According to the WHO, adolescents comprise about 19 % of the world’s population( 1 ). Although this population needs specific attention due to both adolescents’ rapid growth and the influence of their dietary patterns on later life, it is usually neglected( 1 – Reference Delisle 3 ). Dietary intakes during young ages affect physical health and the prevalence of chronic diseases in the future( Reference Krinke 4 ). Several studies have shown that overweight and obese adolescents are more prone to be obese( Reference Serdula, Ivery and Coates 5 , Reference Whitaker, Wright and Pepe 6 ) and to be at risk for CVD( Reference Bibbins-Domingo, Coxson and Pletcher 7 , Reference Falkstedt, Hemmingsson and Rasmussen 8 ) later in adulthood. It is well known that the risk of many chronic diseases is associated with dietary pattern during the adolescent years( Reference Sandler, Slemenda and LaPorte 9 , Reference Weaver, Peacock and Johnston 10 ).
Diet quality indices are an approach to estimate entire diet quality and to assess a person’s adherence to dietary guidelines( Reference Wirt and Collins 11 ). Dietary diversity score (DDS), the Healthy Eating Index (HEI) and mean adequacy ratio (MAR) are a priori-defined diet quality indices.
DDS, often based on a food guide pyramid, focuses on the variety between and within food groups as well( Reference Mirmiran, Azadbakht and Esmaillzadeh 12 ). Regardless of the fact that eating diverse foods is one key universal prescription for health and nutritional fitness, it has been suggested that diversity within food groups may be better indicator of more healthful outcomes( Reference Wirt and Collins 11 ). On the other hand, studies showed that DDS is associated with obesity and abdominal adiposity( Reference Azadbakht and Esmaillzadeh 13 ), metabolic syndrome( Reference Azadbakht, Mirmiran and Azizi 14 ) and cardiovascular risk factors( Reference Azadbakht, Mirmiran and Esmaillzadeh 15 ). Moreover, it has been suggested that DDS is a good indicator of adequacy of some nutrients( Reference Mirmiran, Azadbakht and Azizi 16 ).
The HEI, which was designed by the US government to maintain health status and decrease the risk of chronic disease, was established to evaluate adherence to the Dietary Guidelines for Americans( Reference Feskanich, Rockett and Colditz 17 ). The HEI considers consumption of both healthy and unhealthy foods like fruits, vegetables, SFA and cholesterol( Reference Feskanich, Rockett and Colditz 17 ). Studies have shown an inverse correlation between HEI score and different diseases such as abdominal obesity( Reference Tande, Magel and Strand 18 ), breast cancer( Reference Shahril, Sulaiman and Shaharudin 19 ), pancreatic cancer( Reference Arem, Reedy and Sampson 20 ) and high blood pressure( Reference Haghighatdoost, Sarrafzadegan and Mohammadifard 21 ). Furthermore, HEI score is positively associated with serum folate, vitamin A and vitamin C( Reference Weinstein, Vogt and Gerrior 22 ).
Nutrient adequacy ratio (NAR) and MAR compare the individual’s intake of nutrients with the reference intake. NAR and MAR are specific to age and gender, and therefore may provide more accurate assessment of a person’s diet quality( Reference Rouhani, Mirseifinezhad and Omrani 23 ). Studies have linked NAR and MAR to some unhealthy variables like gastric cancer( Reference Lim, Cho and Kim 24 ), C-reactive protein( Reference Kuczmarski, Mason and Allegro 25 ) and being overweight( Reference Yu and Lee 26 ).
Recent evidence shows that the prevalences of overweight and obesity( Reference Hajian-Tilaki and Heidari 27 ) and the metabolic syndrome( Reference Esmaillzadeh, Mirmiran and Azadbakht 28 ) in Iranian adolescents are relatively high; 15·1 %, 8·3 % and 10·1 %, respectively. Moreover, the nutrition transition that is taking place in Iran results in an imbalance of dietary intake( Reference Ghassemi, Harrison and Mohammad 29 ). Regarding these mentioned issues, the risk status of Iranian adolescents and the importance of nutritional measures become revealed.
To our knowledge, studies assessing diet quality indices have mostly been conducted in the adult population, whereas such studies in adolescents are limited. Therefore the aim of present study was to assess diet quality indices including DDS, HEI, MAR and NAR among Isfahani adolescents.
Materials and methods
Participants
In the present cross-sectional study, female students aged 11–13 years were recruited by a systematic cluster-random sampling method as a representative sample of female adolescents in Isfahan, Iran. At first, to include districts with different socio-economic status, all districts were involved in sampling. Then several schools were randomly chosen from selected districts. Finally, according to the list of student records of each school, participants were randomly chosen by using a computer-based random sequencing program. At the end, 265 students were enrolled for the statistical analysis.
Assessment of dietary intake
To evaluate dietary intake, a self-administered semi-quantitative FFQ that included fifty-three food items was applied. In the previously published paper among this population, the reliability and validity of this FFQ have been proved( Reference Rouhani, Mirseifinezhad and Omrani 23 ).
Dietary diversity score
To score dietary diversity a method introduced by Kant et al. was applied( Reference Kant, Schatzkin and Ziegler 30 ). Five main food groups were divided into twenty-three subgroups which show the dietary diversity. Using twenty-three subgroups instead of five main food groups resulted in counting diversity between and within food groups. In addition, a wider range of DDS (i.e. DDS of 10 points rather than 5 points) may be better for comparison of the lowest with the highest group according to diversity. The bread/grains, vegetables, fruits, meat and dairy food groups were categorized into seven (refined bread, biscuits, macaroni, wholegrain bread, corn flakes, rice and refined flour), seven (vegetables, potato, tomato, other starchy vegetables, legumes, yellow vegetables and green vegetables), two (fruit and fruit juice, berries and citrus), four (red meat, poultry, fish and eggs) and three (milk, yoghurt and cheese) subgroups, respectively. To calculate the score for each food group, the sum of scores of the total subgroups was divided into the number of subgroups in its group and then multiplied by 2. Thus DDS could range between 0 and 10 points.
Healthy Eating Index
The method described by Kennedy et al. was used to calculate HEI( Reference Kenndy, Ohls and Carlson 31 ). To calculate HEI, there are ten different components. The first five components concern the quantity of five groups including grains, vegetables, fruits, milk and meat. The sixth, seventh and eighth components are scored according to the percentage of total fat, SFA and cholesterol intakes. For each component, a maximum score (i.e. 10 points) is given to the diet with <30 % of energy from total fat, <10 % energy from SFA and <300 mg cholesterol. The ninth component concerns dietary variety and the last concerns sodium intake.
Nutrient adequacy ratio
To calculate NAR, the estimated daily individual intake of each nutrient was divided by the standard recommended amount for the participant’s sex and age category according to the nutrient’s Dietary Reference Intake (DRI)( Reference Escott-Stump and Mahan 32 ). In the present study NAR values of ten nutrients including Zn, Fe, Ca, vitamin C, vitamin D, niacin, vitamin B12, riboflavin, vitamin B6 and vitamin A were assessed.
Mean adequacy ratio
MAR was calculated as the ratio of the sum of NAR to the number of nutrients (n 10)( Reference Mirmiran, Azadbakht and Esmaillzadeh 12 ).
Definition of terms
Overweight and obesity were considered according to the WHO guidelines as BMI=85th–95th percentile and BMI > 95th percentile, respectively.
Statistical analysis
For analysing dietary intakes Nutritionist IV software was used. The SSPS statistical software package version 12 was used for statistical analysis. Cut-off points for DDS, HEI and MAR were calculated and results are presented across tertiles of indices as well. Multivariate ANOVA was used to compare means of continuous variables and the χ 2 test was applied to assess the prevalence of overweight/obesity and abdominal obesity across tertiles of indices. ANCOVA that was adjusted for energy intake was used for evaluating nutrient intakes among different tertiles. As energy intake is a major confounder for intake of all nutrients and dietary quality indices, it is better to remove the impact of energy intake to better judge the dietary associations.
Results
Of this adolescent population, 31·3 % were overweight or obese and 38·5 % were abdominally obese. Mean DDS and HEI score were 6·15 (sd 1·61) points and 63·90 (sd 19·86) points, respectively. Mean MAR was 1·32 (sd 0·61); mean of NAR of all nutrients was above 1 except for vitamin D intake (0·53 (sd 0·51)).
Characteristics and energy-adjusted distribution of nutrient intakes (except for energy intake) among participants across the tertiles of DDS, HEI and MAR are presented in Tables 1, 2 and 3, respectively. Those in the higher tertile of DDS had significantly lower BMI, waist circumference and hip circumference. Prevalences of overweight/obesity and abdominal adiposity were significantly higher in the lowest tertile of DDS. There was no significant association between anthropometric variables and distribution of participants across tertiles of HEI. Participants in the highest tertile of MAR had higher BMI and waist circumference. Adolescents in the highest tertile of DDS compared with the lowest tertile had lower intakes of energy, total fat and SFA, and higher intakes of protein, vitamin A, vitamin D, vitamin B6, folate, Zn, K, Ca, Mg and vitamin B12. Those in the highest tertile of HEI had significantly higher intakes of protein, vitamin D, vitamin C, folate, vitamin B12, K, Ca, Mg and Zn, and lower intake of cholesterol. Adolescents in the highest tertile of MAR had higher intakes of energy, protein and cholesterol and lower intakes of total fat and SFA.
Table 1 Characteristics and energy-adjusted distribution of nutrient intakes across tertiles of dietary diversity scoreFootnote * (DDS) among female adolescents (n 265) aged 11–13 years, Isfahan, Iran

* Tertile cut-off points of DDS are as follows: first, ≤4·999; second, 5·000 to 7·999; third, ≥8·000.
† P values resulting from ANOVA for quantitative variables and from χ 2 test for qualitative variables.
‡ Energy was not adjusted for energy intake.
§ Values are presented as mean and standard deviation unless indicated otherwise.
Table 2 Characteristics and energy-adjusted distribution of nutrient intakes across tertiles of the Healthy Eating IndexFootnote * (HEI) among female adolescents (n 265) aged 11–13 years, Isfahan, Iran
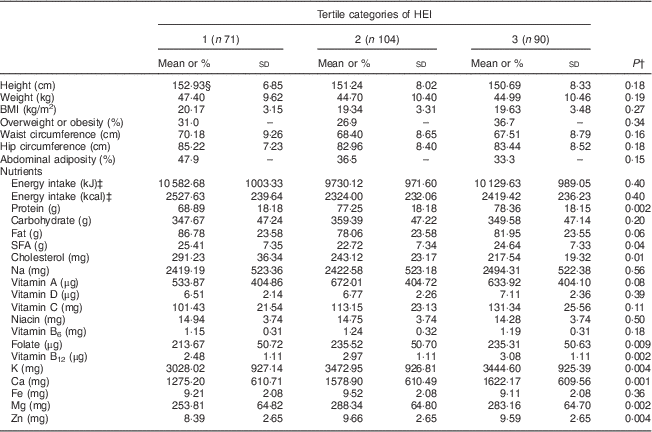
* Tertile cut-off points of HEI are as follows: first, ≤53·999; second, 54·000 to 77·119; third, ≥77·120.
† P values resulting from ANOVA for quantitative variables and from χ 2 test for qualitative variables.
‡ Energy was not adjusted for energy intake.
§ Values are presented as mean and standard deviation unless indicated otherwise.
Table 3 Characteristics and energy-adjusted distribution of nutrient intakes across tertiles of mean adequacy ratioFootnote * (MAR) among female adolescents (n 265) aged 11–13 years, Isfahan, Iran
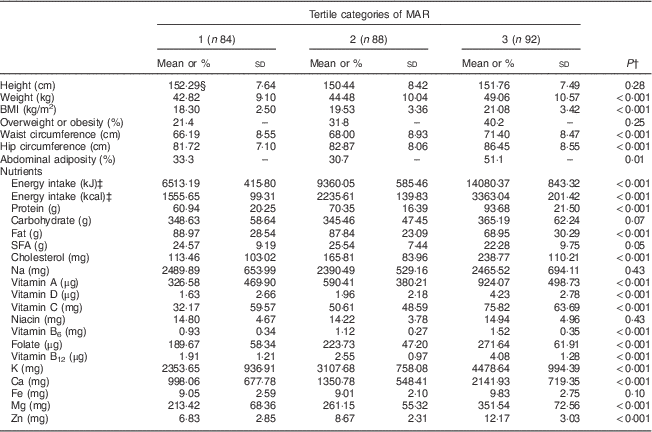
* Tertile cut-off points of MAR are as follows: first, ≤0·979; second, 0·980 to 1·389; third, ≥1·390.
† P values resulting from ANOVA for quantitative variables and from χ 2 test for qualitative variables.
‡ Energy was not adjusted for energy intake.
§ Values are presented as mean and standard deviation unless indicated otherwise.
Discussion
In the present study dietary indices approximately had significant associations with anthropometric variables and nutrient intakes. DDS had the most favourable associations.
One study conducted in Tehran, Iran showed similar mean DDS for adolescents, although 24 h recalls were used in the mentioned study that might be associated with a large amount of under-reporting and over-reporting( Reference Mirmiran, Azadbakht and Esmaillzadeh 12 ). Kersting et al. reported that 50–80 % of 13–16-year-old adolescents had the full score of 5 for DDS; however, that group also used a 24 h recall for assessing nutritional intake( Reference Kersting, Sichert-Hellert and Vereecken 33 ). Different scoring patterns could be the reason for different results. DDS calculated in the present paper has two strengths. The first is that, in contrast to most previous studies, DDS was quantified based on FFQ rather than dietary recall. To evaluate DDS, FFQ is the more applicable instrument since diet diversity is regarded as a long dietary exposure. The second point is that to calculate DDS we derived twenty-three subgroups from five main food groups; counting twenty-three food subgroups resulted in reaching more accurate DDS as it represented the diversity of food groups as well as the diversity within food groups.
The mean HEI score in the present study is consistent with some earlier studies that were carried out with the same method to calculate HEI in this age group( Reference Hurley, Oberlander and Merry 34 , Reference Mirmiran, Azadbakht and Azizi 35 ). One study reported 51·5 (sd 9·07) points as the mean HEI score( Reference Tek, Yildiran and Akbulut 36 ). However, those authors used 24 h recall and HEI-2005 to evaluate dietary intakes. Feskanich et al. introduced the Youth Healthy Eating Index (YHEI) that is a modified HEI to assess diet quality in adolescents( Reference Feskanich, Rockett and Colditz 17 ). Apart from HEI components, YHEI also focuses on foods that are high sources of trans-fatty acids or added sugars or low sources of fibre. Therefore YHEI can range from 0 to 100 points and consists of thirteen components including whole grains, vegetables, fruits, dairy, meat ratio, snack foods, soda and drinks, multivitamin use, margarine and butter, fried foods outside home, visible animal fat, and eat breakfast and dinner with family; components 1 to 7 and 8 to 13 score up to 10 and 5 points, respectively( Reference Feskanich, Rockett and Colditz 17 ). In their study on 16 452 participants aged 9–14 years, mean HEI was 68·9 to 73·2 points, whereas mean YHEI was 58·6 and 59·6 points (for boys and girls, respectively). Information regarding the trans-fat content of foods is not available in Iran, so it is impossible to calculate YHEI scores unless accounting for trans-fat sources instead of trans-fat content of foods.
In a study with 2855 Spanish subjects aged 2 to 24 years, most NAR were 1·0 or below but near 1, except for vitamin D (0·36), vitamin E (0·66) and vitamin A (0·67) which had the lowest NAR( Reference Serra-Majem, Ribas-Barba and Pérez-Rodrigo 37 ). In the present study also vitamin D and vitamin A had the lowest NAR values but both were above 1. In addition, it should be noted that in Serra-Majem et al.’s study there was a wide variation in energy and nutrient intakes by age group, among which the 14–17-year-olds and then 10–13-year-olds were at higher risk for inadequate nutrient intakes. So it could be concluded that adolescents had the lower NAR compared with children and young adults. With regard to excluding just under-reporters in the previous study, higher NAR of the present study might be conflicting to some extent.
In our study population, those in the highest compared with the lowest tertile of the three indices had higher protein and carbohydrate intakes, and lower fat and SFA intakes. In contrast, cholesterol pattern was inconsistent between the three dietary indices; cholesterol intake was higher in the third tertile of DDS and MAR whereas across tertiles of HEI cholesterol intake decreased. These could be explained by components of scoring, as DDS was calculated based on sources of protein and carbohydrate but sources of fat did not count in diversity scoring. Moreover, sources of cholesterol like eggs are included in DDS; meanwhile, main sources of SFA such as hydrogenated oil and butter did not enter into the scoring pattern. Participants in the third tertile of HEI had lower fat, SFA and cholesterol intakes in comparison to the lowest tertile because the mentioned items are independent components of the HEI. Patterns observed across tertiles of MAR could be probably attributed to the sources of the evaluated vitamin; for example, meat is the source of Zn and Fe and the highest tertile of MAR received more protein, or although eggs are a source of vitamin A they contain a high amount of cholesterol.
There are some limitations to the present study. To evaluate dietary intakes an FFQ with fifty-three food items was applied; it may not reflect the exact intakes to some extent. However, the reliability and validity of this questionnaire were proved. Results of the present study may not accurately extrapolate to all Iranian adolescents due to small sample size as well as single gender. In addition, the age range is narrowed down to 11–13-year-old girls. All three indices assess overall diet quality while each index considers a different aspect of diet. Generally, DDS is more focused on the dietary diversity, HEI is focused on food intakes and MAR assesses the micronutrient intakes. MAR is important because the adequacy of each micronutrient is necessary for metabolic pathways in the body. On the other hand, due to the interaction between different components of diet, more comprehensive observations should be considered like HEI and DDS. Therefore regarding these three indices together could result in a better judgement of diet quality.
Conclusion
The quality of Iranian adolescents’ diet needs improvement; indeed, dietary quality is the major problem, not dietary quantity, in Isfahani adolescents.
Acknowledgements
Acknowledgements: The authors are grateful to the participants of the present study for their enthusiastic cooperation. Sources of funding: This study was funded by the Food Security Research Center, Isfahan University of Medical Sciences (IUMS), Isfahan, Iran. The authors would like to express their appreciation to the Research Council of IUMS for financial support of the study. The Research Council of IUMS had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: L.A. and A.E. designed the study and collected the data. F.A. wrote the manuscript. L.A. analysed the data and read the draft of the manuscript, commented on it and revised it. All authors read the final version of the manuscript and approved the final version. Ethics of human subject participation: The Ethical Committee of IUMS has approved this study.





