu and Vaisto10) , but such evidence is limited in general population of school-aged children without nutritional deficiencies or learning disabilities(Reference Haapala, Viitasalo and Venäläinen11,Reference Osendarp, Baghurst and Bryan12) .
Glucose is the most studied carbohydrate in association with cognition, as it is the main source of energy for brain cells(Reference Stephen, Alles and de Graaf13). The results of previous studies indicate that increased blood glucose after breakfast improves attention, memory and information processing in preadolescent children(Reference Márquez Acosta, Sutil de Naranjo and Rivas de Yepez14). A higher total dietary fibre intake has been associated with better verbal memory(Reference Micha, Rogers and Nelson15), and higher dietary intakes of soluble and insoluble fibres have been linked to better selective attention and inhibition(Reference Khan, Raine and Drollette16) in preadolescent children. Furthermore, a recent cross-sectional study found that a lower dietary intake of simple carbohydrates and a higher dietary intake of fibre were associated with better cognition in preadolescent children(Reference Hassevoort, Lin and Khan17). However, most of the existing studies on the associations of dietary intakes of carbohydrates with cognition have only assessed blood glucose levels after meals(Reference Benton, Maconie and Williams18,Reference Cooper, Bandelow and Nute19) . Moreover, evidence on the associations of dietary intakes of other carbohydrates with cognition, particularly in children, is limited(Reference Micha, Rogers and Nelson15,Reference Khan, Raine and Drollette16) .
A higher dietary intake of SFAs and trans-FAs and a lower dietary intake of n-3 PUFAs have been related to poorer cognition in some(Reference Baym, Khan and Monti20) but not all(Reference Zhang, Hebert and Muldoon21) cross-sectional studies among children. A meta-analytic study exhibited that n-3 FA supplements improved cognition in infants but not in older children(Reference Jiao, Li and Chu22). However, recent dietary(Reference Sørensen, Damsgaard and Dalskov23) and supplementary(Reference Johnson, Fransson and Östlund24) intervention studies suggest that increased intakes of n-3 and n-6 PUFAs, and especially increased intakes of EPA and DHA, are linked to improved cognitive skills in children 8–11 years of age. Nevertheless, there is limited knowledge on the associations of dietary intakes of other FAs than EPA and DHA with cognition.
We investigated the association of dietary intakes of carbohydrates and FAs with cognition in a population sample of school children aged 6–8 years. We hypothesised that higher intakes of fibre and PUFAs and lower intakes of SFAs and refined carbohydrates are related to better cognition in children.
Methods
Study population and design
The present analyses are based on the baseline data from the Physical Activity and Nutrition in Children (PANIC) study, which is a controlled physical activity and dietary intervention study in a population sample of children from the city of Kuopio, Finland. We invited 736 children, 6–9 years of age, who started the first grade in sixteen primary schools of Kuopio in 2007–2009 to participate in the study. Altogether 512 children (248 girls, 264 boys), who accounted for 70 % of those invited, participated in the baseline examinations in 2007–2009. The participants did not differ in age, sex or BMI-sd score from all children who started the first grade in the city of Kuopio in 2007–2009 based on the data from standard school health examinations performed for all Finnish children before the first grade. After excluding children with developmental disabilities and attention-deficit hyperactivity disorder, 487 (237 girls, 250 boys) children had complete data used in the analyses.
Assessment of dietary intakes of carbohydrates and fatty acids
We assessed energy and nutrient intakes by food records filled out by the parents on four predefined consecutive days(Reference Eloranta, Lindi and Schwab25). Of the food diaries, 99·5 % included two weekdays and two weekend days, and 0·5 % included three weekdays and one weekend day. A clinical nutritionist instructed the parents to record all food and drinks consumed by their children at home, at school, in afternoon care and elsewhere outside home using household or other measures, such as tablespoons, decilitres and centimetres. She reviewed the food records at return and completed the records with the parents using a picture booklet of portion sizes(Reference Paturi, Nieminen and Reinivuo26), if needed. A clinical nutritionist also asked the catering company about the details of food and drinks, such as menus, cooking fat and spread on bread, served at schools and in afternoon care. Moreover, she disaggregated all prepared foods and mixed dishes into ingredients according to the recipes used. We calculated total energy and nutrient intakes from the collected food record data using the Micro Nutrica® dietary analyses software, version 2.5 (The Social Insurance Institution of Finland), that utilises Finnish and international data on nutrient concentrations of foods(Reference Rastas, Seppänen and Knuts27).
The current analyses include the daily intakes of energy (KJ), total carbohydrates, glucose, fructose, sucrose, starch, total fibre, soluble fibre, insoluble fibre, total fat, SFAs, MUFAs, PUFAs, palmitic acid (C16), stearic acid (C18), linoleic acid (C18:2), α-linoleic acid (C18:3), arachidonic acid (C20:4), EPA (C20:5n-3) and DHA (C22:6n-6).
Assessment of cognition
We assessed nonverbal reasoning using Raven’s Coloured Progressive Matrices (RCPM)(Reference Raven, Raven and Court28) that was administered by one trained researcher. The RCPM includes three sets of twelve items. Each test page includes a large item or a pattern of items and six small items. The children were asked to select the correct small item, which completes the large item or the set of items. The test score was the number of correct answers, ranging from 0 to 36.
Assessment of body size and composition
A research nurse twice measured the body weight of children – first, after fasting for 12 h and, second, after emptying their bladder and standing in light underwear – using a calibrated InBody 720® bioelectrical impedance device (Biospace, Seoul, Korea) to an accuracy of 0·1 kg. We used the mean of these two values in the analyses. A research nurse thrice measured the stature of children in the Frankfurt plane without shoes using a wall-mounted stadiometer to an accuracy of 0·1 cm. We measured body fat percentage and lean body mass using the Lunar® dual-energy X-ray absorptiometry device (GE Medical Systems)(Reference Eloranta, Lindi and Schwab25,Reference Tompuri, Lakka and Hakulinen29) .
Other assessments
The parents were asked to report their annual household income (30 000 €, 30 001–60 000 €, and 60 001 €) and their highest completed or ongoing educational degrees (vocational school or less, polytechnic and university) in a questionnaire. We used the educational degree of the more educated parent in the analyses. The parents were also asked to report the medically diagnosed developmental disorders and attention-deficit hyperactivity disorder of their children.
Statistical methods
We analysed the data using the statistical software package IBM SPSS Statistics version 21.0 (IBM Corp.). We used the Student’s t test and the χ 2 test to compare basic characteristics between boys and girls, and multivariate linear regression analyses adjusted for age, sex, body fat percentage, household income, parental education, total physical activity and daily energy intake to investigate the associations of dietary intakes of carbohydrates and FAs with the RCPM score. Because the results of previous studies suggest that the associations of the components of diet quality with cognition are stronger in boys than in girls(Reference Haapala, Viitasalo and Venäläinen11), we conducted the analyses separately for boys and girls. However, we performed the analyses also for all children, because sex did not statistically significantly modify the associations of the measures of diet quality with the RCPM score (P > 0·05 for interactions).
Results
Basic characteristics
Boys were taller, had lower body fat percentage and came from families with higher annual household income compared to girls (Table 1). Boys had a higher daily energy intake and higher intakes of total carbohydrates, sucrose, starch, total fibre, insoluble fibre and total fat compared to girls. Boys also had higher intakes of most FAs, except EPA and DHA, compared to girls (Table 2).
Table 1 Characteristics of children*

RCPM, Raven’s Coloured Progressive Matrices.
* Data are means (sd’s) from the Student’s t test for continuous variables, and numbers (percentages) from the χ 2 test for categorical variables.
Associations of dietary carbohydrate and fatty acid intakes with cognition
In all children, a higher intake of fructose was associated with a higher RCPM score (adjusted r 2 = 0·049) after adjusting for age, sex, body fat percentage, household income, parental education, total physical activity and daily energy intake (Table 3). This association was slightly weakened after further adjusting for the consumption of fruit and berries (β = 0·11, P = 0·100). None of the dietary FAs was associated with the RCPM score in all children.
Table 3 Association of dietary intakes with the RCPM score
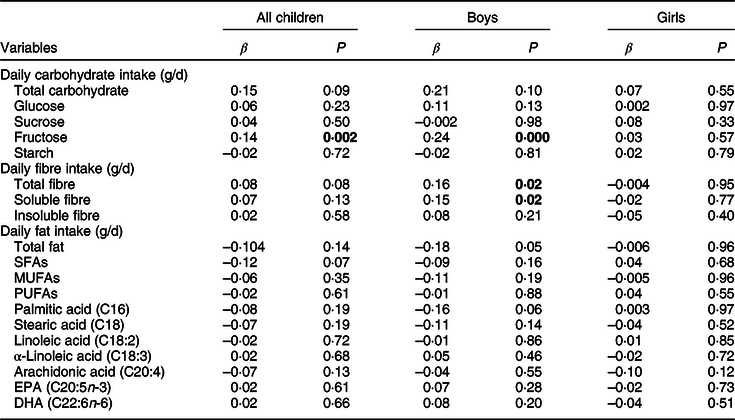
The values are from linear regression models after adjustment for age, sex, body fat percentage, household income, parental education, total physical activity and daily energy intake. P-values <0·05, indicating statistically significant associations, are bolded.
In boys, higher intakes of fructose (adjusted r 2 = 0·045), total fibre (adjusted r 2 = 0·011) and soluble fibre (adjusted r 2 = 0·012) were related to a higher RCPM score after adjusting for age, body fat percentage, household income, parental education, total physical activity and daily energy intake (Table 3). A further adjustment for the consumption of fruit juices (β = 0·15, P = 0·02; adjusted r 2 = 0·025) strengthened the association of soluble fibre with the RCPM score. Similarly, a further adjustment for the consumption of high-fibre bread (β = 0·21, P = 0·01; adjusted r 2 = 0·015) or fruit juices (β = 0·16, P = 0·02; adjusted r 2 = 0·025) strengthened the association of the intake of total fibre with the RCPM score. However, adjustment for the consumption of fruit and berries (β = 0·19, P = 0·040) or fruit juices (β = 0·23, P = 0·006) slightly attenuated the association of the intake of fructose with the RCPM score. Similarly, further adjustment for the consumption of fruit and berries (β = 0·07, P = 0·37) or vegetables (β = 0·12, P = 0·08) attenuated the association of the intake of total fibre with the RCPM score. Further adjustment for the consumption of fruit and berries (β = 0·03, P = 0·72), vegetables (β = 0·11, P = 0·12) or high-fibre bread (β = 0·16, P = 0·03) attenuated the association of the intake of soluble fibre with the RCPM score. None of the dietary FAs was related to the RCPM score in boys.
In girls, the intakes of carbohydrates or FAs were not associated with the RCPM score after adjusting for age, body fat percentage, household income, parental education, total physical activity and daily energy intake (Table 3).
Discussion
We found that higher dietary intakes of fructose, total fibre and soluble fibre were associated with better cognition in boys but not in girls. However, dietary FAs were not related to cognition in boys or in girls.
Our finding on the association between a higher dietary intake of fructose and better cognition in boys is in contrast to previous observations on an inverse association between dietary fructose and cognition in adults(Reference Ye, Gao and Scott30). One reason for these contrasting findings may be that a higher intake of fructose from refined sugars may impair cognitive performance, whereas a higher intake of fructose from natural sources, such as fruits, vegetables and grain products, may be neuroprotective and support cognitive functions(Reference Ye, Gao and Scott31–Reference Akbaraly, Faure and Gourlet33). We previously observed in the present study population that carbohydrates were mainly obtained from low-fibre grain products, and the main sources of fibre were high-fibre bread and vegetables(Reference Eloranta, Lindi and Schwab25) and that fruit and fruit juice consumption was directly related to cognition(Reference Haapala, Viitasalo and Venäläinen11). Natural sources of fructose, such as fruits, include also other neuroprotective nutrients, such as B vitamins and antioxidants, which may partly explain these observations(Reference Tucker, Qiao and Scott32,Reference Akbaraly, Faure and Gourlet33) . Accordingly, we found that the consumption of fruit and berries partly explains the positive association between dietary fructose and the RCPM score.
Our observation on the positive associations of dietary total and soluble fibres with cognition in boys is in accordance with few previous findings in children(Reference Khan, Raine and Drollette16) and in older adults(Reference Ortega, Requejo and Andrés34). We previously found that a higher consumption of high-fibre grain products is related to better cognition in children(Reference Haapala, Viitasalo and Venäläinen11). A higher intake of fibre may support normal cognitive development, because it was found to amplify the effect of neuroprotective brain-derived neurotrophic factor(Reference Schroeder, Lin and Crusio35). A higher dietary fibre intake has also been associated with a decreased concentration of neuroinflammatory markers, such as IL-1β and TNF-α, in the brain(Reference Sherry, Kim and Dilger36).
We found positive associations of dietary fructose and fibre with cognition in boys but not in girls. The results of some previous studies suggest that dietary factors have a stronger effect on brain development in boys than in girls(Reference Isaacs and Oates7,Reference Craft, Murphy and Wemstrom37) . Isaacs and co-workers observed that a higher use of protein-enriched formula during infancy is associated with a larger volume of caudate nucleus and intelligence quotient in adolescence among boys but not among girls(Reference Isaacs and Oates7). The explanation of these sexually dimorphic findings is not known, but they may relate to differences in the stage and rate of neural maturation between boys and girls(Reference Isaacs and Oates7). However, few studies have reported their data in boys and girls separately. Therefore, further studies on sex differences in the associations of dietary factors with cognition are warranted.
We observed no associations of dietary glucose, sucrose, starch or insoluble fibres with cognition in children. One explanation may be that different carbohydrates have varying effects on diverse types of cognition. Papanikolaou and co-workers found that a carbohydrate-rich meal with a high glycaemic index is associated with deterioration in verbal memory but not working memory, measured by a digit span test, among adults(Reference Papanikolaou, Palmer and Binns38). Another study in children showed that working memory was poorer after consuming a meal with a high glycaemic index than after a meal with a low glycaemic index(Reference Benton, Maconie and Williams18). However, neither of these meals had effects on sustained attention(Reference Benton, Maconie and Williams18).
We found no statistically significant associations of dietary FAs with cognition in children. Our observations are in line with those of some previous studies that dietary intake or supplementation of FAs had a weak, if any, association with cognitive performance in well-nourished children with no psychological or reading disabilities(Reference Osendarp, Baghurst and Bryan12,Reference Johnson, Fransson and Östlund24,Reference Ryan, Astwood and Gautier39,Reference Muthayya, Eilander and Transler40) . It is possible that the intake of FAs(Reference Eloranta, Lindi and Schwab25) and the proportion of plasma FAs(Reference Venäläinen, Schwab and Ågren41) among children in our study was adequate to support normal development of cognition(Reference Diamond6,Reference Rask-Nissilä, Jokinen and Terho42) .
The other reason for no associations of dietary FAs with cognition in the present study may be that dietary FAs have different impacts on various cognitive domains. The dietary intake of n-3 FAs has been directly associated with relational memory, but not with item memory in children(Reference Baym, Khan and Monti20). Furthermore, the dietary intake of SFA has been inversely associated with relational and item memory in children aged 7–9 years(Reference Baym, Khan and Monti20,Reference Kanoski and Davidson43) . The reason for these observations may be that PUFAs, such as n-3 FAs, have a pronounced positive effect on cognitive functions that are regulated in the hippocampus, such as relational memory, whereas SFAs have more global negative effects on memory(Reference Kanoski and Davidson43).
The strengths of the present study include a relatively large population sample of children and valid methods used to assess dietary factors and cognition. The parents were asked to record all food and drinks consumed by their children at home, at school, in afternoon care and elsewhere outside home on four predefined and consecutive days, including two weekdays and two weekend days, which provides a better view on overall nutrition than using weekdays only. Moreover, a clinical nutritionist reviewed the food records at return and completed the records with the parents using a picture booklet of portion sizes(Reference Paturi, Nieminen and Reinivuo26), if needed. Our analyses are based on cross-sectional data, and we, therefore, cannot provide evidence for a causal link between dietary factors and cognition. We neither had dietary data from infancy or early childhood, which are important phases of cognitive development(Reference Diamond6) and could, therefore, have an effect on our results. Furthermore, it would have been optimal to use multiple cognitive tests to study the associations of dietary factors with different aspects of cognition.
In conclusion, higher dietary intakes of fructose, total fibre and soluble fibre were associated with better cognition in boys 6–8 years of age. However, dietary fats were not associated with cognition in children. These findings suggest that increasing the consumption of fruit and fruit juices, which are natural sources of fructose, and high-fibre food products, such as grain products, may support normal cognition in boys. Longitudinal studies and optimal dietary intervention studies are needed to provide further evidence for the role of dietary carbohydrates and fats in cognition to investigate whether gender modifies the associations of dietary factors with cognition.
Acknowledgements
Acknowledgements: The first author acknowledges the work of all co-authors especially Dr. Haapala and Prof. Timo Lakka for their support throughout. Financial support: The current study was financially supported by grants from the Jenny and Antti Wihuri Foundation, the Sinikka and Sakari Sohlberg Foundation, the Ministry of Social Affairs and Health of Finland, the Ministry of Education and Culture of Finland, the Finnish Innovation Fund Sitra, the Social Insurance Institution of Finland, the Finnish Cultural Foundation, the Juho Vainio Foundation, the Yrjö Jahnsson Foundation, the Foundation for Pediatric Research, the Paavo Nurmi Foundation, the Paulo Foundation, the Diabetes Research Foundation Kuopio, the Kuopio University Hospital (EVO funding number 5031343) and the Research Committee of the Kuopio University Hospital Catchment Area (the State Research Funding), Yrjö Jahnsson Foundation. Conflict of interest: The manuscript has been approved by all authors, and there are no conflicts of interest regarding it. Authorship: N.S., L.T.A. and H.E.A. were responsible for study design; S.N. drafted the manuscript and analysed the data. V.T., E.A.M., A.T., J.H., L.V. and L.T.A. collected the data and contributed the intellectual content and interpretation of results. L.T.A. is the principal investigator of the PANIC study. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving study participants were approved by the Research Ethics Committee of the Hospital District of Northern Savo, Kuopio. Written informed consent was obtained from the parents of all subjects.






