As low physical activity levels are linked with adverse health effects, such as CVD, obesity, diabetes mellitus type 2, hypertension and certain types of cancer(Reference Blair and Morris1, 2), international physical activity guidelines to maintain and improve health have been formulated(2). Adults aged between 18 and 65 years are recommended to do at least 150 min of moderate-intensity physical activity weekly, or 75 min of vigorous-intensity aerobic physical activity weekly, or an equivalent combination of moderate- and vigorous-intensity aerobic activity(2). Unfortunately, a considerable amount of the American (56 %)(2), Australian (57 %)(Reference Bauman, Armstrong and Davies3) and European (56–80 %)(Reference Sjöström, Oja and Hägströmer4) adult population is not meeting these health-related physical activity guidelines.
These insufficient levels of physical activity will result in an increased risk for negative health effects, and will in turn have a negative economic impact on health-care costs(Reference Wang, Pratt and Macera5). WHO estimated that 1·5–3·0 % of total direct health-care costs are accounted for by low physical activity levels(Reference Oldridge6). Consequently, it is important to develop and implement physical activity interventions that are not only effective (does the intervention result in increased physical activity and consequently increased health?), but also cost-effective (does the money spent on the intervention result in maximal health gains?)(Reference Roux, Pratt and Tengs7). Therefore, cost-effectiveness analyses of physical activity interventions have been suggested recently to assess their feasibility on a broad population basis(Reference Muller-Riemenschneider, Reinhold and Nocon8). Providing information about both the effectiveness of interventions and their cost-effectiveness will assist (national) decision makers and could persuade them to make conscientious choices, especially in times of economic crisis.
An evaluation of seven exemplar physical activity interventions, including strategies such as community-wide campaigns, social support, individually adapted behaviour change and enhanced access to physical activity, was performed recently(Reference Roux, Pratt and Tengs7). Results showed that all interventions were cost-effective and offered good value for money for a cohort of healthy US adults(Reference Roux, Pratt and Tengs7). Another recent cost-effectiveness study modelled the cost impacts and health outcomes of six physical activity interventions over the lifetime of the Australian population(Reference Cobiac, Vos and Barendregt9). It was found that intervention programmes that encourage pedometer use and mass-media-based community campaigns are the most cost-effective strategies. In the UK, four types of interventions (brief interventions in primary care, pedometer use, exercise referral, and walking and cycling programmes in the community) were found to be ‘dominant’ (cost-saving, or more effective and less expensive compared with usual care)(10).
However, until now, no other European studies could be found examining the cost-effectiveness of physical activity interventions. In addition, further economic evaluations of (European) pedometer-based community projects, which have become more and more popular recently(Reference Bravata, Smith-Spangler and Sundaram11), are needed.
Therefore, the present study evaluated the cost-effectiveness of the European community-based project ‘10 000 Steps Ghent’. Details on the development and implementation can be found elsewhere(Reference De Cocker, De Bourdeaudhuij and Brown12). Effectiveness studies showed that the campaign resulted in a significant decrease in sitting time(Reference De Cocker, De Bourdeaudhuij and Brown13) and a significant increase in self-reported and pedometer-determined physical activity(Reference De Cocker, De Bourdeaudhuij and Brown12). The aim of the present paper is to report the results of a health-economic model estimating the costs, savings and health benefits of the ‘10 000 Steps Ghent’ project, in order to examine whether this community-based intervention is cost-effective.
Methods
Study design
A flexible age- and gender-dependent state-transition Markov model assuming a public payer perspective was used to estimate, for both the intervention group and the control group (no ‘10 000 Steps Ghent’ intervention), the development of diabetes and cardiovascular and oncological life events over time and the associated costs. The model was based on a Markov model published by Annemans et al. (Reference Annemans, Lamotte and Clarys14) and further developed using Microsoft® Excel 2007 (Microsoft Corporation, Redmond, WA, USA).
The model makes use of a closed cohort representing the population reached by the intervention. The target population of the intervention were all 25–75-year-old adults living in Ghent (mid-sized town in Belgium, with approximately 245 000 inhabitants). The efficacy study revealed that 40·3 % of the studied population increased their self-reported total amount of walking. This reached population consisted of 45·6 % men, had a mean age of 48·2 (sd 13·1) years (11·4 % aged 25–29 years, 8·9 % aged 30–34 years, 10·8 % aged 35–39 years, 9·2 % aged 40–44 years, 11·4 % aged 45–49 years, 9·2 % aged 50–54 years, 10·1 % aged 55–59 years, 8·7 % aged 60–64 years, 9·7 % aged 65–69 years, 10·6 % aged 70–74 years), was mostly (64·6 %) highly educated (college or university degree), employed (70·0 %) and in good health (88·7 % reported good to excellent health).
Nine health states were included in the model: (i) being healthy; (ii) having diabetes mellitus type 2; (iii) having CHD (first year); (iv) having CHD follow-up; (v) having stroke (first year); (vi) having stroke follow-up; (vii) having colon cancer (first year); (viii) having colon cancer follow-up; and (ix) dying (see Fig. 1). Transitions between health states were allowed once a year.

Fig. 1 Markov diagram: in this state-transition model all of the different health states are inserted. Each arrow is linked with a certain transition probability. The circles represent possible health states. The following states can be distinguished: being healthy, developing diabetes, developing a stroke, developing a coronary event, developing colon cancer and dying
Within the present study a cost-utility analysis was performed, defined as the ratio of incremental costs to incremental quality-adjusted life years (QALY), also called the incremental cost-effectiveness ratio (ICER), calculated as
where I is intervention and NI is no intervention.
QALY are calculated by multiplying the utility level for a given disease status (this is a health-related quality-of-life weight and ranges between 0 and 1) with the number of years an individual suffers from that particular disease. A utility of 0 is assigned to death, while a utility of 1 represents perfect health. Using this universal measure, international comparisons with other health-economic evaluations are possible. Concerning the costs, both the cost of the intervention and the cost of the diseases are accounted for.
State-transition Markov model: assumptions and description
It was assumed that all individuals started in a health state free of events. A simulation of the evolution of the cohort was made based on the change in walking time and the associated risk of developing an illness. Four diseases were included in the model, supported by existing evidence suggesting that walking is associated with a lower disease risk(Reference Manson, Greenland and LaCroix15–Reference Orsini, Mantzoros and Wolk18). During each cycle (1 year) a healthy individual has a risk of developing diabetes, CHD, ischaemic or hemorrhagic stroke, colon cancer, or dying from another cause. Hence individuals developing diabetes move to the health state ‘diabetes’. Once an individual is diagnosed with diabetes, he or she can only remain in this state or go to the ‘dead’ state. Since these patients have an increased risk for developing both microvascular and macrovascular complications, the prevalence of these complications and the associated costs were taken into account. Patients suffering from stroke move to the ‘stroke 1’ state. Once a patient has had a stroke, he or she can only move to the follow-up ‘stroke 1+’ state or to the ‘dead’ state. Patients who suffer from a fatal stroke move to the ‘dead’ state after being in the ‘stroke 1’ state for one cycle. The prevalence and the associated costs and utilities of fatal and non-fatal stroke were taken into account in the ‘stroke 1’ state. Patients being affected by CHD (‘CHD 1’ state), including myocardial infarction (MI), stable or unstable angina, can only move to the follow-up ‘CHD 1+’ state or to the ‘dead’ state. Those developing a fatal MI move to the ‘dead’ state after being in the ‘CHD 1’ state for one cycle. The prevalence and the associated costs and utilities of fatal and non-fatal CHD are taken into account in the ‘CHD 1’ state. Finally, individuals suffering from colon cancer can only move to the follow-up ‘colon cancer 1+’ state or to the ‘dead’ state after being in the ‘colon cancer 1’ state for one cycle.
Once an individual enters the ‘dead’ state, no further transitions are possible, since this is an absorbing state. The model was extended over a time period of 20 years. Hence individuals started in a given age category as stated above and remained in the model for 20 years or until they died. The risk of developing a disease or dying throughout the model was specified with transition probabilities.
Clinical data inputs
Disease transition probabilities
The age- and gender-dependent transition probabilities for the Belgian population are based on recent epidemiological studies (see Table 1). The risk of developing diabetes varies between 0·024 % and 0·805 % depending on age and gender(19, Reference Wens, Van Casteren and Vermeire20). The prevalence of microvascular and macrovascular complications in diabetic patients was based on a study by Williams et al.(Reference Williams, Van Gaal and Lucioni21). The risk of developing CHD or stroke varies between 0·001 % and 1·351 % and between 0·0 % and 0·555 % per annum, respectively. These data are based on Belgian registries(Reference De Backer22, 23). The incidence of colon cancer was derived from the Belgium National Cancer Registry and ranges between 0·001 % and 0·258 %(24).
Table 1 Age- and gender-dependent probabilities for developing one of the disease states; death probabilities associated with a certain disease state and overall mortality probabilities other than caused by diabetes, CHD, stroke or colon cancer

Mortality probabilities
Annual age-specific mortality probabilities (death from other cause) for the overall population were based on nationally available data(25). Mortality probabilities associated with a certain disease state were not readily available; hence they were either calculated by multiplying average national mortality probabilities with the mortality increase associated with a given disease(19, 23, Reference Ubink-Veltmaat, Bilo and Groenier26–Reference Moholdt, Wisloff and Nilsen28) or literature findings were used (Table 1). Ubinck-Veltmaat et al. (2003) reported a 40 % mortality increase among diabetics(Reference Ubink-Veltmaat, Bilo and Groenier26). Stroke patients have a twofold mortality increase compared with the general population(23). For CHD, an annual mortality probability of 8·98 % for men and 8·68 % for women was used based on a study of Moholdt et al. (2008)(Reference Moholdt, Wisloff and Nilsen28). Finally, colon cancer patients have a 1-year mortality probability of 14·09 %(Reference Gatta27).
Effect of the programme
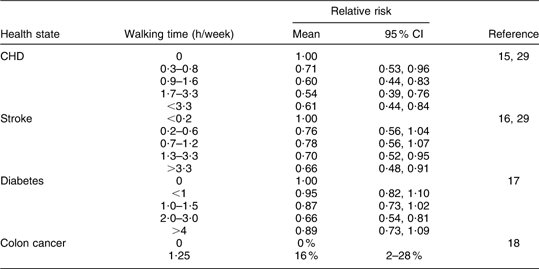
The probability that the ‘10 000 Steps Ghent’ intervention programme increases physical activity levels is drawn from the effectiveness study(Reference De Cocker, De Bourdeaudhuij and Brown12) showing an increase in walking of 66 min/week (95 % CI 28, 104 min/week) for the intervention group compared with a control group (F = 10·4, P = 0·001). Studies of good methodological quality (including large cohorts) showed that increased walking was linked with relative risk reductions of experiencing each health state(Reference Manson, Greenland and LaCroix15–Reference Orsini, Mantzoros and Wolk18). A linear relationship was assumed between the walking time and the associated risk reduction (see Table 3, relative risk reductions). Walking for a total of 66 min/week decreases the risk of developing diabetes by 11·46 %, the risk of developing CHD by 34·4 %, the risk of developing a stroke by 24·46 % and the risk for colon cancer for men (not for women) by 2·01 %(Reference Manson, Greenland and LaCroix15–Reference Orsini, Mantzoros and Wolk18, Reference Oguma and Shinoda-Tagawa29). The risk reductions were applied to both genders, unless there was evidence of no significant risk reduction for a specific gender, as was the case for colon cancer(Reference Spence, Heesch and Brown30). The original data can be found in Table 2.
Table 2 Original data extracted from the literature that served as a basis for the calculation of the relative risk reductions
Cost data input
Disease costs
A public payer perspective was assumed to estimate disease costs; therefore only direct medical costs were taken into account (see Table 3, expressed in 2009 Euros). Recruitment costs, research costs, travel and time costs to be physically active were not included in the model. The proportion of diabetics with no complications, diabetics with microvascular complications, diabetics with macrovascular complications and diabetics with both micro- and macrovascular complications and the costs associated with these complications were used to calculate the overall cost of diabetes(Reference Williams, Van Gaal and Lucioni21). For CHD and stroke a first-year cost and a follow-up cost were calculated since the cost for an individual with a newly diagnosed CVD differs from the cost for those who suffer longer from a CVD(Reference Annemans, Marbaix and Webb31, Reference Lamotte, Caekelbergh and Annemans32). For colon cancer an equal cost per year was assumed, since evidence suggests that both the first year after the diagnosis and the last year (end of life) are associated with the highest costs(Reference Van Gelder33). A decrease of costs can be found for the period in between, hence an average cost over the years was used(Reference Van Gelder33). No costs are linked to the ‘dead’ state, since the costs of fatal events are already accounted for in the relevant disease state.
Table 3 Parameters used for base case and sensitivity analyses

RR, relative risk.
Intervention costs
The intervention costs include promotional materials, the development and maintenance of a website, ½ full-time equivalent staff and a pedometer for those who reported having used a pedometer during the intervention (16·4 %)(Reference De Cocker, De Bourdeaudhuij and Brown12, Reference De Cocker, De Bourdeaudhuij and Brown34). It was assumed that the intervention has to be implemented each year in order to maintain the effect.
An effective duration of 5 years was considered for the pedometers. Therefore the cost of the intervention per inhabitant amounts to 3·51€ in the first year. In the following years (second year until fifth year) a cost of 0·23€ was taken into account. This 5-year cycle was repeated over a time horizon of 20 years. All future costs were discounted to present values at 3 % annually (see Table 3).
Health-related quality of life: QALY
‘Loss of utility’ data (penalty linked to a given disease state) associated with the different disease states were drawn from recent literature(Reference Heyworth, Hazell and Linehan35, Reference Uyl-de Groot, Vermorken and Hanna36). Age-specific utility levels for healthy individuals were based on Belgian data(Reference Greiner, Weijnen and Nieuwenhuizen37, Reference Szende and Williams38). Hence, utilities were calculated for each disease state within the different age categories (see Table 3). Afterwards, QALY were calculated by multiplying these utilities with the number of life years. Future QALY were discounted at 1·5 % as suggested by the Belgian Health Care Knowledge Centre (KCE).
Main outcome measure of the cost-utility analysis: ICER
As explained before, the ICER was calculated by dividing the difference in costs by the difference in QALY. The ‘10 000 Steps Ghent’ intervention was considered cost-effective if the ICER was no more than the KCE-recommended threshold of 30 000€/QALY.
Sensitivity analyses
In order to capture the uncertainty associated with some parameters, both one-way sensitivity analyses and probabilistic sensitivity analyses (Monte Carlo) were performed. The latter allowed to asses the uncertainty for all parameters by varying them concurrently, each with their own probability distribution. The one-way sensitivity analyses made it possible to assess the effect of each parameter on the ICER, by varying them separately.
Cost data were assumed to follow a gamma distribution, utilities followed a beta distribution and risk reductions a lognormal distribution(Reference Briggs, Sculpher and Claxton39). Standard errors for each parameter were based on literature findings (see Table 3).
The base case model assumes a life-long programme with a life-long intervention effect. However, additional, more conservative analyses were conducted implementing a life-long intervention programme with only a 5-year intervention effect and a 1-year intervention effect.
Results
Base case (life-long intervention effect)
For the no-intervention situation the average discounted QALY amount to 12·07 and 12·66, with a cost of 3539€ and 2881€, for men and women, respectively. Implementing the ‘10 000 Steps Ghent’ intervention improved the average QALY by 0·16 to give 12·23 QALY for men and by 0·11 to give 12·77 QALY for women. The total costs decreased by 576€ to 2963€ and by 427€ to 2454€, respectively. Hence for both genders the intervention programme was more effective and less expensive than the no-intervention situation and is therefore called ‘dominant’ or cost-saving.
One-way sensitivity analyses
One-way sensitivity analyses were performed to assess the effect of the uncertainty of parameters on total QALY and total costs. The results of these analyses are shown in Tornado diagrams (see Figs 2 and 3). Varying the utility values and the intervention effect within their uncertainty range had only a small impact on the difference in QALY between intervention and control groups. The uncertainty associated with the relative risk reductions, however, had a more pronounced effect on the change in QALY. Nevertheless, the intervention remained dominant. The results were similar for both genders (Fig. 2).

Fig. 2 (colour online) Tornado diagrams of the ‘10 000 Steps Ghent’ intervention v. no intervention: results on QALY for (a) men and (b) women (QALY, quality-adjusted life years; RR, relative risk)

Fig. 3 (colour online) Tornado diagrams of the ‘10 000 Steps Ghent’ intervention v. no intervention: results on costs for (a) men and (b) women (RR, relative risk)
Figure 3 shows the influence of the different parameters on the change in cost. Here again the uncertainty related to the risk reduction data have the most important impact. Varying the intervention effect, intervention costs or the cost of colon cancer, on the other hand, had only a minor impact on the total change in cost. Once again, the results were similar for both genders.
Probabilistic analyses
The results of the Monte Carlo analyses, performed to assess the effect of the uncertainty related to all parameters, were favourable. As can be seen in Figs 4(a) and 4(d), the life-long effect of the intervention based on 5000 simulations remained dominant, because the costs and effects points were situated in the south-east quadrant of the cost-effectiveness plane.

Fig. 4 (colour online) Results of the probabilistic sensitivity analysis whereby all variables are varied simultaneously assuming: (a) life-long intervention effects for men; (b) 5-year intervention effects for men; (c) 1-year intervention effects for men; (d) life-long intervention effects for women; (e) 5-year intervention effects for women; (f) 1-year intervention effects for women
The additional analyses with a shorter intervention effect still showed favourable results (see Figs 4(b), 4(c), 4(e) and 4(f)). Taking into account a 5-year intervention effect, the intervention remained dominant for both genders. The 1-year intervention effect simulation showed a substantial decrease in QALY gain and cost savings, however the overall result remained favourable.
Discussion
In the present study, a health-economic model was described providing information about the costs of the ‘10 000 Steps Ghent’ intervention, the health benefits and savings related to this intervention, and the cost-effectiveness of this community-based programme. Compared with no intervention, the ‘10 000 Steps Ghent’ programme is more effective and associated with a decrease in cost, taking into account the avoided disease costs, and thus the intervention is called ‘dominant’. Variation in the different parameters of the Markov model did not alter the results significantly; hence the intervention remained ‘dominant’ at all times. Only the additional analyses assuming a 1-year intervention effect showed a borderline dominance.
Present results demonstrate the economic value of primary prevention for public health, even though the costs and health benefits of the present intervention were compared with a ‘no-intervention’ situation. Furthermore, the findings confirm results found in other health-economic studies of physical activity interventions. Despite differences in analysis methods and assumptions, making profound comparison difficult, overall conclusions are similar. In the USA, it was found that community-wide campaigns were also cost-effective ($US 14 000–69 000/QALY) compared with a no-intervention alternative(Reference Roux, Pratt and Tengs7). In the UK, four types of interventions, including pedometer use, were found to be dominant when compared with ‘usual care’(10). Also, in Australia, intervention programmes that encourage pedometer use and mass-media-based community campaigns were both found to be dominant against current practice for physical activity intervention(Reference Cobiac, Vos and Barendregt9). Our intervention combined both strategies (encouraging pedometer use and a media campaign) and was also found to be dominant.
Some weaknesses concerning the present study need to be discussed. First, low physical activity levels are not only associated with diabetes, CHD, stroke and colon cancer. However, only these were included in the model, as for these the strongest evidence exists associating walking with lower disease risk. Still, low physical activity levels are also an independent risk factor for several other related diseases such as obesity, osteoporosis and depression, which are not explicitly included in the Markov model. People suffering from these diseases will be considered ‘healthy’ in the model, which underestimates the potential impact of low physical activity levels on health. However with regard to obesity, it can be assumed that this condition will be implicitly caught by the other disease states, since it is associated with an increased risk for developing diabetes, CVD and cancer(Reference McGee40). Second, within the current study we accounted only for the total increase in minutes walking, and not for frequency or intensity of walking. Current physical activity guidelines recommend activities of at least moderate intensity – however ‘some activity is better than none’ – whereas frequency is not specified(2). Still, future models could take into account these aspects. Third, it was assumed that the intervention was re-implemented each year (taking into account a survival duration of the pedometers of 5 years), maintaining the same positive effect seen after 1 year of intervention. However, there is no evidence available yet with regard to effects of the intervention change over time. It might be that the effect increases (e.g. more people are reached) when the intervention is implemented year after year, but the positive effect may equally diminish (e.g. people get used to the campaign and show less interest). Hence some additional analyses were conducted taking into account a 5-year intervention effect and a 1-year intervention effect. The results of these additional analyses remained favourable. Fourth, the Markov model was based on some assumptions. The model uses a public payer perspective only assuming direct medical costs. Furthermore, the disease transition and mortality probabilities were based on recent epidemiological publications or national available data. For certain age-dependent incidence rates like diabetes, Belgian data were missing, so information from neighbouring countries was used. In addition, the risk reductions were applied to both genders, even if some were reported for men or women only, unless no evidence was found for a significant risk reduction in a specific gender. Fifth, also the relative risk reductions, disease costs and health-related quality-of-life measures were based on earlier studies. However, the problem of inaccurate or uncertain parameters was countered with two types of sensitivity analyses, which is a major strength of the present study. In these sensitivity analyses, disease and intervention costs, risk reductions, utility levels and the intervention effect were varied separately (one-way sensitivity analyses) and concurrently (probabilistic sensitivity analyses – Monte Carlo). A second strong point is the fact that the present Markov model is dependent on age and gender. Disease state probabilities, mortality probabilities and utility levels were specific for ten different age categories and for both genders. Third, the measures of intervention effects are strong as they are provided by a thorough and high-quality effectiveness study with a quasi-experimental pre–post design. Fourth as mentioned above, different scenarios were conducted where the intervention effect lasted life long, for 5 years and for 1 year. Finally, to our knowledge this is the first study examining the cost-effectiveness of a European community intervention based on ‘10 000 Steps’.
Conclusion
In conclusion, this comprehensive cost-effectiveness analysis suggests that the community intervention ‘10 000 Steps Ghent’ will lead to health benefits and cost savings over a time horizon of 20 years. The present results could help to convince decision makers of the valuable role that community ‘10 000 Steps’ programmes can play in the prevention of chronic diseases, and help to use public health funds appropriately.
Acknowledgements
The study received no specific grant from any funding agency in the public, commercial or not-for-profit sector. No author had interests that might be interpreted as influencing the research or perceived (financial) conflicts of interest related to the research reported in the present manuscript. D.D.S. and K.D.C. contributed equally to the manuscript. K.D.C., I.D.B. and G.C. contributed to the conception and design of the study, while D.D.S. and L.A. designed the statistical analyses and interpreted the data. K.D.C. and D.D.S. led the writing of the paper and wrote the manuscript, while L.A., I.D.B. and G.C. provided significant feedback on the manuscript. Equal first authors D.D.S. and K.D.C. contributed to the conception and design of the study, analysed the data and led the writing of the paper and wrote the manuscript. I.D.B. and G.C. contributed to the study conception and design, and provided feedback on the manuscript. L.A. participated in the statistical analyses (model development) and interpretation of the data.









