Vitamin D is a secosteroid that is produced cutaneously through solar UV-B irradiation of 7-dehydrocholesterol present in the skin( Reference Liu 1 , Reference Pilz, Kienreich and Rutters 2 ). The second source of vitamin D is via food intake and like for Ca, the greatest contribution to intake comes from milk and other dairy products. Vitamin D undergoes two hydroxylation steps, one in the liver and one in the kidney. The final hydroxylation step in the kidney converts 25-hydroxyvitamin D (25(OH)D) to its active metabolite, 1,25-dihydroxyvitamin D (1,25(OH)2D), and the enzyme 1-α-hydroxylase catalyses this conversion( Reference Wamberg, Christiansen and Paulsen 3 ). Interestingly, expression of the nuclear vitamin D receptor (nVDR) and 1-α-hydroxylase is present not only in the kidneys but also many other tissues of the body( Reference Dusso, Brown and Slatopolsky 4 ), including the pancreas( Reference Pilz, Kienreich and Rutters 2 ) and immune cells( Reference Bouillon, Carmeliet and Lieben 5 – Reference Calton, Keane and Soares 8 ). Thus many tissues have the ability to locally synthesize 1,25(OH)2D from 25(OH)D and the potential to contribute to circulating concentrations( Reference Dusso, Brown and Slatopolsky 4 ). The active metabolite can then bind to the nVDR, where it forms a heterodimer with the retinoid X receptor( Reference Pilz, Kienreich and Rutters 2 ). It is now recognized that nVDR regulates approximately 3 % of the human genome (~700 genes)( Reference Pilz, Kienreich and Rutters 2 , Reference Bouillon, Carmeliet and Lieben 5 ) and, together with its wide distribution, this provides some foundation for the study of extra-skeletal benefits of vitamin D.
The metabolic syndrome (MetS) is a clustering of risk factors that greatly increases the risk of CVD and type 2 diabetes mellitus (T2DM). Insulin resistance (IR) is a key player in the development of MetS; however, factors other than IR are also important. Clinical diagnosis of MetS is based on the presence of three or more of the following markers of chronic disease: (i) greater waist circumference; (ii) raised fasting plasma glucose; (iii) hypertension (elevated systolic blood pressure or diastolic blood pressure); and (iv) dyslipidaemia (raised TAG and low HDL cholesterol)( Reference Alberti, Eckel and Grundy 9 ). The prevalence of MetS in Australia is high with ~30 % of adults classified as having the syndrome( Reference Cameron, Magliano and Zimmet 10 ); a figure comparable to that in other developed countries( Reference Beltran-Sanchez, Harhay and Harhay 11 , Reference Grundy 12 ). For a sun-drenched country with abundant milk supplies, it is surprising that both vitamin D insufficiency and low Ca intake are highly prevalent in Australia( Reference Daly, Gagnon and Lu 13 – 15 ). Inadequate vitamin D status has been implicated as a causal factor in many chronic conditions( Reference Peterlik, Boonen and Cross 16 ) including T2DM( Reference Brock, Huang and Fraser 17 ), MetS( Reference Brenner, Arora and Garcia-Bailo 18 , Reference Gagnon, Lu and Magliano 19 ), CVD( Reference Anderson, May and Horne 20 ), hypertension( Reference Kunutsor, Apekey and Steur 21 , Reference Scragg, Sowers and Bell 22 ) and IR( Reference Chiu, Chu and Go 23 , Reference von Hurst, Stonehouse and Coad 24 ). We have previously discussed the potential for Ca and vitamin D to regulate body weight( Reference Zemel, Shi and Greer 25 ) and influence the risk of chronic disease( Reference Soares, Chan She Ping-Delfos and Ghanbari 26 – Reference Soares, Ping-Delfos and Sherriff 29 ). Documented pathways include Ca’s stimulation of fat oxidation, heightened diet-induced thermogenesis, increased faecal fat excretion( Reference Soares, Murhadi and Kurpad 27 ), reduced circulating TAG( Reference Major, Alarie and Dore 30 ) and the potential for vitamin D to increase resting metabolism( Reference Calton, Pathak and Soares 31 ). Emerging data also support a beneficial effect on IR and T2DM( Reference Soares, Murhadi and Kurpad 27 , Reference Pittas, Dawson-Hughes and Li 32 – Reference Drolet, Richard and Sniderman 34 ). However, a consensus document produced by the Institute of Medicine found little convincing evidence available at the time in support of extra-skeletal effects of vitamin D( 35 ). The aim of the present study was to investigate whether there was a link between population-based measures of vitamin D status, dietary Ca intake and the prevalence of MetS.
Methods
To fulfil our objectives we used a state-wide representative survey of Victorian adults: the Victorian Health Monitor (VHM)( 36 ). The VHM was conducted between May 2009 and April 2010. A stratified cluster sample was selected, based on census collection districts within the eight Victorian Government Department of Health regions. Fifty randomly selected census collection districts were included in the sample, twenty-five from metropolitan and twenty-five from rural Victoria. One eligible person (aged 18–75 years) from each household in each census collection district was randomly selected to participate. The VHM was approved by the Human Research Ethics Committee (HREC) of the Baker IDI Heart and Diabetes Institute, Melbourne, Victoria( Reference Kelsall, de Gooyer and Carey 37 ). The analysis of the VHM database was also approved by the HREC at Curtin University (HREC approval number: SPH-19-2014).
The VHM involved an initial household visit to participants to collect demographic information, followed by a participant visit to a local test site to collect risk factor information and undergo biomedical and physical examination. Participants were then asked to complete three 24 h dietary recall interviews, which were conducted over a 6-week period. The overall response rate for the VHM was 38 % and a final sample of 3653 participants was achieved.
The response rate in the VHM survey is comparable to similar Australian surveys including the Australian Health Survey: Biomedical Results 2011–12 (response rate 37·1 %)( 38 ) and the Australian Diabetes, Obseity and Lifestyle Study (response rate 37 %)( Reference Dunstan, Zimmet and Welborn 39 ). To identify any potential selection bias in the VHM between participants and non-participants, key demographic characteristics were compared. A minimal level of difference was found between the two groups( Reference Kelsall, de Gooyer and Carey 37 ). Demographic characteristics of participants of the VHM survey were also similar to those from the annual Victorian Population Health Surveys conducted in 2010 (n 7535) and 2011–12 (n 33 673) by the Victorian Government, which had response rates of 73 %( 40 ) and 67 %( 41 ), respectively. This would suggest that the level of bias in the VHM is probably no different from that in the larger Victorian Population Health Survey.
Test sites for the collection of biomedical and physical measures were set up specifically for the purposes of the study in census collection districts included in the sample. The procedures used for the biomedical examination were closely aligned with the protocol recommended by the WHO( 42 ). Participants provided written informed consent upon arrival at test sites and were asked to stay until all tests were complete. Abnormal test results were reviewed by a study doctor who determined whether a result warranted follow-up with a participant. Further details on the survey protocols and procedures can be found in the VHM report( 36 ) and the VHM food and nutrition report( 43 ).
Sample
In the present study, we excluded: (i) participants with missing glycosylated Hb (HbA1c) data (n 31); (ii) those with HbA1c≥6·7 % as they were classified as having T2DM according to the American Diabetes Association cut-offs (n 39)( 44 ); (iii) those with diagnosed T2DM (n 140); (iv) those with type 1 diabetes (n 9); (v) participants on diabetic medications (n 25); and (vi) those with missing metabolic components for MetS diagnosis (n 5). Hence, a total of 3404 participants were included in the analysis. Information on the use of supplements (Ca or vitamin D) was not available in this survey.
Assessment of vitamin D status
Blood samples were collected via venepuncture after an overnight fast of 10 h or more. Blood was immediately transported to an accredited central laboratory in Melbourne, Australia. The measurement of serum 25(OH)D concentration was based on the DiaSorin Corporation Liaison® 25(OH)D total assay. The assay is an automated, direct competitive chemiluminescent immunoassay that measures ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3) to provide a total value for circulating vitamin D in nmol/l. The detection limit was 10 nmol/l. The ALTM (All Laboratory Trimmed Mean) was not computed by the laboratory, nor were results compared with a TV (‘Target Value’) assigned by the NIST (National Institute of Standards and Technology) Reference Measurement Procedure.
Assessment of dietary calcium intake
Dietary intake data were obtained by multiple-pass 24 h diet recall using computer-assisted telephone interviews. The first diet recall interview was conducted within 5 to 7 d of the participants attending the biomedical examination. Two subsequent diet recall interviews were conducted at 2-week intervals following the first diet recall interview. A total of 3653 participants attended and participated in survey components at test sites. Three dietary recalls were conducted, with a total of 10 307 dietary recalls completed, where 96 % completed one dietary recall, 94 % completed two dietary recalls and 92 % completed three dietary recalls. Details of the dietary recall and post-interview processing methodology employed are described in the VHM food and nutrition report( 43 ).
All dietary recall interviews were conducted by certified dietitians from the Department of Nutrition and Dietetics, Monash University. Interviewers were trained to assure competency and consistency in collected dietary recall information. Interviewers used a food model book to aid participants with their description of portion sizes of the foods and beverages they had consumed. The food model book prompted dietary recall by including frequently forgotten foods and eating occasions, and assisted with portion size estimation with ‘to scale’ photographs of food and beverage containers, measuring spoons and cups( 43 ).
The FoodWorks® nutrition software (FoodWorks® Interview) was employed for implementation of dietary recalls. The dietary recall used a multiple-pass approach to assist participants to sufficiently recall their food and beverage intakes. The software includes a scripted guide for interviewers to help prompt participants for food recall in each interview. Interviewers were able to interrupt and prompt for further details on food items if required. Further information on the multiple-pass dietary recall process has been described in detail in the VHM food and nutrition report( 43 ).
On completion of the interviews, volume conversion factors were developed to convert food volumes into food weights. Conversions of food volumes to weights were done by ‘reference to published data, by measuring the weight and volume of specific foods, or by considering the food as very similar to another food for which a volume conversion factor was already available’( 43 ). The AUSNUT 2007( 45 ) nutrient composition data were used to calculate nutrient intakes based on estimated food intakes. The mean intake for each nutrient was computed for each participant based on information collected from three 24 h dietary recalls and was used in the analysis. This information was used to get a single measure of nutrient intake for each participant( 43 ).
Physical activity level
The following criteria were used to define each participant’s level of physical activity: (i) sufficiently physically active (≥150 min of ‘physical activity time’ per week); (ii) insufficiently physically active (1–149 min of ‘physical activity time’ per week); and (iii) physically inactive (0 min of ‘physical activity time’ per week)( 36 ). ‘Physical activity time’ was calculated as the sum of the time spent walking or performing moderate activity plus double the time spent in vigorous physical activity (to reflect its greater intensity)( Reference Armstrong, Bauman and Davies 46 ).
Anthropometric measurements
Anthropometric measurement methods for weight, height and waist circumference have been previously described in the VHM report( 36 ).
Biomedical measurements
Blood collection was conducted via venepuncture after an overnight fast of 10 h or more. Blood samples were assessed for the following factors: total cholesterol, HDL cholesterol, TAG, Hba1c and fasting plasma glucose levels. Blood samples were centrifuged on site and were analysed at a separate central laboratory on a Siemens ADVIA 2400 Clinical Chemistry System. Blood components were measured as following: total cholesterol using enzymatic (oxidase/peroxidase) methods; HDL cholesterol using the elimination/catalase method; TAG using the GPO Trinder reagent set with serum blank; blood glucose using the hexokinase method; and HbA1c was measured by immunoassay (Roche Integra chemistry analyser)( 36 ).
Blood pressure measurements
Sitting blood pressure measurements (GE Dinamap 8100 Vital Signs Monitor) were performed in triplicate on each participant, after a rest period of 5 min. The average of the two closest measurements (<10 mmHg for systolic blood pressure and <6 mmHg for diastolic blood pressure) were used in the analysis. Further details have been presented in the VHM report( 36 ).
Classification of metabolic syndrome
MetS was classified according to the criteria from the joint interim statement of several major organizations( Reference Alberti, Eckel and Grundy 9 ). Individuals were classified as having MetS if they had three or more of the following five components: (i) elevated TAG ≥1·7 mmol/l (≥150 mg/dl); (ii) reduced HDL-C <1·0 mmol/l (<40 mg/dl) in males and <1·3 mmol/l (<50 mg/dl) in females, or on lipid-lowering therapy; (iii) hypertension (systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg), or on anti-hypertensive medications; (iv) elevated fasting plasma glucose ≥5·6 to 6·9 mmol/l (≥100 to 124 mg/dl) but free of diabetes; and (v) elevated waist circumference ≥94 cm for males or ≥90 cm for Aboriginal and Torres Strait Islander, Asian and South American males and ≥80 cm for females. In the current analysis participants were categorized into having or not having MetS (yes/no).
Statistical analysis
The main outcome variable was the status of MetS (yes/no). The primary exploratory variables of interest were serum 25(OH)D concentration and Ca intake, which were both categorized into tertiles: low 25(OH)D (range 10–44 nmol/l; median 33 nmol/l), medium 25(OH)D (range 45–65 nmol/l; median 54 nmol/l) and high 25(OH)D (range 65–204 nmol/l; median 77 nmol/l); and low Ca (range 72–719 mg/d; median 579 mg/d), medium Ca (range 720–1009 mg/d; median 858 mg/d) and high Ca (range 1010–3726 mg/d; median 1233 mg/d). The association between all possible combinations of serum 25(OH)D concentration and Ca intake tertiles (thereby nine levels in total) and MetS was examined in the present study, with mutual adjustment for the other components. Serum 25(OH)D concentration was also tested as a continuous variable for every 10 nmol/l increment, while Ca intake was tested as a continuous variable for every 500 mg/d increment.
In the first stage of the analysis, demographic statistics and differences between the serum 25(OH)D concentration and Ca intake tertiles were tested by the independent-samples t test and frequency tabulation. Furthermore, to investigate the effect of the categorical predictors of interest on the risk of having MetS and higher value of its components, a χ 2 test and simple binary logistic regression analysis were then conducted to obtain the crude unadjusted odds ratios and corresponding 95 % confidence intervals.
Multiple logistic regression analysis was then carried out to calculate the adjusted odds ratios (AOR) and 95 % confidence intervals for the relationships between serum 25(OH)D concentration or Ca intake and having MetS. Analyses were conducted using the statistical software package IBM SPSS Statistics for Windows, Version 21.0. Complex samples analysis was applied to adjust for the unequal selection probability due to the multistage stratified cluster-sampling procedure used in the VHM survey. Appropriate clustering and weighting variables were used to compute appropriate standard errors and confidence intervals in the complex samples analysis procedure. A P value of less than 0·05 was accepted as statistical significance.
Confounders
In our analysis we considered and tested several risk modifiers, based on our experience( Reference Markwick, Ansari and Sullivan 47 ) and that of others( Reference Brenner, Arora and Garcia-Bailo 18 , Reference Hypponen, Boucher and Berry 48 – Reference Reis, von Muhlen and Miller 50 ). Accordingly we included the following demographic factors: weight, age, gender, country of birth, income, education level, physical activity level, smoking status and season. Dietary factors included intakes of: alcohol, dietary fibre, energy, Mg, retinol, 25(OH)D concentration (Ca intake model only) and Ca intake (25(OH)D concentration model only). Age, weight, alcohol, dietary fibre, energy intake, Mg, retinol, 25(OH)D concentration and Ca intake were entered into the regression model as continuous variables. Country of birth was identified according to those born in Australia and those born overseas. Education level was categorized according to three levels: tertiary education, TAFE/diploma/certificate and high school or less. Smoking status was assessed on the basis of three categories: current smoker, ex-smoker and non-smoker. Income levels were categorized according to four categories: ≥$AU 70 000, $AU 30 001–70 000, <$AU 30 000 and don’t know/refused. Season of biomedical examination was categorized as summer, autumn, winter and spring.
Rationale of analysis
In the current analysis we examined the relationship of serum 25(OH)D concentration and Ca intake on MetS through a series of questions that resulted in different models:
-
1. What was the unadjusted relationship between 25(OH)D and Ca intake with MetS? (crude model).
-
2. What was the confounding influence of sociodemographic factors on the relationship of 25(OH)D/Ca intake with MetS? (model 1).
-
3. What was the potential influence of dietary factors on the relationship of 25(OH)D/Ca intake with MetS? (model 2).
Results
The present study population consisted of a total of 3404 adults with a mean age of 49 years. The overall prevalence of MetS was 21·6 %, with a larger proportion of males (22 %) having MetS than females (14 %; P<0·001). The mean serum 25(OH)D concentration of those with MetS was 49·6 nmol/l, significantly lower than that of participants without MetS which was 57·5 mmol/l (P<0·001). The mean dietary Ca intake was 849 mg/d in those with MetS and 926 mg/d in those without MetS (P<0·001; Table 1).
Table 1 Demographic and clinical characteristics by the presence/absence of metabolic syndrome (MetS) among non-diabetic adults (n 3404) aged 18–75 years from the Victorian Health Monitor survey, May 2009–April 2010

Data are presented as mean estimate (weighted) % for categorical variables, and mean estimate (weighted) and se for continuous variables. Differences in the continuous and categorical variables between groups were assessed by the independent-samples t test and the χ 2 test, respectively.
† Median of the tertile group.
Association between tertiles of serum 25-hydroxyvitamin D concentration, calcium intake and presence of metabolic syndrome
Every 10 nmol/l increment in serum 25(OH)D concentration reduced the likelihood of having MetS by 15 % (model 2; Table 2). The crude model indicated that those in the highest tertile of serum 25(OH)D concentration had a 60 % lower odds of having MetS. After adjusting for sociodemographic variables (model 1), the significant inverse association between serum 25(OH)D concentration and presence of MetS remained. After adjustment for dietary variables (alcohol, dietary fibre, energy, Mg, Ca and retinol), participants in the highest 25(OH)D tertile had a 65 % lower odds of having MetS compared with those in the lowest 25(OH)D tertile (model 2; Table 2).
Table 2 Odds ratio of having metabolic syndrome by tertiles of serum 25-hydroxyvitamin D (25(OH)D) concentration among non-diabetic adults (n 3404) aged 18–75 years from the Victorian Health Monitor survey, May 2009–April 2010
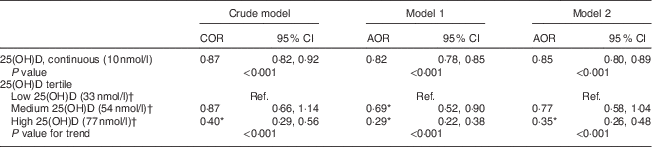
COR, crude odds ratio; AOR, adjusted odds ratio; Ref., lowest 25(OH)D tertile served as the reference group.
Model 1: adjusted for age, gender, country of birth, income, education, smoking and season.
Model 2: adjusted for model 1 covariates plus energy intake, physical activity level, body weight, alcohol, dietary fibre, Mg, Ca and retinol.
* Significant in comparison to the reference group at 5 % significance level.
† Median of the tertile group.
Table 3 shows that every 500 mg/d increment in dietary Ca intake reduced the likelihood of having MetS by 25 % after adjusting for sociodemographic variables in model 1, but the reduction became non-significant after adding dietary variables (alcohol, dietary fibre, energy, Mg and serum 25(OH)D concentration) in model 2. If we did not control for serum 25(OH)D in the latter model, the AOR approached significance (AOR=0·81, 95 % CI 0·64, 1·02; P=0·073) but was non-significant on controlling for 25(OH)D (Table 3, model 2; P=0·141). Those in the highest tertile of dietary Ca intake had significantly reduced odds of having MetS by 39 % in the crude model and 37 % in model 1 in comparison with those in the lowest tertile of dietary Ca intake; however, the comparison was not significant when dietary factors were added to model 2 (Table 3). Based on previous evidence we tested for potential interactions between serum 25(OH)D concentration, Ca intake and age, gender, smoking status, physical activity, county of birth and education level; however, no significant interactive effects were found( Reference Daly, Gagnon and Lu 13 ). Furthermore, interactions between serum 25(OH)D concentration, Ca and dietary variables (alcohol, dietary fibre, energy, Mg and retinol) were tested but none were significant.
Table 3 Odds ratio of having metabolic syndrome by tertiles of dietary calcium intake among non-diabetic adults (n 3404) aged 18–75 years from the Victorian Health Monitor survey, May 2009–April 2010
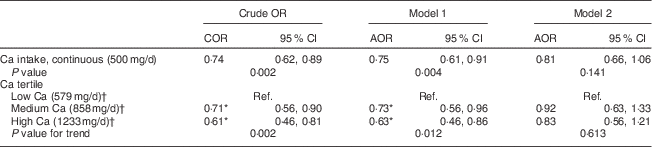
COR, crude odds ratio; AOR, adjusted odds ratio; Ref., lowest Ca tertile served as the reference group.
Model 1: adjusted for age, gender, country of birth, income, education, smoking and season.
Model 2: adjusted for model 1 covariates plus energy intake, physical activity level, body weight, alcohol, dietary fibre, Mg and 25-hydroxyvitamin D concentration.
* Significant in comparison to reference group at 5 % significance level.
† Median of the tertile group.
Association between combined effects of serum 25-hydroxyvitamin D concentration and calcium intake and presence of metabolic syndrome
In view of finding no significant interaction between serum 25(OH)D status and Ca intake (P=0·651), the regression analysis was extended to examine the effect of combining serum 25(OH)D concentration and Ca intake tertiles on MetS (Fig. 1). The combination of low serum 25(OH)D tertile (median 33 nmol/l) and low Ca intake tertile (median 579 mg/d) was the reference group. After controlling for confounding factors, the combination of high serum 25(OH)D and low, medium or high Ca intake significantly reduced the odds of having MetS by 72, 70 and 66 %, respectively (Fig. 1).

Fig. 1 Combined effects of serum 25-hydroxyvitamin D (25(OH)D) concentration (median of the tertile group given in parentheses) and dietary calcium intake (![]() , low calcium, median 579 mg/d;
, low calcium, median 579 mg/d; ![]() , medium calcium, median 858 mg/d;
, medium calcium, median 858 mg/d; ![]() , high calcium, median 1233 mg/d) on the presence of metabolic syndrome (MetS) among non-diabetic adults (n 3404) aged 18–75 years from the Victorian Health Monitor survey, May 2009–April 2010. Adjusted odds ratios (AOR), with the upper limit (UL) of the 95 % confidence interval represented by vertical bars, adjusted for age, gender, country of birth, income, education, smoking, season, physical activity level, weight, alcohol, dietary fibre, magnesium, retinol and energy intake. ‘Ref.’ indicates that the lowest 25(OH)D and lowest calcium tertile served as the reference group; *significant in comparison to reference group at 5 % significance level
, high calcium, median 1233 mg/d) on the presence of metabolic syndrome (MetS) among non-diabetic adults (n 3404) aged 18–75 years from the Victorian Health Monitor survey, May 2009–April 2010. Adjusted odds ratios (AOR), with the upper limit (UL) of the 95 % confidence interval represented by vertical bars, adjusted for age, gender, country of birth, income, education, smoking, season, physical activity level, weight, alcohol, dietary fibre, magnesium, retinol and energy intake. ‘Ref.’ indicates that the lowest 25(OH)D and lowest calcium tertile served as the reference group; *significant in comparison to reference group at 5 % significance level
Discussion
We investigated the individual and combined association of serum 25(OH)D concentration with dietary Ca intake on MetS. In addition to many confounders, we controlled for Ca intake in the 25(OH)D model and for 25(OH)D concentration in the Ca model, to investigate their effect, independent of each other. The results of this representative sample of adults from an Australian state have indicated that higher serum 25(OH)D concentration per se was associated with significantly reduced odds of MetS (Table 2). However, this was not statistically significant for every model of Ca intake tested (Table 3). As a continuous variable the overall pattern for Ca was in the same direction as 25(OH)D and with a lower AOR (Table 3). If we did not control for 25(OH)D in the Ca intake continuous model, the AOR approached significance (AOR=0·81, 95 % CI 0·64, 1·02; P=0·073) but on controlling for 25(OH)D (Table 3, model 2; P=0·141), this was non-significant. Such outcomes suggest that prevailing serum 25(OH)D concentrations could modulate the potential effect of Ca on MetS.
Our findings are consistent with other cross-sectional and prospective studies where an inverse association between 25(OH)D concentration, Ca intake and MetS was observed( Reference Brenner, Arora and Garcia-Bailo 18 , Reference Gagnon, Lu and Magliano 19 , Reference Hypponen, Boucher and Berry 48 , Reference Ford, Ajani and McGuire 51 – Reference Vitezova, Zillikens and van Herpt 53 ). One cross-sectional study found a 67 % reduction in the odds of having MetS among those in the highest 25(OH)D tertile (68–231 nmol/l) v. the lowest tertile (9–45 nmol/l)( Reference Hypponen, Boucher and Berry 48 ). Our study obtained relatively similar results where the highest tertile of 25(OH)D was found to contribute a 65 % reduced odds for MetS in comparison to the lowest tertile (Table 2). The study by Hypponen et al.( Reference Hypponen, Boucher and Berry 48 ) had double the sample size but adjusted only for gender, month and hour of blood measurement. In comparison we controlled for additional sociodemographic, anthropometric and dietary covariates. A more recent prospective study in the elderly also found an inverse association between MetS and high 25(OH)D (≥75 nmol/l), although the magnitude of their findings was much lower( Reference Vitezova, Zillikens and van Herpt 53 ). Furthermore, a large prospective study reflected our results and found a 36 % reduction in odds of having MetS in the highest Ca intake group (1005–2596 mg/d) in comparison to the lowest Ca group. Overall, despite differences between such studies in sample sizes, study design (cross-sectional v. prospective), age of subjects and confounders used, the protective effect of vitamin D in reducing the odds of having MetS appears consistent.
We also examined the potential additive effects of tertile combinations of serum 25(OH)D concentration and Ca intake on MetS (Fig. 1). The outcomes were interesting since they suggested that at low and medium tertiles of 25(OH)D, there was a trend for increasing Ca intake to reduce AOR of MetS (Fig. 1). However, in the highest 25(OH)D tertile this trend disappeared, with significantly reduced AOR across the range from low to high Ca intakes. This was suggestive of a plateau effect, raising the possibility of a threshold to the interplay between Ca and 25(OH)D on functional outcomes.
It is now well known that increasing Ca intake increases passive Ca absorption from the gastrointestinal tract( Reference Heaney 54 ). A higher Ca intake also increases the half-life of 25(OH)D in circulation( Reference Lips 55 ) and together these actions may explain the effect of high Ca in the lowest 25(OH)D tertile (Fig. 1). However, a key physiological function of 25(OH)D is the maintenance of Ca homeostasis via active intestinal Ca absorption( Reference Heaney 54 , Reference Norman 56 , Reference Shapses, Sukumar and Schneider 57 ). So an improvement in vitamin D status from the low to medium tertile (Fig. 1) would further increase active Ca absorption and possibly allow for a greater effect of Ca on MetS. In support of such a paradigm was the observation that the overall effect of Ca in the medium 25(OH)D tertile was stronger than in the low 25(OH)D tertile (Fig. 1). While 25(OH)D and Ca absorption have a positive relationship, there is a plateau to this effect. Above ~80 nmol/l, active Ca absorption does not respond to further increases in 25(OH)D( Reference Heaney 54 ). It is notable that the latter concentration falls within the highest tertile of 25(OH)D in the present study and may explain why increasing Ca intake ceases to have any added benefit in the highest tertile (Fig. 1).
There is another related and important facet to these relationships. A raised parathyroid hormone concentration is associated with an increased risk of MetS( Reference Soares, Ping-Delfos and Sherriff 29 , Reference Ahlström, Hagström and Larsson 58 , Reference Huang, Shapses and Wang 59 ). Increases in dietary Ca and in serum 25(OH)D would lower circulating parathyroid hormone. Recent data have described the exponential decline in parathyroid hormone with increases in 25(OH)D( Reference Durazo-Arvizu, Dawson-Hughes and Sempos 60 ). The analysis indicated two inflection points in the relationship, with the second plateau at 25(OH)D concentrations above ~70 nmol/l where parathyroid hormone was maximally suppressed( Reference Durazo-Arvizu, Dawson-Hughes and Sempos 60 ). We acknowledge that this threshold value of 25(OH)D is not universally accepted( Reference Lucas and Neale 61 ) and that further work is necessary. However, it serves the argument that, at the highest tertile of 25(OH)D in the present study, the negative effects of a raised parathyroid hormone level on MetS could be significantly diminished relative to the previous tertiles. Overall, our results argue that Ca intake has an added effect with 25(OH)D on reducing MetS, but this applies only up to the medium tertile of 25(OH)D (Fig. 1). Above the latter the observed effects are due mainly to 25(OH)D per se. There is some evidence in the literature in support of threshold effects, especially for outcomes that impinge on MetS. A randomized controlled trial has demonstrated that following vitamin D supplementation, significant increases in insulin sensitivity (HOMA%S) were observed only in those who achieved a 25(OH)D concentration of 80 nmol/l and had maintained that value for 6 months( Reference von Hurst, Stonehouse and Coad 24 ). In a weight-loss randomized controlled trial, participants who achieved 80 nmol/l at 12 months demonstrated significantly greater losses in weight, percentage fat mass and waist circumference, compared with those who did not( Reference Mason, Xiao and Imayama 62 ). We cannot predict the threshold value of 25(OH)D from our study. Moreover, as the outcomes of these randomized controlled trials were derived from post hoc analyses, they only support the hypothesis rather than validate an 80 nmol/l cut-off.
Potential mechanisms
There are many mechanistic pathways to support our observations of a protective effect of 25(OH)D concentrations on MetS. An animal study suggests an independent effect of 25(OH)D on β cells, with improvements in impaired glucose tolerance and insulin secretion, despite prevailing plasma Ca concentrations( Reference Cade and Norman 63 ). 1,25(OH)2D has a role in insulin secretion( Reference Cavalier, Delanaye and Souberbielle 64 ), where it stimulates the expression of the insulin receptor and increases the responsiveness to glucose transport. During vitamin D deficiency β-cell function is inhibited, leading to a decrease in insulin secretion( Reference Norman, Frankel and Heldt 65 ). In addition, inadequate 25(OH)D concentration is associated with IR( Reference Mathieu, Gysemans and Giulietti 66 – Reference Procopio and Borretta 68 ). While we acknowledge that IR does not always explain all of MetS( Reference Cozzolino, Ketteler and Zehnder 69 – Reference Vaidya, Forman and Williams 71 ), it is a key feature in the pathophysiology of the syndrome( Reference Nasser 72 ). The nVDR and 1-α-hydroxylase enzyme are found in tissues not related Ca metabolism, such as in cardiac myocytes, endothelial and smooth vascular muscle cells( Reference Merke, Milde and Lewicka 70 ); potentially underscoring a role of 25(OH)D in cardiovascular health. The renin–angiotensin system is important in the regulation of blood pressure( Reference Schmieder, Hilgers and Schlaich 73 ) and low 25(OH)D concentration may dysregulate control of the renin–angiotensin system( Reference Vaidya, Forman and Williams 71 ). In this context lower 25(OH)D concentration has been found to be inversely correlated with measures of arterial stiffness and also to increased arterial resistance, hypertension and endothelial dysfunction( Reference Ullah, Uwaifo and Nicholas 74 – Reference Alyami, Soares and Sherriff 77 ). Moreover higher vitamin D status could also reduce islet β-cell damage by reducing islet renin–angiotensin system activity, thereby reducing the risk of hyperglycaemia( Reference Leung 78 ).
The beneficial effect of Ca on features of MetS may arise from both its absorbed fraction and its unabsorbed fraction in the gastrointestinal tract( Reference Soares, Murhadi and Kurpad 27 ). There is now increasing evidence that Ca intake may influence fat balance and hence energy balance. Dietary Ca increases whole-body fat oxidation and this could, potentially, reduce circulating fatty acids/lipids( Reference Soares, Murhadi and Kurpad 27 , Reference Gonzalez, Rumbold and Stevenson 79 ). Unabsorbed Ca is not without metabolic effects( Reference Soares, Murhadi and Kurpad 27 ). A meta-analysis indicates that for dairy Ca intake of ~1200 mg/d, an increase of ~5 g/d in faecal fat can be expected( Reference Christensen, Lorenzen and Svith 80 ). This arises from the interaction of non-absorbed Ca and dietary fat in the gastrointestinal lumen, leading to Ca–fatty acid soap formation and hence its eventual excretion. These outcomes may contribute to lower circulating TAG and other lipid fractions seen with Ca supplementation( Reference Major, Alarie and Dore 30 ). Finally, as with other chronic non-communicable conditions, MetS is a low-grade chronic inflammatory state. We, and others, are of the opinion that adequate vitamin D has a significant role in ameliorating the inflammatory state in chronic disease( Reference Calton, Keane and Soares 8 , Reference Lai and Fang 81 , Reference Calton, Keane and Newsholme 82 ).
Study limitations
The cross-sectional design has permitted only an examination of associations between Ca intake, vitamin D status and MetS. Although we have controlled for recognized confounders, we cannot establish which came first, lower 25(OH)D concentration and Ca intake or having MetS. An increased requirement for these nutrients in chronic conditions like MetS is a possibility and may account for a reverse causation. Unlike some European countries, there is no mandatory fortification of the Australian food supply for these nutrients. Unfortunately the VHM survey did not include information on Ca and vitamin D supplement usage. Such information would have potentially allowed us to tease out the effect of food-derived Ca and sunlight-derived vitamin D status (since vitamin D in Australian foods is low) v. pharmacological intake. However, we approached the potential confounding effect of supplement Ca intake by using random generated surrogate data for different age groups, based on the Ca supplement intake percentages collected in the Australian Health Survey 2011–12( 83 ). We found that the change between crude and adjusted effect estimates was much less than 10 %; a cut-off criterion for being a sizeable confounder in epidemiology research( Reference Kurth and Sonis 84 ). Hence, we do not anticipate significant confounding by supplement-derived Ca intake on the association between dietary Ca intake and the risk of MetS in the current study.
Serum 25(OH)D can be affected by genetic variation of the major transporter, the vitamin D-binding protein( Reference Speeckaert, Huang and Delanghe 85 – Reference Boucher 87 ). This is seen as variations in vitamin D-binding protein concentration( Reference Lauridsen, Vestergaard and Hermann 86 , Reference Fu, Yun and Oczak 88 ) as well as some vitamin D-binding protein phenotypes potentially having stronger binding abilities than others( Reference Arnaud and Constans 89 ). Serum 25(OH)D can also differ due to genetic variation in its key activation enzyme, CYP27B1( Reference Hypponen, Berry and Wjst 90 ), that converts 25(OH)D to the active form. Such genetic variant information was not collected in the VHM survey so is a potential confounding factor. Future studies in this area could include this information to provide a more complete picture.
A small proportion of our sample was from South Asia (1·6 %, n 56), an ethnic group associated with high rates of betel nut chewing. Chewing betel nut could increase the risk of developing T2DM( Reference Tseng 91 ) and animal studies have indicated that betel nut ingestion in male parents may contribute to inheritable glucose intolerance in their offspring( Reference Boucher, Ewen and Stowers 92 ). Such data are not available for Australia and were not collected as part of the VHM survey. However, exclusion of these cases (n 56) did not change the direction or magnitude of our results. We therefore anticipate minimal confounding from such a potential habit in our South Asian participants.
Study strengths
We have used a large, representative, population-based sample of one Australian state that covered an age range 18–75 years. The dietary data were collected through a multiple-pass 24 h dietary recall which is the current standard and all blood analysis was conducted centrally by one laboratory based on standard methodology. Our analysis has considered and adjusted for many sociodemographic and nutrient confounders, with further adjustment for energy intake. We acknowledge that this field of research would benefit from the confirmation of a causal role for Ca and vitamin D in MetS. While randomized controlled trials provide Level 1 evidence, they are not necessarily the mainstay of the evidence base for public health nutrition and in deciding nutrition priorities for better health( Reference Mann 93 , Reference Truswell 94 ).
Conclusions
The present study demonstrates that high serum 25(OH)D concentration was associated with significant reductions in the odds of MetS. We raise the possibility that the benefit of Ca is restricted to low and medium serum 25(OH)D concentrations, and this may represent a threshold to the interplay between Ca and 25(OH)D on functional outcomes. Overall, these population-based results contribute to the evidence in favour of a role for vitamin D and Ca in modulation of MetS risk.
Acknowledgements
Acknowledgements: M.J.S. acknowledges the School of Public Health, Curtin University for research infrastructure and support, and the Victorian Department of Health and Human Services for use of the VHM survey data set. The authors thank the reviewers and the editorial board for their constructive comments. The opinions and analysis in this manuscript are those of the authors and not those of: the Department of Health and Human Services, Victoria; the Victorian Government; the Secretary to the Department of Health Victoria or the Victorian Minister for Health. Financial support: P.K.P. is the recipient of an Australian Postgraduate Award. Conflict of interest: None. Authorship: P.K.P. analysed data and wrote the first draft. M.J.S. generated the idea, planned the analysis and co-wrote the manuscript. Y.Z. cross-checked the analysis and co-wrote the manuscript. L.S.P. and A.Z. critically reviewed all aspects of the manuscript. Ethics of human subject participation: The VHM was approved by the Human Research Ethics Committee (HREC) of the Baker IDI Heart and Diabetes Institute, Melbourne, Victoria. The analysis of the VHM database was also approved by the HREC at Curtin University (HREC approval number: SPH-19-2014).







