The nutrition transition with progressive Westernisation of diets and sedentary lifestyles, propelled by socio-economic and technological development as well as globalisation and accelerated urbanisation, has led developing countries, and particularly their urban areas, to experience a rise of non-communicable diseases(Reference Drewnowski1–Reference Popkin3). Among these non-communicable diseases, CVD and diabetes as cardiometabolic risk factors (CMRF)(Reference Despres, Cartier and Cote4) and the metabolic syndrome (MetS) have been recognised to be directly related to nutrition(5). CMRF show an escalating trend in most developing countries and are becoming leading contributors to the burden of diseases, death and disability(Reference Abegunde, Mathers and Adam6, Reference Misra and Khurana7). A recent meta-analysis reported that over a period of 15 years (1990 to 2005), the trend of obesity prevalence in West Africa increased by 114 %, with urban women being 4·79 times more affected than men(Reference Abubakari, Lauder and Agyemang8). Although studies on CMRF in Sub-Saharan Africa are scarce, they suggest that CMRF prevalence rates are similar to those reported in Western countries, with for instance hypertension reaching 29·4 % and 40·2 % in urban adults in Ghana and Burkina Faso, respectively(Reference Agyemang9, Reference Niakara, Fournet and Gary10), and diabetes reaching 6·8 % in Nigeria and 6·3 % in Ghana(Reference Abubakari, Lauder and Jones11).
Meanwhile, global and micronutrient malnutrition remains highly prevalent, even in adults. Indeed, studies in Sub-Saharan Africa have reported that more than 20 % of women have BMI < 18·5 kg/m2, 57·1 % are anaemic and 18·5 % are deficient in vitamin A(Reference Black, Allen and Bhutta12, Reference West13).
Research usually focuses on either malnutrition or CMRF, seldom on both. Evidence suggests that the nutrition transition in developing countries is occurring rapidly, which contributes to the double burden of malnutrition, i.e. the overlap of CMRF and malnutrition within the same population group, the same household or the same individual(Reference Asfaw14–Reference Popkin17). Few studies have addressed the issue of this double nutritional burden, and they have primarily considered the overlap of overweight/obesity and undernutrition in populations as a whole(Reference Mendez, Monteiro and Popkin18, Reference Monteiro, Conde and Popkin19) or within households(Reference Barquera, Peterson and Must15, Reference Doak, Adair and Bentley16). The double burden of malnutrition and CMRF in individuals has been little studied(Reference Eckhardt, Torheim and Monterrubio20, Reference Vorster and Kruger21), particularly in Sub-Saharan Africa where it is poorly documented(Reference van der Sande, Ceesay and Milligan22).
Since WHO recognised non-communicable diseases as an epidemic in the developing world, public health concern has increased. However, the need to halt the epidemic should not be at the expense of nutritional deficiency disorders. The challenge of these programmes is especially complex in settings where epidemiological data related to population-attributable risk for various factors are not readily or reliably available at present. Relevant research in a country-specific manner should be implemented to comprehend the dimensions, as well as the dynamics, of the double burden of malnutrition. A cross-sectional study carried out in Ouagadougou, Burkina Faso was designed to assess cardiometabolic risk and nutritional deficiency markers in adults. One of the hypotheses was that the double burden of malnutrition is frequent, particularly among women. The present paper reports on the double burden phenotypes and occurrence according to sociodemographic parameters.
Methods
Population and sample
The study was carried out in 2010 in the northern part of Ouagadougou where a population observatory has been in operation since 2008, with periodic collection of socio-economic, sociodemographic and health data in a population sample of 80 000 people. This part of the capital city is a vulnerable area according to data from national and international institutions(23). The study sample of 330 adults aged 25–60 years and stratified by income was selected using the observatory database. The availability of data on this part of Ouagadougou such as the household identification, socio-economic and demographic data argued for our study location. The database included 13 021 households with at least one individual between 25 and 60 years of age. A proxy of household income was derived using principal component analysis, with twelve discriminatory household asset variables (ownership of house, telephone, television, DVD, fridge, motorbike, car, type of household toilet, electricity, type of cooking fuel, and type of floor, roof and walls). Households were split into three tertiles of this income proxy. For each tertile, 110 households were randomly selected, with fifty additional individuals as alternatives. Only one individual per household was enrolled. The field team consisted of a medical doctor (the first author) and an experienced laboratory technician and two research assistants trained by the first author.
Eligible participants were born-Burkinabè adults aged 25 to 60 years who had been living in Ouagadougou for at least 6 months and would remain there until the end of the study. Persons with prior hypertension or diabetes were retained in the study. Pregnant or lactating women, as well physically and mentally disabled persons, were excluded.
A sample size of 300 adults aged 25 to 60 years was adequate on the basis of an estimated prevalence of 10 % of concurrent overweight/obesity and micronutrient deficiency within the same individual, taking into account the overweight/obesity prevalence of 33 %(Reference Niakara, Fournet and Gary10) and the limited access to micronutrient-rich foods in 65·6 % of households in Ouagadougou(24). The precision was ±3 %, and the confidence interval was 95 % with α < 0·05 using the software PASS (Power Analysis and Sample Size) supplied by NCSS (Kaysville, UT, USA). The size of the sample was increased by 10 % up to 330, to provide for drop-outs, missing participants and incomplete data sets.
Variables
After enrolment, personal interviews were performed with participants to collect data on age, parity, education level, psychosocial factors and lifestyle patterns. Anthropometric and clinical data as well as blood samples were also collected. Psychosocial and lifestyle data are not presented here.
Anthropometrics
Body weight was measured to the nearest 100 g, with participants in light clothing and without shoes, using a portable electronic scale of 150 kg capacity (Seca 803 Clara Scale, Semur-en-Auxois, France). Height was measured to the nearest 0·5 cm using a portable locally built stadiometer, with the participants standing upright on a flat surface without shoes and with the back of their heels and the occiput against the stadiometer. Waist circumference (WC) was measured to the nearest 0·1 cm with a flexible, non-stretchable and tension-regulated steel tape (Gulick measuring tape©; Creative Health Products, Inc., Plymouth, MI, USA), at the midpoint between the lowest rib and the iliac crest, while participants were standing and breathing normally(Reference Lohman, Roche and Martorell25). The averages of two separate measures of body weight, height and WC were used in the analyses. BMI was calculated as weight (in kilograms) divided by the square of height (in metres). BMI was categorised as follows(26): underweight, BMI < 18·5 kg/m2; normal weight, BMI = 18·5–24·9 kg/m2; overweight, BMI = 25·0–29·9 kg/m2; obese, BMI ≥ 30·0 kg/m2. Abdominal obesity was defined as WC ≥ 102 cm for men and WC ≥ 88 cm for women(27).
Blood pressure
Blood pressure was measured by the first author with a calibrated aneroid sphygmomanometer on the right arm of seated participants after a minimum of 10 min rest. Systolic and diastolic pressures were measured twice with an interval of 10 min between the first and the second measurement. The mean of the two readings was used in the analyses. Hypertension for individuals without a prior diagnosis was defined as systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg(Reference Chalmers, MacMahon and Mancia28).
Blood sampling and laboratory measures
Venous blood samples were drawn, after an overnight fast of at least 12 h, into 10 ml EDTA tubes and 10 ml dry tubes for plasma and serum collection, respectively. Blood samples were immediately stored in cold boxes and brought to the laboratory within 2 h. Samples were centrifuged at 3000 rpm for 10 min, aliquoted into cryotubes and frozen at −32°C. Fasting glucose was immediately determined on plasma samples using the glucose oxidase method at the University of Ouagadougou. Hyperglycaemia was defined as fasting plasma glucose >6·1 mmol/l for individuals without a prior diagnosis(29). Plasma concentrations of HDL cholesterol (HDL-C), LDL cholesterol (LDL-C) and TAG were determined with enzymatic methods. Cut-offs for low HDL-C were <0·9 mmol/l for men and <1·0 mmol/l for women. The cut-off for high plasma LDL-C was >3·37 mmol/l. Hypertriacylglycerolaemia was defined as plasma TAG concentration >1·7 mmol/l(27). The ratio of total cholesterol (TC) to HDL-C (TC:HDL-C) was calculated and a value >5 was defined as ‘high TC:HDL-C’(30). Serum insulin concentration was measured by RIA (Cisbio Bioassays, Bagnols sur Ceze, France) and the homeostasis model assessment (HOMA) equation ((fasting glycaemia × serum insulin)/22·5) was used as an index of insulin resistance; insulin resistance was defined as HOMA-IR ≥ last quartile in the whole studied population(Reference Matthews, Hosker and Rudenski31). Serum retinol was measured using HPLC at the University of Ouagadougou, with serum retinol level <0·7 mmol/l being indicative of vitamin A deficiency(32). Plasma ferritin level was measured using chemiluminescence with a cut-off of <15 μg/l for iron depletion, and Hb was directly measured in the field with a drop of whole blood using a HemoCue® (Hemocue HB 201+, Angelholm, Sweden) with anaemia being defined as Hb<12 g/dl in men and <13 g/dl in women(33). Insulin, ferritin and blood lipid determinations were carried out at the University of Nancy (France).
Clustering of deficiency indicators and cardiometabolic risk factors
Nutritional deficiency indicators considered when assessing the double burden of malnutrition were underweight, iron depletion and vitamin A deficiency, for a maximum of three indicators. Individual CMRF were the following, for a maximum count of four: overweight or obesity or abdominal obesity; hypertension; hyperglycaemia or insulin resistance or diagnosed diabetes; and dyslipidaemia (high LDL-C or low HDL-C or hypertriacylglycerolaemia or high TC:HDL-C). We also considered MetS as the clustering within an individual of hyperglycaemia or insulin resistance or diagnosed diabetes with at least two of the following risk factors: overall obesity (BMI ≥ 30·0 kg/m2), hypertriacylglycerolaemia, low HDL-C and high blood pressure or treated hypertension(27). We used the WHO MetS definition since the cut-offs for individual CMRF were those of WHO.
Statistical analysis
Data were analysed using the IBM SPSS statistical software package version 18·0 (IBM, Armonk, NY, USA). Results are expressed as means and standard deviations for continuous variables, or as percentages with 95 % confidence intervals for categorical variables. The differences between men and women were assessed using independent t tests or χ 2 tests as appropriate. Differences between socio-economic levels were computed using the χ 2 test. The level of statistical significance was P < 0·05.
Ethical considerations
The study was approved by the Ethics Committee of the Faculty of Medicine, University of Montreal, and the Ethics Committee for Health Research of Burkina Faso. The study objectives were clearly explained to participants, selected household heads and local authorities. A written informed consent was obtained from each participant prior to enrolment. They were given back their results on blood pressure and glycaemia, and those with abnormal values were referred for diagnosis and treatment.
Results
A total of 310 adults (51·9 % women) completed the study, with a response rate of 94 %. Mean age of the population under study was 36·4 (sd 9·0) years (Table 1), and women were less educated than men (P = 0·05).
Table 1 Sociodemographic characteristics, nutritional deficiencies and cardiometabolic risk factors by sex* among adults (n 310) aged 25–60 years, northern district of Ouagadougou, Burkina Faso, 2010
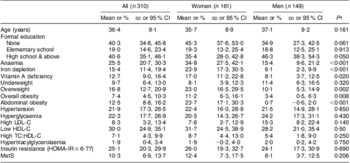
LDL-C, LDL cholesterol; HDL-C, HDL cholesterol; TC:HDL-C, ratio of total cholesterol to HDL-C; HOMA-IR, homeostasis model assessment–insulin resistance index; MetS, metabolic syndrome.
*This includes twenty-one participants with prior hypertension and two participants with prior diabetes.
†Significance of the difference between women and men as determined by Student's t test or the χ 2 test.
Malnutrition and cardiometabolic risk factors
The overall prevalence of anaemia, iron depletion and vitamin A deficiency was 25·5 %, 15·4 % and 12·7 %, respectively (Table 1). Anaemia was twice as high in women as in men (P < 0·001). Iron depletion was four times higher in women than men (P < 0·001). Vitamin A deficiency was also significantly higher in women than men (P = 0·02). Underweight (BMI < 18·5 kg/m2) prevalence was 9·7 %, with no sex difference. Low-income and non-educated participants exhibited significantly higher prevalence of vitamin A deficiency (Table 2) compared with high-income and more educated participants (25·7 % v. 4·1 %, P < 0·001; 21·1 % v. 5·6 %, P = 0·001, respectively).
Table 2 Nutritional deficiencies and cardiometabolic risk factors according to income level and formal educationFootnote * among adults (n 310) aged 25–60 years, northern district of Ouagadougou, Burkina Faso, 2010

HDL-C, HDL cholesterol; TC:HDL-C, ratio of total cholesterol to HDL-C; MetS, metabolic syndrome.
a,b,c Values within a row (for income level and formal education separately) with unlike superscript letters were significantly different using the χ 2 test (P < 0·05).
* This includes twenty-one participants with prior hypertension and two participants with prior diabetes.
The prevalence of insulin resistance, hypertension and hyperglycaemia including previously diagnosed cases was 25·1 %, 21·9 % and 22·3 %, respectively, with no statistical differences between women and men. The prevalence of low HDL-C, high LDL-C, hypertriacylglycerolaemia, high TC:HDL-C ratio and MetS was 30·0 %, 8·3 %, 1·9 %, 7·1 %, and 10·3 %, respectively, with no sex difference (Table 1). Hypertension did not vary either with income or education and was above 18 % in all groups. Hyperglycaemia was significantly more prevalent in the groups with low and medium income compared with the high income group (P < 0·001), and also was significantly higher in less-educated participants compared with those who attended high school (P = 0·001). There was no statistical difference in the prevalence of low HDL-C, high LDL-C, high TC:HDL-C ratio, insulin resistance and MetS according to income or education (Table 2).
The prevalence of overweight, overall obesity and abdominal obesity was 16·8 %, 7·4 % and 12·5 %, respectively, and consistently higher in women than men (Table 1). Overweight, overall obesity and abdominal obesity were 24·5 %, 13·3 % and 21·4 %, respectively, for participants in the high income group and significantly higher compared with low and middle income groups (P = 0·045, 0·025 and 0·003, respectively). Participants with high school education level exhibited more overall obesity than those with no formal education (11·9 % v. 2·8 %, P = 0·010; Table 2).
Coexistence of malnutrition and cardiometabolic risk factors
As shown in Fig. 1, 72·9 % of the participants had at least one CMRF with 36·1 % having at least two risk factors. Additionally 27·3 % and 5·2 % of the participants had one and two nutritional deficiencies, respectively. Overall 23·5 % exhibited a double burden of malnutrition, with significantly more women than men (30·4 % v. 16·1 %, P = 0·008; Fig. 2).

Fig. 1 Clustering of nutritional deficiencies (underweight, iron depletion and vitamin A deficiency) and cardiometabolic risk factors (CMRF) among adults (n 310) aged 25–60 years, northern district of Ouagadougou, Burkina Faso, 2010

Fig. 2 The occurrence of nutritional deficiencies (underweight, iron depletion and vitamin A deficiency) and cardiometabolic risk factors (CMRF) according to sex among adults (n 310) aged 25–60 years, northern district of Ouagadougou, Burkina Faso, 2010 (![]() $$$$, no nutritional deficiencies;
$$$$, no nutritional deficiencies; ![]() $$$$, at least one nutritional deficiency;
$$$$, at least one nutritional deficiency; ![]() $$$$, at least one CMRF;
$$$$, at least one CMRF; ![]() $$$$, double burden of nutritional deficiency and CMRF). Mean values were significantly different from those of men (as determined by the χ 2 test): *P < 0·05, **P < 0·01
$$$$, double burden of nutritional deficiency and CMRF). Mean values were significantly different from those of men (as determined by the χ 2 test): *P < 0·05, **P < 0·01
Table 3 portrays the five most common phenotypes of the double burden of malnutrition, with 9·0 % showing one or two micronutrient deficiencies and at least one CMRF other than overweight/obesity (Phenotype 1); 5·2 % with one or two micronutrient deficiencies combined with overweight/obesity and at least one other CMRF (Phenotype 2); 5·2 % with underweight and at least one CMRF (Phenotype 3); 2·6 % with one or two micronutrient deficiencies plus MetS (Phenotype 4); and finally 1·6 % with underweight plus one micronutrient deficiency and at least one CMRF (Phenotype 5). Phenotypes 1 and 2 were significantly higher in women than in men (P = 0·030 and 0·050, respectively).
Table 3 The most common phenotypes of the double burden of malnutrition and CMRF by sex among adults (n 310) aged 25–60 years, northern district of Ouagadougou, Burkina Faso, 2010

CMRF, cardiometabolic risk factors; MetS, metabolic syndrome.
*Significance of the difference between women and men as determined by the χ 2 test.
Compared with participants who had high school education level, those without formal education (Fig. 3) showed the highest rate of double burden: 32·0 (95 % CI 23·9, 40·1) % v. 15·1 (95 % CI 8·9, 21·3) % (P = 0·002). Similarly, the occurrence of the double burden according to income (Fig. 4) was significantly higher (P = 0·005) in the group with low income, 32·1 (95 % CI 25·2, 42·7) %, compared with the high income group, 13·3 (95 % CI 6·6, 20·0) % (P = 0·005).
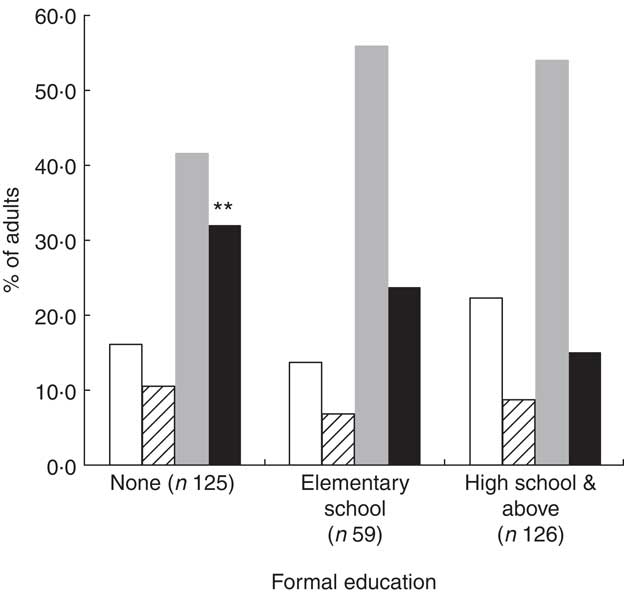
Fig. 3 The occurrence of nutritional deficiencies (underweight, iron depletion and vitamin A deficiency) and cardiometabolic risk factors (CMRF) according to formal education among adults (n 310) aged 25–60 years, northern district of Ouagadougou, Burkina Faso, 2010 (![]() $$$$, no nutritional deficiencies;
$$$$, no nutritional deficiencies; ![]() $$$$, at least one nutritional deficiency;
$$$$, at least one nutritional deficiency; ![]() $$$$, at least one CMRF;
$$$$, at least one CMRF; ![]() $$$$, double burden of nutritional deficiency and CMRF). Mean values were significantly different from those for high school and above (as determined by the χ 2 test): **P < 0·01
$$$$, double burden of nutritional deficiency and CMRF). Mean values were significantly different from those for high school and above (as determined by the χ 2 test): **P < 0·01

Fig. 4 The occurrence of nutritional deficiencies (underweight, iron depletion and vitamin A deficiency) and cardiometabolic risk factors (CMRF) according to income level among adults (n 310) aged 25–60 years, northern district of Ouagadougou, Burkina Faso, 2010 (![]() $$$$, no nutritional deficiencies;
$$$$, no nutritional deficiencies; ![]() $$$$, at least one nutritional deficiency;
$$$$, at least one nutritional deficiency; ![]() $$$$, at least one CMRF;
$$$$, at least one CMRF; ![]() $$$$, double burden of nutritional deficiency and CMRF). Mean values were significantly different from those for high income level (as determined by the χ 2 test): **P < 0·01
$$$$, double burden of nutritional deficiency and CMRF). Mean values were significantly different from those for high income level (as determined by the χ 2 test): **P < 0·01
Discussion
To our knowledge, the present study one of the first in adults on concurrent nutritional deficiencies and nutrition-related CMRF in Sub-Saharan Africa. It shows that the double burden of malnutrition within individual adults is widespread in Ouagadougou, and more in women, as well as in less-educated and poorer individuals. The results highlight the fact that CMRF are taking the lead as nutrition-related problems in adults, while indicators of underweight and micronutrient deficiencies remain highly prevalent, particularly in women. Women are more vulnerable to nutritional deficiencies because of their high nutritional requirements for pregnancy and lactation, and also because of gender inequalities in poverty(Reference Delisle34). Cultural factors may also contribute to the higher prevalence of overweight in women(Reference Holdsworth, Gartner and Landais35). The shift towards CMRF in many developing countries has promoted the misconception that diets are moving away from problems of food shortage towards problems of excess food, with nutritional deficiencies becoming a problem of the past(Reference Eckhardt, Torheim and Monterrubio20). Furthermore, the synergistic interaction of ‘undernutrition’ and ‘overnutrition’ is all too often overlooked. Besides, ‘overnutrition’ may be a misnomer for nutrition-related chronic diseases risk since a sizeable proportion of our participants with normal weight or WC showed one or more CMRF (31 %).
Few African studies have reported this double burden of malnutrition beyond the combination of overweight with underweight in the same population. Fewer studies still focus on the double burden in the same individuals. In a study among women of three developing countries (Mexico, Peru and Egypt), Eckhardt et al.(Reference Eckhardt, Torheim and Monterrubio20) reported that overweight overlapped with anaemia. Studies carried out in developed countries and Latin American countries have reported that lifestyle changes including increasing intake of energy-dense but micronutrient-poor foods, such as high-sugar beverages and high-fat salty snacks, coupled with low intake of micronutrient-rich foods, are pieces of the puzzle that could contribute to explain such overlap of obesity and micronutrient deficiencies(Reference Drewnowski and Specter2, Reference Eckhardt, Torheim and Monterrubio20, Reference Drewnowski and Darmon36, Reference Murphy and Allen37). However such evidence is lacking in Sub-Saharan Africa, until eating patterns are better documented.
The high prevalence of hypertension in our study is consistent with previous reports in urban Africa(Reference Agyemang9, Reference Amoah38, Reference Edwards, Unwin and Mugusi39); however it seems lower than that previously reported in Ouagadougou (21·9 % v. 40·2 %(Reference Niakara, Fournet and Gary10)), perhaps because of the younger age range in our study. A major concern is that in developing countries, only half of hypertensive individuals are aware of their condition, and of these, only 50 % are under medical treatment, with only 50 % being improved(Reference Whitworth40). In our study only 30 % of hypertensive participants were aware of their condition with less than half under medical treatment (data not shown).
The prevalence of hyperglycaemia was also high, compared with recent studies in Benin(Reference Ntandou, Delisle and Agueh41, Reference Sodjinou, Agueh and Fayomi42), even if we excluded previously diagnosed cases of diabetes like in the latter studies. The prevalence was significantly higher in the low income group, wherein underweight prevalence was also the highest, suggesting that this metabolic risk factor is no longer a matter of affluence. These results also appear somewhat consistent with the clinical features (i.e. poverty, protein–energy malnutrition, generalised wasting, insulin resistance) of malnutrition-related diabetes or fibrocalculous pancreatic diabetes(Reference Mohan, Nagalotimath and Yajnik43) mostly reported from South Asia(Reference Mohan, Farooq and Deepa44), but with still limited evidence in Sub-Saharan Africa(Reference Alemu, Dessie and Seid45). This should be of particular concern, especially when we are aware that estimates by the International Diabetes Federation suggest the number of adults with diabetes in Sub-Saharan Africa will increase by 98 % from 12·1 million in 2010 to 23·9 million in 2030(46).
The low prevalence of hypertriacylglycerolaemia and the rather high prevalence of low HDL-C in our study are noteworthy and consistent with previous studies in Benin (West Africa)(Reference Ntandou, Delisle and Agueh41, Reference Sodjinou, Agueh and Fayomi42). An extensive review on the African diaspora in the UK(Reference Zoratti47) and a recent study including West Africans and African Americans(Reference Sumner, Zhou and Doumatey48) reported a lower propensity of African people to adverse blood lipid profile. However, low HDL-C was rare in the diaspora, which suggests that lifestyle patterns including diet may explain this difference, considering that West Africans are the ancestors of African Americans who have as much as 70 % of their gene pool of West African origin(Reference Tishkoff, Reed and Friedlaender49). In the present study, the higher prevalence of low HDL-C in the low income group (as shown) and in overweight participants (data not shown) is consistent with an epigenetic or environmental hypothesis, portraying low HDL-C as a particular feature of ‘dysnutrition’(Reference Delisle and Receveur50) (encompassing both ‘undernutrition’ and ‘overnutrition’) to be investigated in urban Africans.
The prevalence of overweight, overall obesity and abdominal obesity increased significantly with rising income in our study, suggesting that these populations are at early stages of the nutrition transition, with excess weight primarily among the more affluent, before it progressively shifts to lower-income groups as demonstrated in middle-income countries(Reference Monteiro, Moura and Conde51). Women were at least four times more affected by overall obesity than men, which is in line with previous reports in Ouagadougou(Reference Ouedraogo, Fournet and Martin-Prevel52) and other African cities(Reference Abubakari, Lauder and Agyemang8, Reference Sodjinou, Agueh and Fayomi42, Reference Amoah53–Reference Ntandou, Delisle and Agueh55). Abdominal obesity, which is known to be more deleterious to health(Reference Montague and O'Rahilly56) than overall obesity, also prevails in women. Despite this gender difference in overall and abdominal obesity, risk factors like hypertension, hyperglycaemia and dyslipidaemia did not differ between women and men, challenging the relevance of BMI and WC cut-offs for African populations. Several studies in populations of Asian(Reference Lear, Humphries and Kohli57) and African(Reference Camhi, Bray and Bouchard58, Reference Katzmarzyk, Bray and Greenway59) origin have highlighted discrepancies in the relationship between BMI, WC and body fat among different ethnic groups(Reference Deurenberg, Yap and van Staveren60–Reference Desilets, Garrel and Couillard62). This calls for revisiting the obesity cut-off points according to race/ethnic group(Reference Carroll, Chiapa and Rodriquez63), which would require long-term longitudinal studies.
Our hypothesis on the persistence of nutritional deficiencies in the midst of a high prevalence of so-called ‘overnutrition’ was confirmed in the present study. Anaemia and iron depletion were very highly prevalent, both reaching the level of a public health problem, mainly among women. The prevalence rate of vitamin A deficiency in our study was also above the threshold of public health significance of 15 %(32). Moreover, evidence is increasingly supportive of an interaction between micronutrient deficiencies and CMRF(Reference Eckhardt, Torheim and Monterrubio20, Reference Block, Jensen and Norkus64, Reference Ford, Will and Bowman65). The role of micronutrient malnutrition in non-communicable diseases is a compelling area for research, particularly in developing countries. Such research could contribute to knowledge on emerging cardiovascular risk factors, while providing further evidence for action.
Several limitations can be identified in our study. The cross-sectional design of the study does not allow any inference to be drawn with respect to the causal relationship between variables. Furthermore, the study is only representative of one district in Ouagadougou and the results cannot be extrapolated to the whole urban population of Burkina Faso without caution. Despite these limitations, the study provides useful data on both nutritional deficiencies and CMRF separately in the population as well as the prevalence of the double burden of malnutrition in connection with sociodemographic parameters.
The present study shows that the upward trend of nutrition-related chronic diseases is an overwhelming issue in adults of Ouagadougou. Worse still, it overlaps with micronutrient deficiencies, mainly among women, and among the uneducated and the poor. The prohibitive cost of curative treatment of nutrition-related chronic diseases, for both the patient and the health system mainly in developing countries, requires that increased attention be paid to preventive measures in adults but also throughout the life cycle. It is increasingly important for public health programmes to focus on healthy diets and lifestyle patterns (and their accessibility) that lead to optimal health outcomes at both ends of the spectrum. To develop such programmes, more research is required to indentify location-specific diets that may be protective against both nutritional deficiencies and CMRF. From now on, rather than focusing deficiencies or chronic diseases, it would be helpful to address both conditions in simultaneous strong public messages.
Acknowledgements
This work was supported by the Canadian International Development Agency. The authors declare that they have no competing interests. A.N.Z. developed the study protocol as his PhD project, he collected and analysed the data and wrote the manuscript. H.F.D. designed the study, supervised data analysis and thoroughly revised the manuscript. G.R. was involved in the study design and paper revision. B.S. conducted the local laboratory analyses and revised the manuscript. B.B. provided the sampling database, assisted with statistics and revised the manuscript. The authors gratefully acknowledge the Institut de Recherche en Sciences de la Santé (IRSS) and the Institut Supérieur des Sciences de la Population (ISSP) in Burkina Faso for technical and field support; and extend special thanks to Professor Somé Issa of the University of Ouagadougou and Professor Jean-Louis Guéant of the University of Nancy for laboratory analyses. They also thank the study participants and the field workers.









