Diarrhoeal disease remains, with respiratory disease, among the two main causes of childhood morbidity and mortality in middle- and low-income countries( 1 ). The long-term consequences for growth and cognition are being increasingly recognized as the burden associated with diarrhoeal disease( Reference Lorntz, Soares and Moore 2 ). Interventions to reduce diarrhoea burden include the provision of safe water and sanitation, breast-feeding promotion, rotavirus vaccination and therapeutic Zn supplementation during diarrhoea episodes. Prophylactic Zn supplementation has shown efficacy in many trials, but translation of these findings to public health programmes is limited by difficulties in establishing Zn status and efficacy in different populations and sparse knowledge of feasible strategies for delivery( Reference Brown, Peerson and Baker 3 ).
Zn supplementation for 10–14 d is currently included in standard diarrhoea case management guidelines( 4 ). Prophylactic Zn is not currently included in preventive child health programmes. A decision to implement universal Zn supplementation requires knowledge of clinical efficacy, population Zn status and cost-effectiveness relative to existing programmes that in many developing countries already include routine vitamin A supplementation.
While there are reports on the global effect of prophylactic Zn on disability-adjusted life years (DALY)( Reference Edejer, Aikins and Black 5 ), policy makers may still find it difficult to translate this information for use in diarrhoea prevention programmes, where efficacy of Zn supplementation and burden of diarrhoeal illness differ locally. Prevalence of stunting, with initial verification using Zn intake data, could serve as a crude marker of population Zn status( 6 ). Knowledge of cost-effectiveness of routine Zn supplementation at varying prevalences of stunting would therefore be informative to planners, for example to plan geographic targeting within a country.
The objectives of the present study were to: (i) describe the cost of diarrhoeal illness in children aged 6–24 months in a rural South African community; and (ii) explore a theoretical threshold prevalence of stunting at which universal Zn plus vitamin A supplementation (VAZ) would be more cost-effective than vitamin A alone (VA). The latter aim arises from the finding in several micronutrient trials, and in a recent meta-analysis, that Zn supplementation preferentially reduces diarrhoea incidence in stunted children( Reference Brown, Peerson and Baker 3 , Reference Chhagan, Van den Broeck and Luabeya 7 , Reference Umeta, West and Haidar 8 ). We maintain universal vitamin A supplementation as a standard of care given that this public health intervention has an evidence base that has already resulted in its general acceptance in child health programmes( Reference Ching, Birmingham and Goodman 9 ).
Methods
Sources of information for cost of diarrhoeal illness
The cost of diarrhoeal illness was estimated from three sources of data: (i) a micronutrient trial conducted in rural South Africa (referred to below as KZN trial)( Reference Luabeya, Mpontshane and Mackay 10 ); (ii) a health service costing survey conducted in urban and rural sites in a South African district by the Health Economics and HIV/AIDS Research Division at the University of KwaZulu-Natal (referred to below as HEARD project)( Reference Parikh and Veenstra 11 , Reference Veenstra and Oyier 12 ); and (iii) the WHO CHOosing Interventions that are Cost Effective (CHOICE) project database of the cost of interventions and the costs of in-patient and out-patient visits by region (referred to below as WHO-CHOICE)( Reference Adam, Evans and Murray 13 , 14 ). For these international sources that reported costs for base year 2000, the South African Consumer Price Index adjustment factor was used to convert these to year 2004 costs( 15 ). All costs were reported as international dollars ($Int) with 2004 as the base year. The international dollar accounts for differences in purchasing power across countries and can be converted to domestic currency that would purchase the same quantity of services as $1 could purchase in the USA( Reference Evans, Edejer and Adam 16 ).
The KZN trial captured direct and indirect costs borne by families. Data on health service utilization and out-of-pocket costs were collected prospectively from participants. A pre-designed economic questionnaire was completed at weekly home visits conducted for active morbidity surveillance. In the South African public health sector there are no user fee charges for children aged below 5 years. These public health sector care costs were obtained from the HEARD project. The HEARD survey captured direct service provider costs, including consultation time, investigations and drug costs, hospitalization costs, length of stay and referral pattern at different levels of care for different age groups and diagnoses( Reference Veenstra and Oyier 12 ).
HEARD data were collected only at public health facilities. KZN trial data were collected within communities and households and captured additional health-seeking behaviours such as consultation with private practitioners or traditional healers and purchase of over-the-counter medications. HEARD described costs from the health provider's perspective, while the KZN trial also captured the burden on families. Both sources captured costs in South African Rand (ZAR). For our analysis we used the exchange rate in 2004 of ZAR 6·88/$Int 1.
The WHO-CHOICE project estimated average out-patient and hospitalization costs for the Afro-E region using a standardized approach( 14 ). Afro-E, one of fourteen geographic sub-regions defined by global burden of disease estimates, includes countries in sub-Saharan Africa, where HIV/AIDS has a large impact on child (under 5 years) and adult mortality. Hospitalization costs are for public hospitals at 80 % occupancy and include the hotel component of costs. It provides cost per visit for primary-care facilities in this region. It includes capital items but excludes drugs. The latter was obtained from the first two sources.
Calculation of cost per episode of diarrhoea
Direct medical costs included family and health sector costs, and comprised medications, including over-the-counter medication purchases, health workers and traditional healer consultations, primary health-care clinic and hospital out-patient visits, and hospitalization. It included use of public and private sector facilities. Scheduled study visits for data collection were not included as part of the cost of health-seeking. If participants consulted study staff for treatment then a cost equivalent to a visit to a primary health-care facility was assigned.
Non-medical and indirect costs included transport, food purchased at health facilities and cost of child care. Information on productivity losses was estimated from time spent travelling and time lost from work or from school for older siblings, although we are aware of the inherent difficulties in assigning a cost to productivity losses( Reference Olsen and Richardson 17 ). For children who had no health service utilization and zero costs captured for their diarrhoea episodes, we examined the influence of assigning a basic cost for each diarrhoea episode. This basic cost included cost of two weeks of Zn supplementation according to current treatment guidelines and two packets of oral rehydration powder. The rationale for allocating these basic costs was to avoid underestimating costs of illness simply because a basic standard of care was not available or accessible to all children with diarrhoea. The current restructuring of the community health worker programme in South Africa is already incorporating these interventions for wide scale-up.
Combining the information from these sources, we estimated the cost per episode as total direct costs plus total indirect costs averaged over the number of diarrhoea episodes for which cost information was collected, including those episodes that had zero cost recorded. The cost of illness analysis was conducted from a societal perspective; that is, costs were included irrespective of who was paying. We calculated the arithmetic mean as well as the bootstrap mean and standard error of the mean derived from 1000 bootstrap replications because of the skewed nature of the cost data.
In assigning monetary values to productivity losses, we used the minimum wage in South Africa in 2004 to assign a cost for time missed from work during a child's diarrhoea episode.
Cost of supplementation
We referred to WHO-CHOICE for cost of universal VA supplementation( Reference Edejer, Aikins and Black 5 ). These programme-level costs are meant to include all resources required to establish and maintain the intervention, and include those of administration, publicity, training and delivery of the intervention. To date there is sparse information available on programmatic costs of preventive Zn supplementation. For preventive Zn supplementation we therefore used published sources of costing information for micronutrient powders( Reference Horton, Shekar and McDonald 18 ). The costs described in these sources applied to 95 % and 100 % coverage, respectively. We estimated that 80 % coverage would cost about 30 % lower than 95 % coverage( Reference Horton, Shekar and McDonald 18 ).
Efficacy of supplementation
For base case scenarios, measures of the effect on incidence of diarrhoea were obtained from the KZN trial. The trial found that stunted children had fewer diarrhoea episodes if supplemented with VAZ compared with VA( Reference Chhagan, Van den Broeck and Luabeya 7 ). There were no detectable differences in non-stunted children. Since this trial enrolled too few HIV-infected children to detect meaningful differences for this group, we instead used published information on diarrhoeal reduction among HIV-infected children from another Zn supplementation safety trial conducted in South Africa( Reference Bobat, Coovadia and Stephen 19 ). We also examined the possibility of Zn preventing diarrhoea among both stunted and non-stunted children, although with smaller effect in the latter, as described in other international studies. Last, we explored a scenario where stunting prevalence was not considered but diarrhoea reduction with VAZ was assumed to be that described in a recently published meta-analysis( Reference Brown, Peerson and Baker 3 ).
Analytic approach
The measure of cost-effectiveness used in the present analysis is the incremental cost-effectiveness ratio (ICER) of universal VAZ given to all infants starting at age 12 months compared with VA. This is expressed as cost per additional episode of diarrhoea prevented. The following equation was used for ICER:
where
Net cost is the difference in costs of interventions minus the cost of averted disease( Reference Fischer, Anh and Antil 20 ). Negative net costs generally imply savings and support implementation of such interventions. If the intervention were to target stunted children only, then the cost involved in identifying stunted children would need to be included. If the intervention is given universally, but the effect size differs in subgroups of stunted and non-stunted children, then the net effect would depend on the proportionate size of the subgroups. If the intervention was preventive only among stunted children, then the net cost and cases prevented would apply only to stunted children. A dominant intervention would be one that reduces diarrhoea (positive denominator) at a net cost saving (negative numerator). An intervention that reduces diarrhoea at a cost (positive numerator) needs further evaluation of whether the cost of achieving a unit benefit is justified. This usually involves comparison of the ICER with that of other interventions, which requires scaling them to a common denominator such as DALY. The Commission on Macroeconomics and Health recommends that an intervention be considered highly cost-effective if it costs less than the Gross National Income per capita( 21 ). Various cut-offs for cost-effectiveness are used however and these may be highly sensitive to different local and regional factors( 22 ).
Since the preventive effect of VAZ on diarrhoea incidence was observed only among stunted children in the South African trial, we conducted a threshold analysis to determine the theoretical prevalence of stunting at which this intervention would be more cost-effective than VA in preventing diarrhoea. We simulated a hypothetical cohort of 10 000 children aged 12 months supplemented with VA or VAZ for a year. This is based on meta-analyses that showed greatest benefit in terms of diarrhoea prevention accruing after 12 months with Zn supplementation( Reference Brown, Peerson and Baker 3 ). The model included information on effectiveness of Zn plus vitamin A supplementation, cost of diarrhoea episodes and cost of universal supplementation at varying levels of coverage. We calculated ICER for a range of stunting prevalence to assess a level at which universal VAZ would be most cost-effective in preventing diarrhoeal disease assuming that this protective effect is limited to stunted children. All costs were adjusted to base year 2004 for these analyses. We projected all costs over one year and so did not include a discounting rate. We used Microsoft® Office Excel 2010 software for this simulation.
Our main assumptions were: (i) initial coverage target of 80 %; (ii) instead of zero costs, the minimum cost for diarrhoea episodes would be that of two weeks’ supply of Zn and two oral rehydration sachets; (iii) efficacy of interventions vary in direct proportion to coverage, hence we adjusted magnitude of effect for varying coverage levels; and (iv) cost of increasing coverage is anticipated to be 30 % greater for increasing coverage from 80 to 95 %( Reference Horton, Shekar and McDonald 18 ).
We varied several key parameters to examine how robust the threshold analysis was to varying conditions. We performed one-way and multi-way sensitivity analyses varying intervention cost, coverage and cost of illness. We varied magnitude of efficacy using the lower and upper 95 % confidence interval for efficacy from the trial results. We examined scenarios where benefit occurred in both stunted and non-stunted children, with lower effect size in the latter( Reference Umeta, West and Haidar 8 ). We explored doubling the cost of Zn to cater for higher distribution costs. We explored a scenario with 5 % prevalence of HIV among infants, which will be realistic in the South African setting. Lastly, we examined ICER if stunting prevalence was not considered but estimates of diarrhoea reduction reported in meta-analyses were applied to the baseline observed in the South African trial( Reference Brown, Peerson and Baker 3 ).
Ethical review
The Biomedical Research Ethics Committee of the University of KwaZulu-Natal and the Institutional Review Board of Tufts–New England Medical Center approved the KZN trial (ClinicalTrials.gov identifier: NCT00156832). The study was conducted according to the guidelines laid down in the Declaration of Helsinki. Written informed consent was provided by a parent or guardian for all participating children. The HEARD study had approval from the Biomedical Research Ethics Committee, University of KwaZulu-Natal, plus permission from local health authorities and facilities included in the study sample.
Results
Information on health-seeking and costs was available for 318 children contributing 1563 episodes of diarrhoea during the micronutrient trial. For 959/1563 episodes there was no health service utilization and zero cost recorded. The main categories of health care are shown in Table 1. Only 9/318 children had access to private health insurance. Of these nine, four consulted the public sector services. Caregivers missed work for twenty-three episodes of diarrhoea amounting to a total of 57 d, while older siblings missed school for seven episodes amounting to a total of 16 d.
Table 1 Cost of health services for 1563 episodes of diarrhoea among 318 children aged 6–24 months
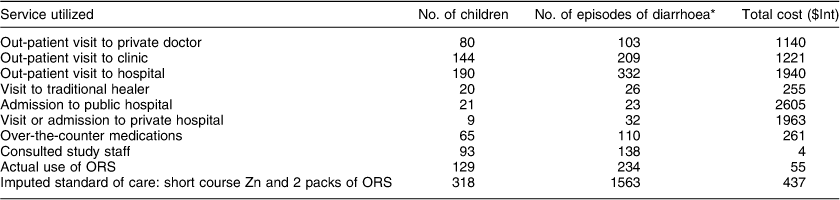
ORS, oral rehydration salts.
*More than one service was used for individual episodes of diarrhoea.
Estimates of costs of illness
The average cost per diarrhoeal episode was $Int 7·34 (Table 2) after assigning a basic cost to reflect standard care for those episodes with zero costs and no health service utilization. Due to many episodes with no health-seeking the cost per episode was highly skewed, with the 10th and 90th centile being $Int 0·28 and $Int 15·63 per episode, respectively. Of the total cost, 65 % was borne by service providers and 35 % by families. Direct costs comprised 91 % of all costs. Doctor consultations and hospitalization contributed most to costs. The average cost per episode was $Int 6·92 if we did not assign a basic cost to reflect standard of care, with the 10th and 90th centile being $Int 0·00 and $Int 15·35, respectively. If we assigned a basic cost plus a monetary value to productivity losses for days lost from work, the average cost increased to $Int 7·80/episode.
Table 2 Cost of diarrhoeal illness among 318 children aged 6–24 months from societal perspective

Estimates of cost of supplementation
Using WHO estimates and after adjusting to base year 2004 Consumer Price Index, we estimated at 80 % coverage a cost for 10 000 children of $Int 37 277 for VA supplementation alone( Reference Edejer, Aikins and Black 5 ); and using cost of micronutrient powders from the World Bank Report, $Int 62 037 for VAZ( Reference Horton, Shekar and McDonald 18 ). At 95 % coverage the cost would be $Int 67 100 and $Int 111 668, respectively. If we doubled the cost of Zn supplementation, then VAZ would cost $Int 82 960 and $Int 149 328 at 80 % and 95 % coverage, respectively.
Estimates of efficacy of Zn supplementation
In the KZN trial stunted children supplemented with VAZ had 2·04 episodes (95 % CI 1·37, 3·05) of diarrhoea per year compared with 3·92 episodes (95 % CI 3·02, 5·09) per year in children who received VA. The difference between non-stunted groups was not statistically significant, so in our base case we included reduction among stunted children only and zero effect among non-stunted. Alternative scenarios included reduction of 1·2 episodes of diarrhoea per child per year among stunted children and 0·36 episodes per child per year among non-stunted children as described by Umeta et al.( Reference Umeta, West and Haidar 8 ). For calculation of ICER without geographic targeting by stunting prevalence, we used relative risk of diarrhoea incidence among the VAZ group of 0·80 compared with VA( Reference Brown, Peerson and Baker 3 ), with the latter having 2·92 episodes per child per year based on the KZN trial.
Incremental cost-effectiveness ratios and threshold analysis
For our base scenario we calculated ICER at 80 % coverage and 15 % prevalence of stunting (Table 3). At 15 % prevalence of stunting VAZ had a relatively low incremental cost of $Int 1·23 per additional case of diarrhoea prevented relative to VA alone. In the threshold analysis, keeping other base case conditions constant, VAZ became cost-saving when stunting prevalence was close to 20 % (Table 4). We varied several parameters in our sensitivity analyses, some of these are presented in Table 5. With increasing stunting prevalence, routine Zn supplementation would result in a greater number of diarrhoea episodes prevented and hence total cost of illness prevented. At 95 % coverage VAZ showed low ICER at 25 % prevalence of stunting, but became cost-saving at a higher prevalence. If we assume that the preventive effect of VAZ is not restricted to stunted children but has a smaller effect among non-stunted children, then fairly low ICER is achieved at stunting prevalence between 10 and 15 %, with cost savings by 20 %. Doubling the cost of VAZ resulted in low ICER at 25 % prevalence of stunting. A scenario of 5 % HIV prevalence showed low ICER at 15 % prevalence of stunting and cost saving by 20 % prevalence. Results were sensitive to cost of diarrhoea episodes and cost and coverage of the intervention (Table 5). Generally, VAZ showed low incremental costs in several sensitivity analyses when the prevalence of stunting was close to 20 %.
Table 3 Calculation of incremental cost-effectiveness ratio (ICER) assuming 80 % coverageFootnote *
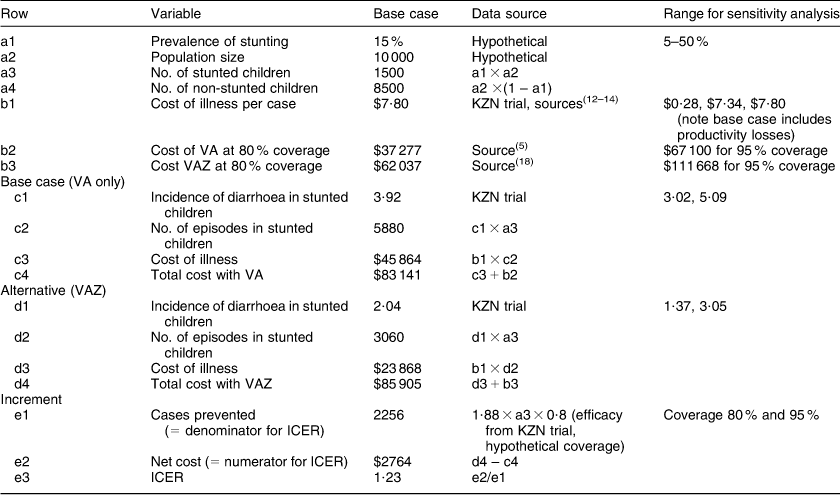
VA, universal vitamin A supplementation alone; VAZ, universal Zn plus vitamin A supplementation.
* All costs are in international dollars adjusted to base year 2004.
Table 4 Incremental cost-effectiveness ratios (ICER) at varying prevalence of stunting with other base case parameters kept constantFootnote *
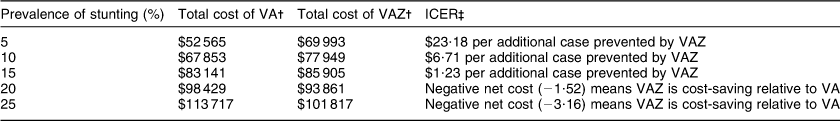
VA, universal vitamin A supplementation alone; VAZ, universal Zn plus vitamin A supplementation.
* All costs are in international dollars adjusted to base year 2004.
† Total cost includes cost of intervention at 80 % coverage, plus cost of diarrhoeal illness in stunted children at $Int 7·80/episode.
‡ ICER calculated as net cost per episode of diarrhoea prevented, assuming that additional Zn supplementation benefits only stunted children. Where net cost is a negative value it means that VAZ is dominant; that is, it is cost-saving relative to VA. In that case a descriptive summary of ICER is provided rather than a cost per episode prevented.
Table 5 Sensitivity analysis: effect of varying assumptions on efficacy and costs of interventionFootnote *
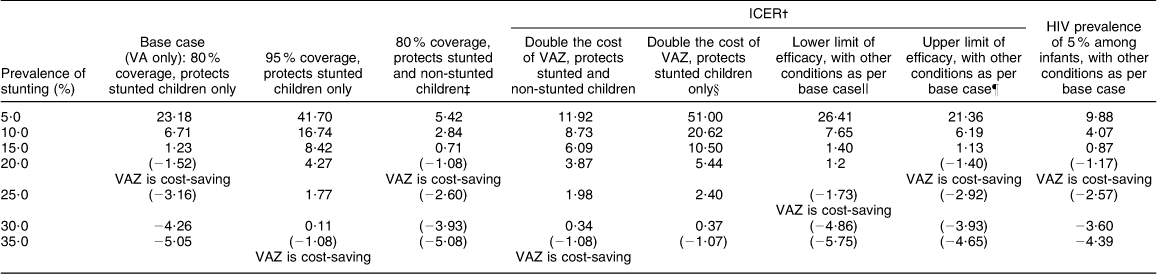
ICER, incremental cost-effectiveness ratio; VA, universal vitamin A supplementation alone; VAZ, universal Zn plus vitamin A supplementation.
* All costs are in international dollars adjusted to base year 2004.
† ICER calculated as net cost per episode of diarrhoea prevented. Where either intervention is dominant a descriptive summary of ICER is provided rather than a cost per additional episode of diarrhoea prevented.
‡ Based on published study by Umeta et al.( Reference Umeta, West and Haidar 8 ) that shows benefit to both groups.
§ Double the cost to cover 5 d of supplement per week and distribution cost.
|| VAZ prevents 1·65 episodes per child relative to VA.
¶ VAZ prevents 2·04 episodes per child relative to VA.
Discussion
Prevalence of stunting in children aged under 5 years, supported by Zn intake data, is an indicator for quantifying Zn deficiency in populations( Reference Hess, Peerson and King 23 ). Zn trials in regions with moderate to high stunting prevalence may show overall benefits or subgroup effects among stunted children( Reference Bhutta, Bird and Black 24 , Reference Rahman, Vermund and Wahed 25 ). This suggests that routine Zn supplementation as a public health intervention would be most effective if targeted to high-risk groups. Individual-level targeting requires feasible case detection strategies that are affected by cost and technical issues such as measurement error and misclassification of stunting. WHO considers stunting prevalence rates of ≥20 % to be of public health concern( Reference Brown and Rivera 26 ). In South Africa the prevalence of stunting varies, with some provinces and rural areas reaching prevalence of 25 % or more – in general reflecting high to intermediate risk of population Zn deficiency( Reference Labadarios, Steyn and Maunder 27 ). Of note is that Zn deficiency prevalence estimates from South African national surveys use data on stunting and Zn intake. In the setting of such information, we propose that a threshold analysis that incorporates stunting prevalence and functional outcomes would be useful to policy makers.
Using the cost of diarrhoeal illness as an outcome of public health importance, we show that by 20 % prevalence of stunting, universal VAZ dominated VA for many scenarios. In other words, it became relatively cost-saving because the additional costs of VAZ compared with VA were negated by the cost of illness prevented by VAZ. This was fairly robust to changes in efficacy but sensitive to high VAZ costs and high coverage needs. At stunting prevalence between 15 and 20 %, the ICER should be interpreted in the context of actual resources available and other competing interventions. For example, at 15 % stunting prevalence, an incremental cost of $Int 1·23 per additional case of diarrhoea prevented (Table 4) could translate crudely to an increment of $Int 18 per additional DALY averted if we assume: (i) a diarrhoea case fatality rate of 2/1000; (ii) that a death in infancy corresponds to 33 DALY (based on WHO DALY estimates); (iii) that duration of an illness is 7 d; and (iv) that disability weighting for a diarrhoeal episode is 0·12( Reference Mathers, Vos and Lopez 28 – Reference Barungi and Kasirye 30 ). This would then be weighed against the DALY averted by other interventions, albeit not taking into account the longer-term impact of diarrhoea, specifically of recurrent diarrhoea on stunting and cognition. Below 15 % prevalence universal VAZ was not advisable. Illness costs were very influential in determining cost-effectiveness, implying that low-cost measures that drastically reduce severity of episodes and avert complications could theoretically displace prophylactic Zn supplementation as a preferred intervention.
Limitations to our model are inherent in our key assumptions. We have not incorporated newer interventions like universal rotavirus immunization, which was introduced subsequent to our study. The impact of HIV may have also changed, with infants now commencing antiretroviral treatment at much earlier ages. It is very likely that we have underestimated the cost of diarrhoeal illness. At higher costs of illness however, VAZ would become even more cost-effective and the threshold stunting prevalence at which VA is dominated may be even lower. The model does not consider other potential effects of Zn supplementation, such as reduced acute respiratory infections, reduced all-cause mortality and potential reduction of stunting, which would strengthen cost-effectiveness( Reference Chhagan, Van den Broeck and Luabeya 31 ). Although the input parameters used were based on a South African trial and health service setting combined with international and regional data, the simulation's structure has applicability to various geographic regions. This can be achieved by using data that may be locally available or updated and retaining sensitivity analyses that are relevant to local or regional settings.
The largest challenge is the paucity of programmatic data for preventive Zn supplementation. We have opted to use costs based on micronutrient powders, which may need further adaptation to accommodate local delivery mechanisms. The adherence with daily micronutrients in a programmatic setting is undeniably crucial to success of the intervention. It is also very likely that the costs of VAZ are underestimated and would require higher operational costs for delivery; however, the current developments in primary health care in South Africa make the delivery mechanism more feasible. South Africa is in the process of strengthening community-based services with community health workers already hired and trained to conduct regular home visits. Growth monitoring visits at primary health facilities or within community sites are another potential delivery point because they are scheduled to occur monthly in infancy and every second month in the second year of life. The service infrastructure therefore can already start considering incorporation of micronutrient supplementation. A pilot site based on geographic prevalence of stunting could then be used to better determine programmatic logistics and costs. This approach will also necessitate periodic surveys (and costs thereof) to monitor population Zn status dissociated by urban/rural and/or other categories.
Conclusion
Where evidence for preventive supplementation under realistic programme conditions is sparse, simulation models, like the one presented here, are useful preliminary exercises to explore cost-effectiveness. Our findings suggest that universal VAZ could replace VA in diarrhoea prevention programmes in South Africa using geographic targeting based on prevalence of stunting. This simulation needs ongoing validation under programmatic conditions where nutrition programmes are integrated with other child health programmes and through incorporation of more extensive programmatic cost data.
Acknowledgements
Sources of funding: This work was supported by grants from the US National Institutes of Health (1 UO1 AI45508-01, 1 K24 AI/HDO1671-01 and D43TW05572-01), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)/Fogarty International Center (FIC) International Maternal and Child Health Training Grant (D43-TW001277-11 to M.L.B.) and the Wellcome Trust (grants 62925 to M.L.B. and 063009 to J.V.d.B.); and an International Nutrition Foundation Fellowship to M.K.C. Conflicts of interest: There are no conflicts of interest to declare. Authors’ contributions: M.K.C. designed and conducted the current analysis and prepared the initial manuscript draft. M.L.B. was the principal investigator and responsible for study design of the micronutrient trial. J.V.d.B. was project director. K.-K.A.L. was project leader and study physician. N.M. supervised field activities and field data coordination. The manuscript was critically reviewed and approved by all of the authors. Acknowledgements: The authors acknowledge the following institution that permitted access to data used in this analysis: the Health Economics and HIV/AIDS Research Division (HEARD) of the University of KwaZulu-Natal, Durban, South Africa. They would also like to acknowledge the following individuals from Tufts University for review of and advice in preparing the manuscript: Drs Katherine Tucker, Jeffrey Griffiths, Parke Wilde and Joshua Cohen.







