Iron-deficiency anaemia (IDA) among pregnant women is associated with an estimated 111 000 maternal deaths each year(1). Anaemia control programmes based on the administration of Fe and folic acid supplements have been implemented in many developing countries; however, the success is very limited and pregnant women are often deficient in a wider range of vitamins and minerals. Of the strategies to prevent iron deficiency (ID), food-based approaches are considered the only sustainable and long-term solution that also provides nutrients other than Fe and adds variety to the diet(2).
The following factors are important for alleviating ID: increasing Fe intake, especially of haem Fe; increasing the intake of absorption enhancers for non-haem Fe, such as vitamin C and meat proteins; decreasing the intake of inhibitors such as phytic acid (e.g. in beans) or tannins (e.g. in tea); and reducing Fe loss (e.g. caused by malaria, intestinal parasites). It is obvious that only an approach that addresses all these factors, which is adapted to local settings (especially with the use of locally available and familiar foods and preparation methods)(Reference Neumann, Bwibo and Murphy3) and does not significantly increase the cost, is feasible in poor communities.
In rural areas in Indonesia, consumption of fleshy food such as meat, poultry and small whole fish with bones, which are readily available sources of haem Fe, Zn and preformed vitamin A, is often limited(Reference Gibson and Hotz4) because of economic constraints. Inexpensive, Fe-rich foods, such as tempeh, are available and have been a part of the daily diet of Indonesians. Tempeh is a non-salted fermented soyabean product that contains 2·3 mg Fe/100 g(Reference Erhardt5) and is consumed as a protein-rich meat substitute by all economic groups.
Hurrell et al.(Reference Hurrell, Reddy and Juillerat6) concluded that complete degradation of phytic acid in legumes may increase Fe absorption up to fivefold. This is in agreement with a study showing that Fe absorption from fermented tempeh was significantly better than that from soya-flour meals(Reference McFarlane, van der Riet and Bothwell7) and that the intake of 208 g of tempeh over 11 d increased the concentration of Fe in the liver in Fe-deficient rats, compared with the intake of unfermented soyabean(Reference Kasaoka, Astuti and Uehara8). The fermentation process leads to a reduction of phytic acid content and to more absorbable Fe. Another study has shown that the remaining phytic acid content in fresh fermented soyabean (tempeh) is 50 % less than that in raw soyabean and further decreases to <20 % in cooked tempeh(Reference Sutardi and Buckle9).
Tempeh is rich in phyto-oestrogens, which have multiple beneficial effects, but animal studies(Reference Takashima-Sasaki, Komiyama and Adachi10) have shown that high amounts can also have adverse effects on offspring. Such effects have not been observed in human trials and the US Food and Drug Administration recommends an intake of 25 g of soya protein daily as part of a healthy diet, which corresponds to 125 g of tempeh(11). A study conducted in Central Java among pregnant women has shown that the daily tempeh intake is only 50 g(Reference Hartini, Winkvist and Lindholm12) and therefore adding tempeh to the usual diet is safe.
To date, relatively little emphasis has been placed on the effectiveness of food-based approaches in the control of ID. Our primary aim was to examine the effect of maternal intervention comprising a daily portion of local fermented soyabean (tempeh) and vitamin C-rich fruits on reducing maternal ID. The effect on birth outcomes was also assessed.
Methods
Study location and participants
We carried out the study from November 2007 to October 2008 in thirty-nine villages in two districts, Karanganyar and Demak, of Central Java Province, Indonesia. The distance between the two districts is about 160 km. The twenty-four villages in Karanganyar district are 325–1500 m above sea level, whereas the fifteen villages in Demak district have an elevation of 0–100 m above sea level. Malaria was not endemic in the study areas. The common diet comprised rice-based meals taken with vegetables and dried salted fish, soya products and occasionally meat.
Pregnant women who attended antenatal clinics in villages were invited to participate in the trial. We also coordinated with the midwives to identify women who had not yet self-reported for prenatal care. The pregnant women identified for the study were asked to gather at the community health centre (Puskesmas) for screening procedures. Requirements for eligibility included: 15–49 years of age; 12–20 weeks of gestational age; no existing severe maternal illness; and predicted singleton neonates. Women were assessed for an estimated gestational age by calculating from the first day of the last menstrual period and were confirmed by palpation on fundal height by a coordinator midwife; physical examination was carried out by a medical doctor from Puskesmas.
The nature and process of the trial were explained to the women at the time of enrolment and only those women who gave written consent were recruited. The ethical and scientific committee for the study of humans of the Faculty of Medicine, University of Indonesia, approved the study protocol. This trial is registered as an International Standard Randomised Controlled Trial (no. ISRCTN13994081).
Procedures
We conducted a community-based interventional trial with randomization of the two groups: the optimized diet group and the control group. The randomization process was carried out at the village level. Our study was conducted as close as possible to the ‘real-life setting’ in the community. All women at 12–20 weeks of gestation were recruited without considering their anaemia status. Both groups had free access to receive tablets containing 60 mg of Fe and 250 μg of folic acid as part of the Ministry of Health package of prenatal care and/or multiple micronutrient supplements from midwives’ clinics. All women at 18–22 weeks of gestation received a single dose of 400 mg albendazole (Kimia Farma, Jakarta, Indonesia) for anthelminthic treatment. Pregnant women allocated to the optimized diet group received supplementary food 6 d/week.
Primary outcome measures included changes in Fe concentrations after the optimized diet intervention. We defined anaemia as an Hb concentration of <110 g/l(Reference Asobayire, Adou and Davidsson13); ID as a plasma ferritin concentration of <30 μg/l(Reference Asobayire, Adou and Davidsson13) or a plasma-soluble transferrin receptor (TfR) concentration of >8·5 mg/l(Reference Sauberlich14); and IDA as concurrent anaemia and ID. Inflammation was indicated when the C-reactive protein (CRP) concentration was >5 mg/l and/or α-1-acid glycoprotein (AGP) concentration was >1 g/l(Reference Thurnham15). We also assessed gestational age, birth weight and infant's length. We identified low birth weight as birth weight <2500 g (≤2500 g was used for scales with 100 g increments) and preterm birth as gestational age <37 weeks. We identified stillbirth as delivery of an infant showing no signs of life movements, breathing or heartbeat after 23 weeks of gestation and early neonatal death as death of a liveborn infant during the first 7 d after birth.
Blood
We took samples of 3 ml of venous blood by means of venepuncture at baseline (12–20 weeks of gestation) and at near term (32–36 weeks of gestation). We drew 0·5 ml of blood into Na-EDTA vacuettes for haematological analyses and measured Hb concentration in blood samples using a haematology analyser (Nihon Kohden MEK-6318K, Tokyo, Japan, or Coulter HmX, Brea, CA, USA). The remaining 2·5 ml was drawn into vacuette heparin tubes (Greiner, Kremsmuenster, Austria) for analyses of plasma ferritin, TfR, CRP and AGP. The tubes were kept in a cooled box with cool packs during transport to the local laboratory. Within 6 h, plasma was separated by centrifugation (MLW T51.1, Germany) at 2750 rpm and room temperature for 10 min. The plasma was then transferred into Eppendorf Safe Lock vials that were stored at −7°C (for 1 month), then at −70°C until further analyses. Frozen plasma concentrations of ferritin, TfR, CRP and AGP were transported in dry ice to DBS-Tech (Legelshurst, Germany) and measured using an in-house sandwich ELISA technique(Reference Erhardt, Estes and Pfeiffer16). We used CRP and AGP as a marker of acute and chronic inflammation since ferritin is elevated in the presence of infection or inflammation(17).
Supplementary food for an optimized diet
The emphasis was on the use of tempeh as the main ingredient of supplementary food for optimizing the diet to provide additional Fe and vitamin C sources to improve the Fe bioavailability. The average weekly supplementary food consisted of 600 g of tempeh, 30 g of red meat, 30 g of dried anchovies, 30 g of chicken liver, 350 g of guava, 300 g of papaya and 100 g of orange. The women were instructed to consume an average of 50 g of tempeh, 15 g of dried anchovies/red meat/chicken liver and 50 g of guava/100 g of papaya together with their lunch meal, 50 g of tempeh for snacks in the evening and 50 g of guava/orange with their dinner meal daily and not to drink tea or coffee within 2 h of meal time. The daily nutrient composition in the food comprised Fe 3·97 mg, vitamin C 173 mg and protein 23 g. Six local recipes of tempeh cooked with different seasoning ingredients were presented in repeating sequence.
Tempeh was purchased from two home industries. We used two brands of soya sauce. Fruit, chicken liver, red meat, dried anchovies and spices were purchased from the local markets. The food was prepared in the study centre and distributed daily until the women gave birth. Cooks were hired from among village women. Each participant received the food in a name-coded plastic box.
Compliance was calculated by expressing the total amount (g) of supplementary food consumed daily as the proportion of the amount assigned to every participant during the intervention period. Uncooked, cooked and leftovers of supplementary food were weighed using food scales with a precision of ±1 g (Soenle-Waagen GmbH, Murrhardt, Germany or Inotec, Essen, Germany) and calibrated daily using fixed weights by the trained enumerators at the study centre.
Duplicate portions of supplementary food were analysed for energy, protein, fat, carbohydrates and Fe at baseline and endline (Table 1). The analyses were conducted at the Center for Research and Development in Food and Nutrition, Bogor, Indonesia. Vitamin C content was taken from the Indonesian food database(Reference Erhardt5).
Table 1 Nutrient content in supplementary foods per 100 g

IQR, interquartile range.
Median and IQR unless otherwise indicated. Nutrient analyses (energy, protein, fat, carbohydrates and Fe) were conducted from duplicate composite samples of four of both Karanganyar and Demak study areas.
†Calculated using the Mann–Whitney U test.
‡Vitamin C content was taken from the Indonesian food database(Reference Erhardt5).
Sensory test
In May 2007 we conducted a 2-week pilot study in which we provided ten pregnant women of median gestational age 7·5 months with a daily target amount of supplementary food to assess the acceptability of the food and compliance with consumption of the target quantity.
Food intake
At baseline and endline, two nutritionists interviewed the participants to obtain records of the food intake of the previous day using a quantitative 24 h recall, except for amounts of the supplementary food that were not recorded at endline. Repeated 24 h recalls within 1 week were obtained from 30 % of participants selected randomly to validate the variation of energy and nutrient intakes.
Stool
We collected fresh stool samples in plastic containers to assess the degree and type of parasite infestation. Within 24 h, using the Kato–Katz technique, we conducted an analysis to quantitatively identify the helminth eggs of hookworm, Ascaris lumbricoides and Trichuris trichiuria (18). In addition, we used the Harada–Mori technique, which is more sensitive compared with the Kato–Katz technique(Reference Oqueka, Supali and Ismid19), to detect living hookworm larvae. We examined the samples at the Laboratory for Parasitology, Sebelas Maret University, Solo, and at the Laboratory for Parasitology, Diponegoro University, Semarang.
Anthropometry
We measured height at baseline accurate to 0·1 cm using a microtoise and monthly body weight to the nearest 0·1 kg using an electronic weighing scale (SECA 890, Hamburg, Germany).
A coordinator midwife and a trained nutritionist visited postpartum women and measured their birth weight and body weight using electronic scales (SECA 890) calibrated before measurement at the study centre. Infants’ length was measured using a length board (SECA 416 or Shorr, Olney, MD, USA). We attempted to establish birth weight and infants’ length within 72 h.
The trained enumerators conducted the interviews and observations through home visits to record socio-economic status.
Statistical analyses
With a power of 90 % and a two-sided significance level of 5 %, baseline and follow-up data of 168 women would be needed to detect a change in the Hb concentration of 5 g/l and an sd of 10 g/l in the optimized diet group relative to the control group. Anticipating a dropout rate of 50 % until delivery, a total of 250 pregnant women were recruited.
We used the Kolmogorov–Smirnov one-sample test to check the normality of data. Data were reported as means and sd for normally distributed variables and as median and interquartile range for non-normally distributed variables. Hb, plasma ferritin, TfR and CRP concentrations and nutrient intake were logarithmically transformed and reported as geometric means and 95 % CI. Continuous Fe and inflammation status was first compared using a t test and then adjusted for potential confounding using univariate analyses. Each model was adjusted for the factors (altitude, maternal gestational week, height, supplement intake) that differed between groups at recruitment, as well as for baseline concentrations of the analyte of interest and for changes in nutrient intake. To test the difference between baseline and endline, the paired t test was used for continuous variables. The Mann–Whitney test was used to examine differences between groups for non-normally distributed variables. The χ 2 test or logistic regression with the Forward Wald method was used to examine differences in proportions. A body Fe store was estimated using the following equation(Reference Cook, Flowers and Skikne20):
Body Fe >0 indicates the amount of Fe in stores and body Fe <0 indicates the deficit in tissue Fe. The effect of inflammation on ferritin is eliminated by multiplying the individual plasma ferritin concentrations of participants in the inflammation group by the respective correction factors. An elevated CRP >5 mg/l indicates incubation × 0·82; a raised CRP and a raised AGP indicate early convalescence × 0·19(Reference Thurnham15). There was no participant with an elevated AGP concentration alone in the present study. P < 0·05 was considered as significant (two-tailed). We used the Statistical Package for the Social Sciences statistical software package version 15·0 (SPSS Institute Inc., Chicago, IL, USA) for statistical analyses. The Nutrisurvey software program was used for entering dietary intake and for converting this information into energy and nutrient intakes on the basis of the Indonesian food database(Reference Erhardt5).
Results
Figure 1 shows the trial profile. Of the 310 women who were enrolled, fifty-eight were excluded from the study. The primary reasons for exclusion were the women's refusal to participate and a gestational age of >20 weeks. Among the remaining 252 women, data on follow-up blood samples were available for 227 (90 %); six women moved elsewhere, thirteen delivered before blood could be taken at near term, two fetuses died after 28 weeks of gestation, three women refused to give blood and one discontinued the consumption of supplementary food. Hb and plasma samples were available for 213 and 227 women, respectively, at endline. We included all liveborn infants in the analyses of birth outcomes. Of the 244 women who delivered, 94·8 % of birth outcomes were available for the analyses of infants, two were stillbirths, one was lost to follow-up and two infants died within 1 week.

Fig. 1 The study profile
Table 2 shows baseline characteristics related to socio-economic status, age and nutritional status and Table 3 shows the characteristics for Fe and inflammation status. Both groups were comparable, except for altitude, gestational age, maternal height and supplement consumption of Fe and folic and/or multiple micronutrient tablets.
Table 2 Baseline characteristics of study participants
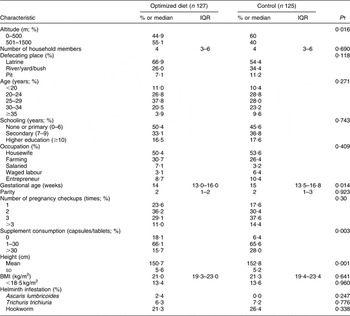
IQR, interquartile range.
% or median and IQR, unless otherwise indicated.
†Calculated using the χ 2 test for categorical variables, the Mann–Whitney U test for non-normally distributed variables or the t test for normally distributed variables.
Table 3 Maternal iron and inflammation status at baseline with two analytic models for differences between group means

Geometric mean and sd unless otherwise indicated.
**P < 0·01 for difference between optimized diet and control group (t test).
†Adjusted difference for altitude, gestational week, maternal height, supplement intakes at baseline (analysis of covariance).
We noted these four potential confounders and ensured that they were controlled for in the main outcome analyses. All participants had access to electricity and went for regular checkups to a midwife. None of the women smoked cigarettes, but 84·5 % of the household members (male) did so. Median BMI was normal at 21 kg/m2. Seventy-six (30·2 %) women were infected lightly with at least one of the three worms assessed, with hookworm infection being the most often detected. The intensity of infection of T. trichiuria was inversely associated with Hb concentration, although weakly (r = −0·174, P = 0·006). After adjusting for altitude, gestational age, maternal height and supplement intake at baseline, Hb, plasma ferritin and TfR concentrations and body Fe values of Fe status and CRP and AGP concentrations of inflammation status did not differ between the optimized diet and control groups, which showed normal values.
Baseline blood samples were collected at a median gestational age of 14 weeks in the intervention group and at 15 weeks in the control group; endline samples were collected at 34·7 weeks in both groups (Table 4). The mean feeding days were 109 (sd 14·8) with a median daily consumption of supplementary food of 231·94 g, with interquartile range of 223·96–235·62 g. The optimized diet group consumed 96·77 %, 98·49 %, 95·68 % and 96·05 % of the total amount of tempeh, animal-source food, vitamin C-rich fruit and supplementary food, respectively, provided to them until the second blood assessment. During the intervention, no significant change in Hb concentration was observed between the groups. Plasma ferritin concentration and body Fe were significantly lower and TfR concentration was significantly higher in both groups. When data were analysed separately for Fe-deficient and Fe-replete women, a significantly smaller decrease in plasma ferritin concentrations was observed in Fe-deficient women but not in Fe-replete women in the optimized diet group compared with the control group (1·42, 95 % CI 1·16, 1·75 μg/l v. 1·85, 95 % CI 1·55, 2·2 μg/l, respectively; P = 0·046). Similarly, compared with the control group, the optimized diet group showed a smaller decrease in Hb (1·02, 95 % CI 0·98, 1·07 g/l, P = 0·058) and body Fe (2·57, 95 % CI 1·71, 3·43 mg/kg, P = 0·073) concentrations. By contrast, in Fe-replete women no differences in Fe status were observed between the optimized diet and control groups. We recorded no significant difference in optimized diet v. control groups with regard to acute inflammation, whereas a larger decrease was seen in Fe-replete women for chronic inflammation in the optimized diet group compared with the control group (0·15, 95 % CI 0·12, 0·18 g/l v. 0·09, 95 % CI 0·06, 0·12 g/l, respectively; P = 0·046).
Table 4 Effect on iron and inflammation status as a result of consuming the optimized diet

Geometric mean and sd unless otherwise indicated.
*P < 0·05, **P < 0·01, ***P < 0·001 for significant change from baseline (paired t test).
†Adjusted for baseline factors: altitude, gestational week, maternal height and supplement intake; Hb concentration, plasma ferritin, soluble transferrin receptor, C-reactive protein and α-1-acid glycoprotein concentrations; and changes in energy, protein, fat, carbohydrates, Fe and vitamin C intakes (analysis of covariance).
‡Mean and sd.
§Median and interquartile range; P values were calculated using the Mann–Whitney U test.
Thirty-six (16·9 %), eighty-six (37·9 %), six (2·6 %) and fourteen (6·6 %) women had anaemia, ID, tissue ID and IDA, respectively (Table 5). A few women (0·9 %) suffered from chronic inflammation and thirty-nine (17·2 %) suffered from acute inflammation, which was of low intensity. At near term, the prevalence of ID, tissue ID and IDA increased in both groups. By logistic regression, there was no significant time × treatment interaction for anaemia, ID, tissue ID and IDA. The prevalence of chronic and acute inflammation remained virtually unchanged in the two groups.
Table 5 Proportion of women with anaemia and low iron stores

TfR, soluble transferrin receptor; CRP, C-reactive protein; AGP, α-1-acid glycoprotein.
†Calculated using logistic regression analysis (the Forward Wald method) with treatment × time interaction, controlled for altitude, gestational week, maternal height and supplement intake at baseline and changes in energy, protein, fat, carbohydrates, Fe and vitamin C intakes.
All but one pregnant woman took Fe-containing supplements; 13·3 % took only Fe–folate supplements from government programmes (60 mg Fe/tablet), 46·1 % took multiple micronutrients containing Fe (12–118 mg Fe/tablet) and the remaining 40·2 % took both supplements. The total intake of Fe from supplements was comparable between the optimized diet and control groups (2502 (sd 177) mg v. 2920 (sd 189) mg, respectively; P = 0·127). Only thirteen women had worm infections at endline, with six being infected with hookworm and seven having T. trichiuria (data not shown).
There was no change in food availability in the study area for the duration of the study (Table 6). Intake of Fe increased significantly in the optimized diet group; intake of protein increased significantly in both groups. When the supplementary food was excluded from the analyses, the changes in nutrient intake did not differ between groups, except for the changes in vitamin C intake. The median intake of tempeh was 56 g.
Table 6 Daily intakes of macronutrients, iron and vitamin C and change from baseline to endline of intervention in participants who answered 24 h recall questionnaire
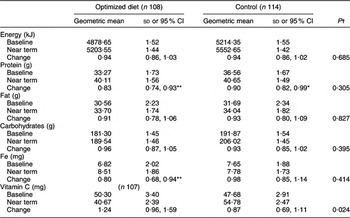
Supplementary foods excluded.
*P < 0·05, **P < 0·01 for significant change from baseline (paired t test).
†Adjusted for baseline factors: altitude, gestational week, maternal height and supplement intake; and energy, protein, fat, carbohydrates, Fe and vitamin C intakes (analysis of covariance).
The mean feeding days until delivery were 136 (sd 21) d and the mean supplementary food consumption was 30703·81 (sd 5493·23) g. None of the differences shown in birth outcomes were significant (Table 7). Although birth weight and length were taken later (P = 0·036) in the control group compared with the optimized diet group, this difference is unlikely to affect birth size measurements of the infants. Compared with the control group, the optimized diet group tended to have a higher percentage of low birth weight, which was mainly because of a shorter mean gestational age in the optimized diet group compared with the control group (37·6 (sd 2·7) weeks v. 39·2 (sd 1·6) weeks, respectively; P = 0·064) among the low birth weight infants. We recorded no differences between the groups with regard to reported complications during pregnancy, such as prolonged labour, retained placenta and postpartum haemorrhage (data not shown).
Table 7 Birth outcomes
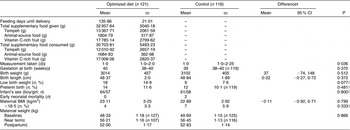
Mean and sd unless otherwise indicated.
†Mean and 95 % CI adjusted for altitude, gestational week, maternal height, supplements intake; P values were calculated using the t test.
‡Median and interquartile range; P values were calculated using the Mann–Whitney U test.
§Geometric mean and sd; P values were calculated using repeated-measures ANOVA.
∥Calculated using logistic regression analysis (Forward Wald method), controlled for altitude, gestational week, maternal height and supplement intake.
Discussion
Consumption of the optimized diet with fermented soyabean (tempeh) and vitamin C-rich fruit in the present study had no effect overall in preventing the worsening of Fe status in Indonesian pregnant women; only in women with ID at baseline could a positive effect on ferritin be found. Our provision of 100 g of tempeh daily, plus a small amount of animal-source food (small dried fish, meat and liver) with fruit rich in vitamin C, was expected to increase both Fe intake and absorption. We estimated that the higher intake of Fe in our intervention (>3·0 mg of non-haem Fe/d, >0·2 mg of haem Fe/d, the improvement in Fe absorption (>130 mg of vitamin C/d, a small amount of meat) and the reduced intake of inhibitors (black tea at meals) could increase the absorbed Fe from 0·4 mg/d (8 mg × 5 %) to 1·68 mg/d (11·2 mg × 15 %), assuming an average intake of 8 mg of Fe/d in the usual diet and an improvement in absorption from 5 % to 15 %. This is not an exact estimation, but 1·68 mg of absorbed Fe/d could be enough to provide pregnant women with their daily Fe requirements(Reference Beaton21). Quantitative measurements of body Fe based on the ratio of TfR to ferritin can be used to calculate the Fe absorption in women, including pregnant women(Reference Cook, Flowers and Skikne20). Compared with the control group, women receiving supplementary food had an overall smaller decline of 0·44 mg/kg of body Fe. With an average body weight of 50 kg, this corresponds to 22 mg more Fe in women who received the supplementary food. In comparison with the additional Fe intake from the supplementary food (24·6 kg over the whole study period, with an average Fe content of 1·755 mg/100 g), this represents an absorption of only 5·1 % of the Fe in the supplementary food provided. The lack of effect in our study may have been caused by the factors given below.
First, the relatively good Fe status and the lower than expected prevalence of ID in our study population might not have been severe enough to detect an effect and may have biased our results. It is well known that Fe absorption becomes more effective as Fe stores become depleted(Reference Andang'o, Osendarp and Ayah22). In our study, treatment effects were most pronounced in women who had ID at baseline. In these women, the effect of our supplementary food on plasma ferritin concentration was greater than the effect in the control group or in Fe-replete women. Three times larger reductions were seen in Fe-replete women compared with Fe-deficient women with regard to plasma ferritin concentration and body Fe stores.
Second, the amount of Fe in our study was low and the duration of our study may have been inadequate to observe the effects on Fe status as our primary outcome. In comparison with the standard supplementation programme for pregnant women in Indonesia with an additional intake of 5400 mg of Fe (60 mg of Fe for 90 d), the additional amount of Fe in the supplementary food (on average 433 mg over the whole study period) was only approximately 8 %; in general, Fe from food is less bioavailable compared with that from chemical preparations.
Third, the women in the present study had relatively good energy and protein intakes, and their habitual diet contained 1·434 mg of Fe/1000 kJ with a relatively high amount of vitamin C (9·799 mg/1000 kJ). Therefore, the additional vitamin C and other nutrients in our supplementary food might not have been as active as in other kinds of diets. For example, in one study even large amounts of ascorbic acid had only a modest effect on Fe absorption from soya(Reference Gillooly, Torrance and Bothwell23).
Fourth, we did not find a high prevalence of intestinal parasites, which could have increased the requirement for Fe. In the present study, 23·8 % of pregnant women had light infestation with hookworms and 6·7 % had trichuriasis; further, at baseline we could see only a weak effect of trichuriasis but not of hookworm on Hb concentrations.
In addition, we found no evidence that supplementary food had an effect on inflammation status and birth outcomes. The prevalence of inflammation was low and mild. The mean birth weight (3057 g) was comparable to that reported in a previous study on another island of Indonesia(Reference Shankar, Jahari and Sebayang24); however, birth weight in our population was much higher than that in a study in Nepal (2733 g), in which an effect on birth weight could be seen with a daily antenatal multiple micronutrient supplement(Reference Osrin, Vaidya and Shrestha25).
In conclusion, increasing Fe and ascorbic acid from tempeh and vitamin C-rich fruits did not improve the Fe status of pregnant women in rural Indonesia with a relatively good traditional diet. It is possible, however, that our optimized diet could produce a detectable increase in Fe stores in some other target groups and in other situations, as shown in our subgroup analyses for pregnant women with ID.
Acknowledgements
The present research was supported by the Nestlé Foundation (Switzerland) for the Study of Problems of Nutrition in the World. The authors have no conflict of interest to declare. M.W.-E. and S.M. contributed to the supervision of data collection; J.G.E. contributed to blood sample analyses; M.W.-E. contributed to data analyses and to manuscript preparation. All authors contributed to the study design, to data interpretation and to critical revision of the manuscript. The authors thank the pregnant women, their infants and their families for their involvement in the trial. They also thank the field team members (Widya Rahmawati, Yuridayati, Keszia Tuni Sulistyo Utami, Ani Noviani and Wawin Misterianingtyas); the local field staff; the staff of the District Health Department of Karanganyar and Demak; the laboratory staff of the Parasitology Sebelas Maret University, Solo, and of the Diponegoro University, Semarang, staff of the Karanganyar District General Hospital, and of the Clinic Patology Diponegoro University, Semarang; and the health staff of Puskesmas Ngargoyoso, Puskesmas Jenawi, Puskesmas Tawangmangu, Puskesmas Bonang 1, Puskesmas Karangawen 2 and Puskesmas Demak 2, for their support.










