During the 20th century there was a dramatic fall in the age at menarche (AAM), widely attributed to improvements in nutrition. However, during the last 30 years or so there has been a levelling and even a slight reversal of this trend, despite increasing rates of childhood obesity. Earlier menarche is associated with an increased risk of developing breast cancer(Reference Gao, Shu and Dai1–Reference Kvale and Heuch3), possibly as a result of increased lifetime exposure to oestrogens. Studies have found that women who started menstruating at or before 12 years of age have an odds ratio for breast cancer of about 1·5–2·0 compared with those started aged 15 years or more(Reference Tavani, Gallus and La Vecchia4, Reference Butler, Potischman and Newman5). Early menarche may also be associated with an increased risk of ovarian cancer and IHD(Reference Cooper, Ephross and Weinberg6), but is protective against osteoporosis(Reference Fox, Magaziner and Sherwin7), a major cause of morbidity and mortality. Identifying factors associated with early menarche may promote a better understanding of the aetiology of these diseases, and understanding the role of modifiable factors such as diet is particularly important.
Although it is known that anorexia nervosa and undernutrition can delay menarche(Reference Dreizen, Spirakis and Stone8, Reference Satyarayana and Nadami Nadu9), the relationship between normal ranges of childhood dietary intakes and AAM is unclear. Associations observed between energy intake and AAM are variable(Reference Moisan, Meyer and Gringras10–Reference de Ridder, Thijssen and Van’t Veer15) and hard to compare between studies due to inconsistent adjustment for body size and for under-reporting by obese subjects. Fat intake affects oestrogen metabolism(Reference Longcope, Gorbach and Goldin16) and has been suggested as a possible risk factor for breast cancer, thus potentially providing a link with AAM, but there is no consistent evidence that intakes of either total fat or different types of fat are associated with AAM(Reference Moisan, Meyer and Gringras10, Reference Petridou, Syrigou and Toupadaki12–Reference Merzenich, Boenig and Wahrendorf14). Dietary fibre intakes relate to plasma oestrogen concentrations(Reference Goldin, Adkercreutz and Gorbach17, Reference Adlercreutz, Höckerstedt and Bannwart18) and international variations in fibre intake correlate with differences in AAM(Reference Hughes19). Foods suggested to affect breast cancer risk and therefore of interest in relation to possible associations with AAM include meat(Reference Lubin, Burns and Blot20) and soya products. Soya is a rich source of phyto-oestrogens, which may exert either pro- or anti-oestrogenic effects. Various micronutrients(Reference Moisan, Meyer and Gringras10, Reference Britton, Wolff and Lapinski21), vegetarian diets and nuts and seeds(Reference Soriguer, Gonzalez-Romero and Esteva22) have also been implicated in the timing of puberty.
Most existing studies of diet and menarche have measured diet close to or after the occurrence of menarche, making it hard to draw conclusions about the causality of any observed relationships with diet. The critical period for diet to impact on pubertal timing may be many years earlier, as suggested by the recently demonstrated associations between early growth and AAM(Reference Sloboda, Hart and Doherty23, Reference Dos Santos Silva, De Stavola and Mann24). Many studies lack information on important potential confounding factors. Moreover, most have investigated macronutrient intakes only and few present information on the intakes of micronutrients or food groups.
The aim of the current study was to relate dietary intakes measured prospectively throughout childhood to the occurrence of menarche in a large population of girls in South-West England.
Methods
Study population
The Avon Longitudinal Study of Parents and Children (ALSPAC) is an ongoing prospective cohort study recruited from all pregnancies in three Bristol-based District Health Authorities with expected dates of delivery between April 1991 and December 1992. A total cohort of 14 541 pregnancies resulted. Ethical approval for the study was obtained from the ALSPAC Law and Ethics Committee and the local research ethics committees. More detailed information on the study(Reference Golding, Pembrey and Jones25) is available on the ALSPAC website (http://www.bristol.ac.uk/alspac). Data have been collected on the parents and their children primarily using questionnaires alongside medical records, biological samples and clinical data. From the age of 7 years all children have been invited to regular research clinics for a variety of clinical assessments. The present study relates to AAM and so looks at girls only.
Assessment of age at menarche
Data on whether or not the girls had started menstruating was collected at clinics run when they were aged about 11·5 years and about 12·5 years. The latter was held between February 2004 and December 2005; the girls were asked during a measuring session in a private room if they had started menstruating, and if so when.
In total 3751 girls attended the clinic at 12·5 years. Mean (sd) age of attendance at the clinic was 12·89 (0·23) years, ranging from 11·30 to 14·34 years. Data were available from 3298 girls on whether or not they had reached menarche; of these 1637 (50 %) stated that they had not. Of the 1661 who had reached menarche, age at first period was available for 1550 and for 1328 white girls from singleton births. Due to the range of ages at which the girls attended the clinic and the fact that 50 % of girls had not yet reached menarche on attending, it was not possible to determine median age at menarche. Thus 12 years 8 months was chosen as a cut-off point for AAM which was likely to be as near as possible to the median, i.e. old enough to maximise the number of girls in the ‘yes’ group for menarche, but young enough to minimise the amount of missing data resulting from girls who had not yet started menstruating but attended the clinic at ages younger than the cut-off point. Figure 1 shows the distribution of age at first period among those girls who reached menarche by 12 years 8 months (n 1043). Girls reaching menarche after 12 years 8 months do not appear in this figure, as data on AAM later than 12 years 8 months are available only on some of those girls attending the clinic late. Some descriptive characteristics of girls with and without data on AAM are given in Table 1. Compared with the whole cohort, girls with data on AAM were less likely to have a mother whose periods started before 12 years of age, a mother with no educational qualifications and a father in social class IV or V. They had higher birth weight, but were shorter and had a lower BMI at 10 years. They were more likely to have been breast-fed and less likely to have received solid foods before 3 months of age, and had a marginally higher energy intake at 10 years.
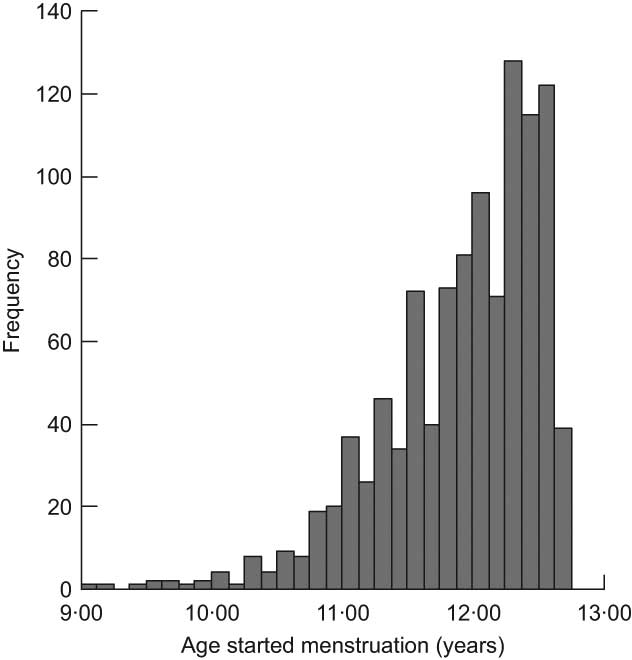
Fig. 1 Distribution of age at menarche among girls who had reached menarche by 12 years 8 months, Avon Longitudinal Study of Parents and Children, Bristol, South-West England
Table 1 Descriptive characteristics of girls with and without data on age at reaching menarche (baseline group for comparison = live births in the ALSPAC cohort), Avon Longitudinal Study of Parents and Children, Bristol, South-West England

*Lower level national school exams at age 16 years.
†Higher level national school exams at age 16 years.
‡A level (national school exams at age 18 years) or university degree.
§P value by t test.
∥P value by χ 2 test.
Assessment of diet
The dietary information used in the present study was collected by two methods: 3 d dietary record and FFQ.
The 3 d dietary records were collected from the whole cohort between February 2002 and October 2003 when the child was aged 10–11 years. Around a week before the child was due to visit a research clinic at the age of 10·5 years, a 3 d food diary was sent to the child to complete at home (for one weekend day and two weekdays) and bring to the clinic. The diary was checked by a nutritionist in the clinic for completeness and clarity, with the child and usually a parent. If the child had not completed a diary, a single 24 h recall was administered in the clinic. The diet records were coded using DIDO (Diet In, Data Out), a coding package developed by the MRC Human Nutrition Research Unit in Cambridge and adapted for use in coding children’s diets(Reference Price, Paul and Key26). The coded data were converted to nutrient intakes using a database consisting of the fifth edition of McCance & Widdowson’s The Composition of Foods and supplements(Reference Holland, Welch and Unwin27–Reference Chan, Brown and Church36), augmented with manufacturers’ information and information from the nutrient database used by the National Diet and Nutrition Survey.
Dietary information on the whole cohort was also collected by FFQ at 3 years (38 months) and 7 years (81 months), which were completed by the child’s main carer. The questionnaires included questions about the weekly frequency of consumption of about sixty food groups and food items; there were five frequency options ranging from ‘never or rarely’ to ‘more than once a day’. Standard portion sizes were used. These questionnaires were used to estimate nutrient intakes by multiplying the weekly frequency of consumption of each type of food by its estimated nutrient content, and summing this across all foods consumed. FFQ giving unrealistic estimates of any nutrient intakes were excluded from analyses. The questionnaires used are available on the ALSPAC website. The FFQ were validated by comparison with the results of 3 d food records taken on a 10 % sub-sample of the cohort at 3 years and 7 years. Similar mean nutrient intakes were obtained by both methods, and correlations between nutrient intakes obtained by both methods were generally about 0·3 for absolute nutrient intakes and about 0·4 for nutrient intakes per MJ (see Appendix 1 and Appendix 2).
Other variables
Data were also collected on several other variables considered to be potential confounders of any associations between diet and menarche. These included child’s ethnic group, maternal educational level, paternal occupational social class, maternal smoking during pregnancy and maternal AAM (assessed by self-completion postal questionnaires sent to the mother during pregnancy), breast-feeding duration and age of introduction to solids (assessed by questionnaires sent to the mothers when the child was 6 months and 15 months old), and birth weight (extracted from routine hospital birth records). Ethnic group was classified as white/non-white; the non-white group of girls was too small (n 109) and ethnically heterogeneous to allow a more detailed classification of ethnic group. The other variables were classified as shown in Table 1. Height and weight were measured at the 10 years clinic and at the 7 years clinic held between September 1998 and September 2000 when the children were aged 7–8 years. BMI was calculated as weight (in kilograms) divided by the square of height (in metres).
Among white singleton girls on whom data on whether or not menarche had occurred by 12 years 8 months were available, data on maternal education were also available for 98 %, on paternal social class for 88 %, on parity for 97 %, on maternal age at menarche for 98 %, on breast-feeding duration for 94 %, on age at introduction of solids on 95 %, and on height and BMI at 10 years for 92 %. The number included in each analysis is given in the tables.
Statistical methods
The girls were divided into groups according to whether or not they had reached menarche by 12 years 8 months. Girls younger than 12 years 8 months at the second clinic visit who were still pre-menarche were excluded from the analyses.
Initial analyses suggested that the proportion of white and non-white girls reaching menarche by 12 years 8 months differed (χ 2 = 3·50, P = 0·061), with more non-white than white girls reaching menarche by this age (49·5 % v. 40·6 %). As the total number of non-white girls (n 109) was too small to analyse separately, all subsequent analyses were restricted to white girls from singleton births.
The relationship between nutrient intakes and AAM was investigated initially by comparing nutrient intakes in girls who had and had not reached menarche using unpaired t tests. Where necessary, nutrient intake distributions were transformed to the natural logarithm or the square root in order to minimize skewness in the distribution. Nutrient intakes (other than energy) were adjusted for energy intake using the residuals method(Reference Willett and Stampfer37). For those nutrient intakes where there was some evidence of an association with AAM (P < 0·10), the odds ratios for menarche associated with a 1 sd increase in energy-adjusted nutrient intake were calculated using binary logistic regression, with and without adjustment for potential confounders. Nutrient intakes investigated were energy, total fat, SFA, MUFA and PUFA, starch, sugar, total, animal and vegetable protein, NSP (a measure of fibre intake), Fe, Ca, Zn, Mg and carotene.
The association between food intakes at 3 years, 7 years and 10 years and the occurrence of menarche by 12 years 8 months was investigated initially using the χ 2 test. At 3 years and 7 years food intakes were grouped by frequency of consumption, and at 10 years by mean daily amount consumed. Food variables investigated included intakes of fruit, vegetables, total and oily fish, meat, dairy products, soya meat/textured vegetable protein, legumes and whether or not the child ate a vegetarian diet (if no meat or fish consumption was reported in the FFQ or food diary, then the child was considered to be a vegetarian). The effect of potential confounders on any associations with food intake found in univariate analyses was investigated using binary logistic regression. To exclude the possibility that any observed relationships with diet merely reflected variation in body size, we also adjusted any associations found with diet at 10 years for height and BMI measured at 10 years, and any associations found with diet measured at 7 years for height and BMI measured at 7 years. No suitable measures of body size were available close to the 3 years diet measurements.
Analyses using dietary record data at 10 years were repeated excluding those children considered to be possible under-reporters on the basis of the agreement between estimated energy requirements (EER) based on age and body weight(Reference Torun38) and reported energy intake (REI)(Reference Rennie, Coward and Jebb39); under-reporters were defined as subjects with a ratio of REI:EER of less than 78 %. (Only 3·6 % of the sample were considered to be possible over-reporters, i.e. an REI of >122 % of EER.) Under-reporting was more likely among overweight children and children of less-educated mothers. Analyses were also repeated excluding children for whom only 24 h recall data were available.
Results
Food and nutrient intakes throughout childhood and occurrence of menarche
Table 2 shows mean nutrient intakes according to the occurrence of menarche by 12 years 8 months, and Table 3 shows the adjusted and unadjusted odds ratios for menarche per 1 sd increase in nutrient intake at 3, 7 and 10 years. Only those nutrients where there was some evidence (P < 0·10) of an association with menarche are shown (full tables of results are available from the corresponding author). Adjustment for potential confounders generally had a minimal effect on any associations found.
Table 2 Mean/geometric mean energy and energy-adjusted nutrient intakes (and 95 % confidence intervals) throughout childhood according to whether menarche was reached before or after 12 years 8 months, Avon Longitudinal Study of Parents and Children, Bristol, South-West England
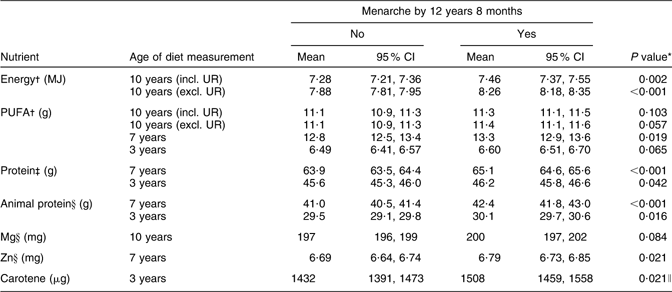
UR, under-reporters.
Sample size according to whether menarche was reached before or after 12 years 8 months: n 1486 for ‘no’ and n 1014 for ‘yes’ including UR, and n 1067 for ‘no’ and n 619 for ‘yes’ excluding UR, for diet at 10 years; n 1419 for ‘no’ and n 951 for ‘yes’ for diet at 7 years; n 1480 for ‘no’ and n 994 for ‘yes’ for diet at 3 years.
*P value by t test.
†Geometric mean shown, as nutrient intakes transformed to the natural logarithm before analysis.
‡Geometric mean shown, as nutrient intakes at 7 years transformed to the square root and nutrient intakes at 3 years transformed to the natural logarithm before analysis.
§Geometric mean shown, as nutrient intakes transformed to the square root before analysis.
∥P value by Mann–Whitney U test.
Table 3 Odds ratios (and 95 % confidence intervals) for reaching menarche by 12 years 8 months per 1 sd increase in energy and energy-adjusted nutrient intakes at 10 years, 7 years and 3 years, Avon Longitudinal Study of Parents and Children, Bristol, South-West England
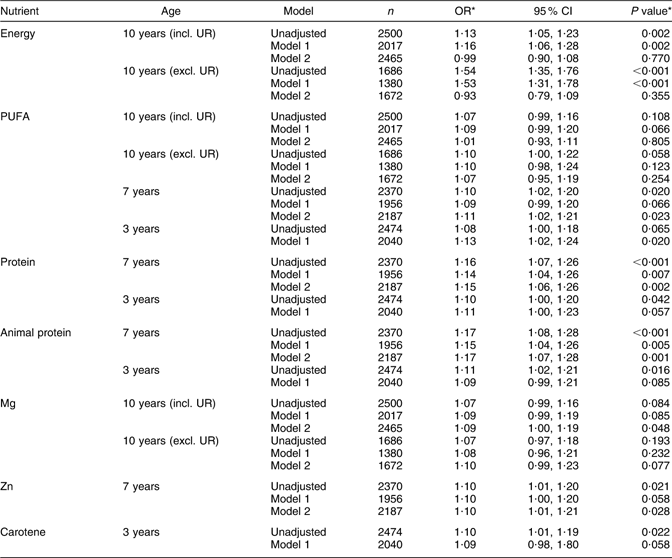
UR, under-reporters.
Model 1: adjusted for maternal education, paternal occupational social class, maternal smoking in pregnancy, maternal age at menarche, parity, duration of breast-feeding, age of introduction to solids and birth weight.
Model 2: adjusted only for height and BMI at time of diet measurement.
*OR and P value obtained by binary logistic regression.
Energy intakes at 10 years were higher in girls reaching menarche by 12 years 8 months, as might be expected given their greater height and weight at 10 years. Energy intake was also strongly positively associated with the OR for menarche. These associations with energy intake were strengthened on excluding under-reporters (adjusted OR: 1·16 per 1 sd increase in energy intake at 10 years, rising to 1·54 on exclusion of under-reporters), but were eliminated on adjusting for height and BMI at 10 years. Energy intakes at 3 years and 7 years were not associated with AAM.
PUFA intakes at 7 years and 3 years were marginally higher among girls who reached menarche by 12 years 8 months, and were also marginally higher at 10 years on excluding under-reporters. PUFA intakes at 10 years, 7 years and 3 years were also weakly positively associated with the OR for menarche. Adjusting for height and BMI eliminated the association with PUFA at 10 years. There was no evidence of an association with intakes of total fat, SFA or MUFA.
Total and animal protein intakes at 3 years and 7 years, but not 10 years, were higher among girls who reached menarche by 12 years 8 months and were also positively associated with the OR for menarche. These associations were strongest for protein intakes at 7 years. There was no evidence for an association with vegetable protein intake, or that starch, sugar or NSP intakes at any age were associated with AAM.
Mg intakes at 10 years were marginally higher among girls who reached menarche by 12 years 8 months, and were weakly associated with the OR for menarche. Zn intakes at 7 years were higher among girls reaching menarche by 12 years 8 months, and were positively associated with the OR for menarche, and the same was true for carotene intakes at 3 years. There was no association with intakes of these nutrients at other ages or with Ca or Fe intakes at any age.
The associations between intakes at 7 years of PUFA, protein, animal protein and Zn and occurrence of menarche were minimally altered by adjustment for height and BMI at 7 years.
Exclusion of children with 24 h recall data only on diet at 10 years had a minimal effect on associations between dietary intakes and AAM.
Table 4 shows the incidence of menarche by 12 years 8 months according to food intakes throughout childhood and the OR for reaching menarche by category of food intake. Only those foods where there was some evidence (P < 0·10) of an association with menarche are shown (full tables of results are available from the corresponding author). Few associations between food intake and menarche were observed, but meat intakes at both 7 years and 3 years were strongly positively associated with the occurrence of menarche. At 7 years, the proportion reaching menarche by 12 years 8 months increased from 35·2 % among those eating less than 4 portions of meat per week to 48·8 % among those eating more than 12 portions, with the OR for menarche being 1·75 in those consuming more than 12 portions compared with <4 portions. There was also weak evidence that higher vegetable consumption at 3 years was positively associated with the occurrence of menarche.
Table 4 Odds ratios (and 95 % confidence intervals) by binary logistic regression for reaching menarche by 12 years 8 months according to levels of food group consumption at 7 years and 3 years, Avon Longitudinal Study of Parents and Children, Bristol, South-West England
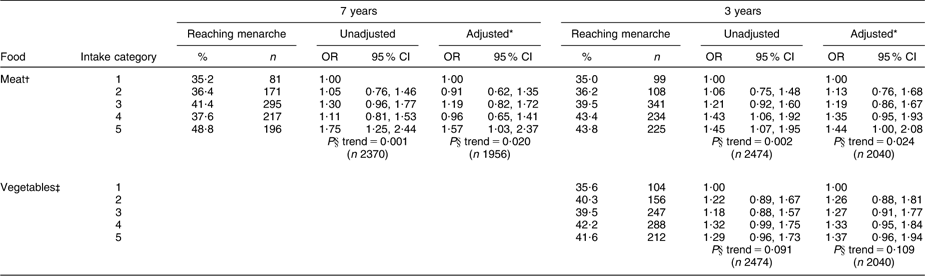
*Adjusted for energy intake, maternal education, paternal occupational social class, maternal smoking in pregnancy, maternal age at menarche, parity, duration of breast-feeding, age of introduction to solids and birth weight.
†Intake categories for meat: at 7 years, 1 = <4 portions/week, 2 = 4–7 portions/week, 3 = >7–9 portions/week, 4 = >9–12 portions/week, 5 = >12 portions/week; at 3 years, 1 = <3 portions/week, 2 = 3–4 portions/week, 3 = >4–6 portions/week, 4 = >6–8 portions/week, 5 = >8 portions/week.
‡Intake categories for vegetables: at 3 years 1 = <3 portions/week, 2 = 3–6 portions/week, 3 = >6–<10 portions/week, 4 = 10–<14 portions/week, 5 = ≥14+ portions/week.
§P for linear trend using food intake category as a covariate.
There was no evidence that the incidence of menarche differed according to the intake at any age of fruit, total fish, oily fish, dairy products, soya products and legumes or between vegetarians and non-vegetarians.
These associations remained similar on adjustment for potential confounders and for body size around the time of diet measurement.
Discussion
In this large group of contemporary girls we have found a number of associations between dietary intakes throughout childhood and the occurrence of menarche. We have confirmed previous findings of higher energy intakes among girls reaching menarche earlier, reflecting their larger body size. We have also found evidence that intakes of meat and total and animal protein, and also possibly PUFA in early to mid-childhood may increase the chances of menarche by 12 years 8 months. However, we found no evidence that the chances of reaching menarche increased with higher total fat intakes, or reduced with higher intakes of fruit, vegetables or NSP. Unexpectedly, higher vegetable intakes at 3 years were associated with an increased chance of reaching menarche, although this may have reflected the positive association between meat and vegetable intakes at 3 years.
Strengths of our study include the large sample size and the prospectively collected data on diet in early and mid-childhood as well as at 10 years, close to the time of menarche. Most other studies have collected data on diet only close to or after menarche, but the associations between early growth and pubertal development(Reference Sloboda, Hart and Doherty23, Reference Dos Santos Silva, De Stavola and Mann24) suggest that the critical exposures predicting puberty may occur earlier in childhood. In addition, measuring diet close to the time of puberty introduces the possibility of reverse causation, whereby puberty may affect dietary intakes rather than vice versa. This may have been an issue with our 10 years dietary data as many of the girls would have entered puberty by this point. A further strength is the wide range of foods and nutrients examined. Conversely, this will have increased the risk of false positive findings. However, several of the associations between dietary intakes and the occurrence of menarche were found for more than one age point throughout childhood, increasing their plausibility. It was also possible to control for a large range of potential confounding variables. This generally had a minimal effect on the strength of the associations, suggesting that the observed relationships with diet were unlikely to reflect unmeasured confounding.
Weaknesses of the study include the fact that the last point at which we were able to assess AAM was around 12 years 8 months, so we were unable to look at effects on mean AAM or create a ‘late’ menarche group. In addition the method of dietary assessment at 10 years differed from the method at 3 years and 7 years, limiting our ability to compare the results obtained at different time points. The dietary data at 3 years and 7 years were by obtained an unquantified FFQ and so results for energy intake at these ages should be treated with caution, although FFQ have a reasonable ability to rank individuals for energy-adjusted nutrient intakes and should discriminate well for frequency of consumption of different foods. Information on diet at 10 years was collected by a 3 d unweighed food record. Unweighed food records have been shown to compare well with the results from weighed intakes(Reference Bingham, Gill and Welch40), generally considered to be the ‘gold standard’ of dietary assessment, and results obtained by this method at earlier ages in ALSPAC have plausible relationships with biological outcomes such as Fe status(Reference Cowin, Emond and Emmett41) and insulin-like growth factor-1 levels(Reference Rogers, Gunnell and Emmett42). There was a relative lack of data on girls from lower socio-economic groups which may have restricted the range of dietary intakes observed and limited our ability to detect associations between diet and AAM. However, unless the observed associations differed according to social class, it is unlikely to have affected the generalizability of our results.
A study with comparable methods to our own was that of Berkey et al.(Reference Berkey, Gardner and Frazier43), who studied sixty-seven Caucasian girls born in Boston in the 1930s and 1940s. Diet was prospectively assessed at ages 1–2, 3–5 and 6–8 years. AAM was related to intakes of energy and energy-adjusted intakes of fat, animal protein and vegetable protein. No association with energy or fat intake throughout childhood was found, but there was a negative association between mean AAM and animal protein intakes at 3–5 and 6–8 years, and a positive association with vegetable protein intakes at 3–5 years. These results are closely comparable to our own, despite the fact that the subjects were born during the Depression and maturing during World War II.
Several early studies also suggested that meat or protein intake had a menarche-promoting effect. In 1950 Kralj-Cercek(Reference Kralj-Cercek44) reported on a study of Slovenian girls whose diet was classified as ‘proteinous’, ‘mixed’ or ‘carbohydratic’, and found that the mean AAM increased from 12·7 years in the ‘proteinous’ group to 14·1 years in the ‘carbohydratic’ group. This association with diet seemed to be independent of the effect of ‘body build’. Two studies of American girls in 1970(Reference Sanchez, Kissinger and Phillips45) and 1987(Reference Kissinger and Sanchez46) found vegetarianism(Reference Sanchez, Kissinger and Phillips45) and higher consumption of ‘meat analogues’ or ‘nuts and beans’(Reference Kissinger and Sanchez46) to be associated with delayed menarche, and meat intake in the upper v. the lower quartile to be associated with reaching menarche 6 months earlier. However, information on dietary assessment methods in these studies was scant, and there was no control for energy intake or anthropometry in the analyses. A recent study of AAM in North Korean refugees also reported that women stating a ‘food preference’ for meat, fish and dairy reported a lower AAM than those with a food preference for rice and starch or fruit and vegetables(Reference Ku, Kang and Kim47). In a cross-sectional study of 8–16-year-old Spanish schoolgirls published in 1995, Soriguer et al.(Reference Soriguer, Gonzalez-Romero and Esteva22) also found that higher intakes of nuts (and seeds) were associated with a reduced risk of menarche, but found no association with meat intake. A large retrospective study of UK vegetarians in the 1990s found no difference in AAM between life-long vegetarians and those becoming vegetarian as adults(Reference Rosell, Appleby and Key48). Several other later studies which measured protein intake around the time of menarche found little evidence of an association(Reference Moisan, Meyer and Gringras10–Reference de Ridder, Thijssen and Van’t Veer15).
These differing results may stem from the relationship between meat and protein intake in the samples studied. It is possible that in historical cohorts (and the North Korean group) lower meat intake would have been associated with greater reductions in protein intake than today, when meat intake is more likely to be replaced with other protein sources. In our study, vegetarians had lower protein intakes, and meat and protein intakes were positively correlated. However, there was no difference between vegetarians and omnivores in the proportion reaching menarche, suggesting that the effect of meat or protein may be manifested at higher intakes and obscured by grouping all meat eaters together. A menarche-promoting role of meat/animal protein would seem logical from an evolutionary standpoint. Meat is a good source of bioavailable Zn and Fe, requirements for which are high during pregnancy, and deficiencies of Zn and Fe are common among pregnant women in developing countries(Reference Huddle, Gibson and Cullinan49, Reference Bothwell50). A meat-rich diet could be seen as indicating suitable nutritional conditions for a successful pregnancy. The secular trends in AAM in the West over the last century might reflect the secular trends in meat consumption.
In summary, in this contemporary population there was evidence that higher intakes of meat and protein might have a menarche-promoting effect, and that diet in early to mid-childhood may be more strongly associated with the occurrence of menarche than diet in later childhood. This implies that dietary alterations in early to mid-childhood might affect menarcheal timing, and possibly breast cancer and osteoporosis risk in later life. However, these findings need to be repeated in other populations before any recommendations for dietary change can be made.
Acknowledgements
Sources of funding: The UK Medical Research Council (grant ref. 74882), the Wellcome Trust (grant ref. 076467) and the University of Bristol provide core support for ALSPAC. The work on diet is further supported by the Arthritic Association. The present work was funded by a grant from the World Cancer Research Fund (grant ref. 2002/22). Conflict of interest declaration: None of the authors had any conflicts of interest. Authors’ responsibilities: I.S.R. coordinated the study, developed the original hypotheses and undertook the writing of the manuscript and some of the analysis. K.N. undertook some of the analysis and assisted in the writing of the manuscript. P.M.E., D.B.D., A.R.C. and A.R.N. helped to develop the hypotheses and assisted in the writing of the manuscript. Acknowledgements: The authors are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
Appendix 1
Spearman correlations (ρ) between nutrient intakes obtained by FFQ and 3 d food record at 3 years (450 boys, 360 girls)
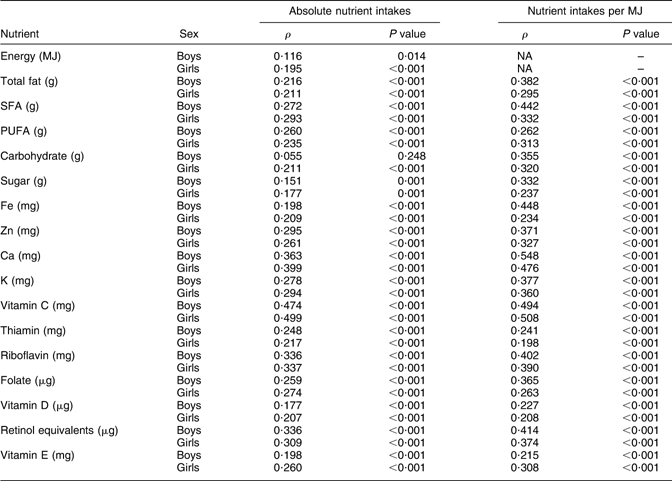
NA, not applicable.
Appendix 2
Spearman correlations (ρ) between nutrient intakes from food records and FFQ in 7-year-old children (n 730)
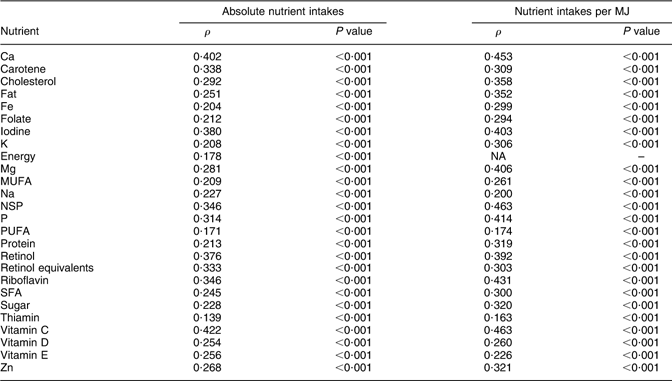
NA, not applicable.







