Small for gestational age (SGA) increases neonatal mortality and various infant morbidities, including chronic lung disease, necrotizing enterocolitis, perinatal acidosis, hypoglycaemia, hypothermia, coagulation abnormalities and selected immunological deficiencies(Reference McIntire, Bloom and Casey1,Reference Pallotto and Kilbride2) . It also leads to chronic diseases in later life such as type 2 diabetes, hypertension, obesity, CVD and mental health problems(Reference Hack, Taylor and Drotar3–Reference Weinstock9). The associated economic costs due to immediate neonatal intensive care, ongoing long-term complex health needs and lost economic productivity can be substantial. Unfortunately, modern obstetrics is still unable to predict, prevent or treat SGA(Reference Lockwood10).
Folate acts as a coenzyme in the biosynthesis of purine nucleotides and deoxythymidylic acid which are essential for DNA and RNA synthesis(Reference Krishnaswamy and Madhavan Nair11). While folic acid supplementation has been recommended for prevention of neural tube defects, its impact on other birth outcomes is not fully understood. Epidemiological studies investigating the associations of folic acid supplementation and dietary folate intake with SGA have provided conflicting results(Reference Baker, Mackie and Lean12–Reference Zheng, Guan and Zhao34). Eleven studies found that folic acid supplementation before and/or during pregnancy reduced the risk of SGA(Reference Catov, Bodnar and Olsen15–Reference Dwarkanath, Barzilay and Thomas17,Reference Hodgetts, Morris and Francis20,Reference Li, Li and Ye22,Reference Papadopoulou, Stratakis and Roumeliotaki26,Reference Rolschau, Kristoffersen and Ulrich28,Reference Timmermans, Jaddoe and Hofman30,Reference Yan, Xu and Su32–Reference Zheng, Guan and Zhao34) , but three studies reported no association between folic acid supplementation and SGA(Reference Bukowski, Malone and Porter14,Reference Nilsen, Vollset and Monsen25,Reference Wang, Ge and Zhu31) . In addition, three studies suggested that a high dosage of folic acid may be associated with an increased risk of SGA at birth(Reference Navarrete-Munoz, Gimenez Monzo and Garcia de La Hera23,Reference Navarrete-Munoz, Valera-Gran and Garcia-de-la-Hera24,Reference Pastor-Valero, Navarrete-Munoz and Rebagliato27) . Two studies found that dietary folate intake during periconception or pregnancy was a protective factor for SGA(Reference Dwarkanath, Barzilay and Thomas17,Reference Pastor-Valero, Navarrete-Munoz and Rebagliato27) , but one study found that SGA was not associated with dietary folate intake during the second trimester(Reference Nilsen, Vollset and Monsen25). Five studies reported that higher folate concentrations in blood (erythrocyte or serum) during preconception or pregnancy had a protective effect on SGA(Reference Baker, Mackie and Lean12,Reference Bergen, Jaddoe and Timmermans13,Reference Furness, Yasin and Dekker18,Reference Goldenberg, Tamura and Cliver19,Reference Kim, Ahn and Ryu21) , but one study found that SGA was not associated with folate concentrations(Reference Ronnenberg, Goldman and Chen29). Today, folic acid supplementation and dietary folate intake are recommended to women in many countries. A recommendation to take folic acid supplements starting from 3 months before pregnancy until the end of the first trimester of pregnancy to prevent neural tube defects has been exercised in China since 2009 and users take 400 µg folic acid daily(35). National health authorities in many countries recommend periconceptional folic acid supplementation, and some countries have introduced mandatory folate fortification of foods(Reference Kloosterman36–40). By taking account of the joint effect of folic acid supplementation and dietary folate intake, it might be possible to define the folic acid supplementation scheme mostly likely to affect SGA risk. However, no study has examined the joint effect of folic acid supplementation and dietary folate intake on the risk of SGA.
Approximately one-fifth of the world’s population is Chinese. The rate of SGA infants has increased over recent years, with the reported rate of SGA ranging from 5·82 to 17·5 %(Reference Li, Li and Ye22,Reference Wang, Ge and Zhu31,Reference Yang, Gu and Wei33,Reference Zheng, Guan and Zhao34) . The typical diet of northern China is characterized by low amounts of fresh vegetables and fruits, particularly during the winter, resulting in low blood concentrations of folate(Reference Ren41). Only 10–15 % of women of childbearing age routinely take folic acid supplements(Reference Liu, Jin and Meng42–Reference Zhang, Ren and Li44). Given the low percentage of folic acid supplementation among women of childbearing age, seasonal variation of dietary folate intake and the lack of folic acid fortification in staple foods in China, the Lanzhou birth cohort study provides a unique opportunity to concurrently study the impact of folic acid supplementation and dietary folate intake on SGA.
Materials and methods
Study population
A birth cohort was conducted in 2010–2012 at the Gansu Provincial Maternity and Child Care Hospital (GPMCCH), the largest maternity and child care hospital in Lanzhou, China(Reference Liu, Lv and Zhang45–Reference Zhao, Qiu and Zhang48). After obtaining written consent, an in-person interview was conducted at the hospital by trained study interviewers using a standardized and structured questionnaire to collect information. Maternal characteristics (age, income, education level, parity, BMI, etc.), social factors (employment status, height of partner, etc.), lifestyle factors (smoking status, drinking, etc.) and pre-existing medical and previous obstetric history (history of preterm birth, caesarean section, etc.) were self-reported. Pregnancy complications and birth outcomes (pre-eclampsia, gestational diabetes, birth weight, gender of live birth, etc.) were based on diagnoses from the medical records. All women were interviewed within 1–3 d after delivery. A total of 14 359 eligible women were approached for participation and 10 542 (73·4 %) women completed in-person interviews, with 10 179 singleton live births. Among 363 fetuses, 323 were multiple fetuses and forty fetuses died in utero (>32 weeks) or were stillborn. All study procedures were approved by the human investigation committees at the GPMCCH and Yale University.
Gestational age at delivery was calculated in completed weeks from the first day of the last menstrual period. All self-reported last menstrual period dates were further verified by ultrasound examinations during antenatal care in the hospital. SGA was defined as a birth weight below the 10th percentile of the gestational-age- and gender-specific birth weight standard for Chinese newborns(Reference Dai, Deng and Li49); large for gestational age was defined as a birth weight greater than the 90th percentile of the standard; while appropriate for gestational age (AGA) was defined as a birth weight between the 10th and 90th percentile of the standard. The range of gestational age in the Chinese national standard was from 28 to 44 weeks(Reference Dai, Deng and Li49). For neonates with a gestational age of 22–27 weeks, the US national reference based on 2009–2010 live births was applied as a surrogate(Reference Duryea, Hawkins and McIntire50). After exclusion of large-for-gestational-age births, 8758 (784 SGA and 7974 AGA) were included in the final analysis.
Folic acid supplementation and dietary folate intake
Data collection on folic acid supplementation and dietary folate intake has been described in a previous study and the FFQ was validated(Reference Wang, Zhao and Qiu47). Folic acid supplementation started from 3 months before pregnancy through to the end of the first trimester of pregnancy and users took 400 µg folic acid daily. Briefly, information on folic acid supplements was asked for the following four time periods: preconception (12 months before pregnancy), first trimester (1–13 weeks), second trimester (14–27 weeks) and third trimester (>27 weeks). For each time period, the duration and frequency of folic acid supplementation alone and of folic acid-containing multivitamins were ascertained. Folic acid supplementation users were defined as those who took folic acid supplements alone or folic acid-containing multivitamins before conception or during pregnancy. Preconception and pregnancy users were defined as those who took folic acid supplements alone or folic acid-containing multivitamins before conception and during pregnancy. Preconception-only users were defined as those who took folic acid supplements alone or folic acid-containing multivitamins before conception only. Pregnancy-only users were defined as those who took folic acid supplements alone or folic acid-containing multivitamins during pregnancy only. Non-users were defined as those who never took folic acid supplements alone or folic acid-containing multivitamins before conception and/or during pregnancy. The final variables included folic acid supplement users and non-users; folic acid supplements were classified into three levels by duration of use: before conception and during pregnancy, before conception only and during pregnancy only.
Dietary information was collected via a semi-quantitative FFQ. Daily dietary folate intake was estimated from the frequency of consumption and portion size of food items using the Chinese Standard Tables of Food Consumption(51).
Statistical analysis
Pearson’s χ 2 tests were used to compare selected characteristics between AGA and SGA. Unconditional logistic regression models were used to estimate the odds ratio and 95 % confidence interval for single associations of folic acid supplementation and dietary folate intake with SGA. The interaction analysis of multiplication models, done by logistic regression modelling, estimated the joint effect of folic acid supplementation and dietary folate intake on SGA. Dose–response relationships (and P trend) were calculated by including those categorical levels. According to the literature and the results of univariate analysis, potential confounders including maternal age, monthly income per capita, maternal education level, smoking, maternal employment, pre-pregnancy BMI, weight gain during pregnancy, pre-eclampsia, parity, caesarean section, height of the child’s father, history of preterm birth, total energy intake, dietary folate intake and folic acid supplementation were adjusted for in the multivariable logistic regression model. In some previous studies, the results showed that high dosage of folic acid may be associated with an increased risk of SGA at birth(Reference Navarrete-Munoz, Gimenez Monzo and Garcia de La Hera23,Reference Navarrete-Munoz, Valera-Gran and Garcia-de-la-Hera24,Reference Pastor-Valero, Navarrete-Munoz and Rebagliato27) . Therefore, we conducted a sensitivity analysis restricted to participants with <24 weeks of gestational of age. We assessed goodness-of-fit of the models in the present study and all showed a good fit by the Homser–Lemeshow test (P > 0·05). All analyses were performed using the statistical software package SAS version 9.4.
Results
Table 1 shows the distributions of selected characteristics in participants with SGA and AGA births. Women who had SGA births were more likely to be younger than 25 years old, have less family income and lower education level, be exposed to smoke, be unemployed during pregnancy, have a lower pre-pregnancy BMI, gain less weight during pregnancy, be diagnosed with pre-eclampsia, be multipara, undergo caesarean delivery and have a history of preterm birth. Paternal height of SGA was more likely to be shorter than 175 cm compared with paternal height of AGA. Distributions of alcohol drinking during pregnancy, gestational diabetes and infant’s gender were similar between SGA and AGA.
Table 1 Distributions of selected participant characteristics, according to small-for-gestational-age (SGA) and appropriate-for-gestational-age (AGA) births, among women (n 8758) and their children enrolled in the 2010–2012 Gansu Provincial Maternity and Child Care Hospital birth cohort, Lanzhou, China

* Fisher’s exact test.
Compared with non-users, folic acid supplement users had a reduced risk of SGA (OR = 0·72, 95 % CI 0·60, 0·86; Table 2). The significant reduced odds was seen mainly for those who had used folic acid supplements for more than 12 weeks (OR = 0·78, 95 % CI 0·70, 0·87, P trend < 0·001; OR = 0·962, 95 % CI 0·945, 0·979 per 2-week increase in folic acid supplement use). After stratifying by time period of use, significant associations were observed for those who took supplements before conception and during pregnancy (OR = 0·58, 95 % CI 0·46, 0·75, P trend < 0·001; OR = 0·955, 95 % CI 0·935, 0·976 per 2-week increase) or during pregnancy only (OR = 0·78, 95 % CI 0·65, 0·94, P trend = 0·002; OR = 0·966, 95 % CI 0·942, 0·991 per 2-week increase). No significant association was observed among women who took supplements before conception only. We did not observe significant associations between dietary folate intake and SGA. In addition, we assessed the joint effect of folic acid supplementation and dietary folate intake on SGA and found no significant interaction between them (P interaction = 0·062). Additionally, in the sensitivity analysis restricted to the participants with <24 weeks of gestational age (see online supplementary material, Supplemental Table S1), the results were consistent with the findings of Table 2; significant associations also were observed for those who took supplements before conception and during pregnancy (OR = 0·62, 95 % CI 0·48, 0·81, P trend < 0·001; OR = 0·953, 95 % CI 0·927, 0·980 per 2-week increase).
Table 2 Associations of folic acid supplementation and dietary folate intake with the risk of small for gestational age (SGA) among women (n 8758) and their children enrolled in the 2010–2012 Gansu Provincial Maternity and Child Care Hospital birth cohort, Lanzhou, China
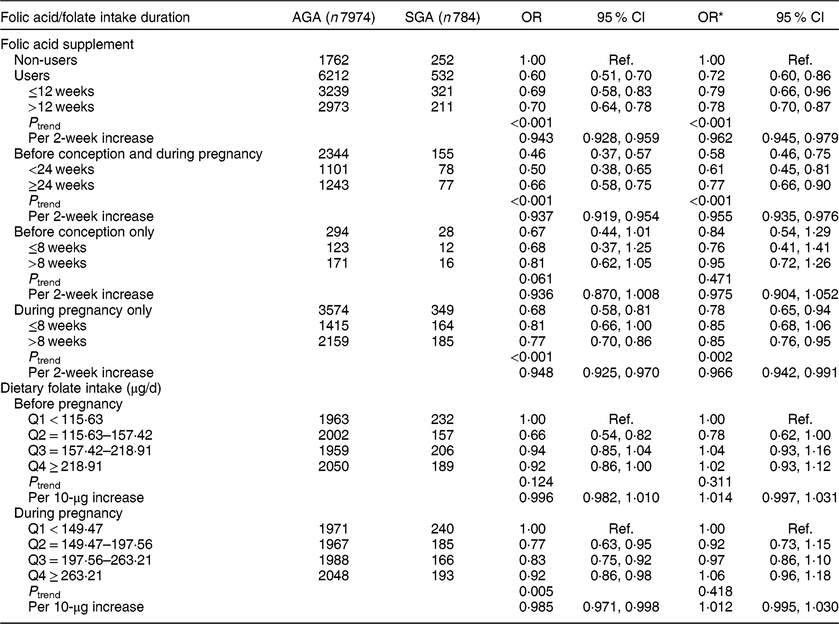
AGA, appropriate for gestational age; Q, quartile; ref., reference category.
Multiplicative interaction on SGA: OR = 0·85 (95 % CI 0·71, 1·01), P = 0·062.
* OR adjusted for maternal age, monthly income per capita, maternal education level, smoking, maternal employment, pre-pregnancy BMI, weight gain during pregnancy, pre-eclampsia, parity, caesarean section, height of child’s father, history of preterm birth, total energy intake, dietary folate intake or folic acid supplement.
We then analysed the data separately for SGA at ≥37 weeks of gestational age and SGA at <37 weeks of gestational age (Table 3). Significant protective effects of folic acid supplement use were seen on SGA at ≥37 weeks of gestational age (OR = 0·70, 95 % CI 0·58, 0·85 for users; OR = 0·59, 95 % CI 0·45, 0·77 for use before conception and during pregnancy, with OR = 0·953, 95 % CI 0·931, 0·976 per 2-week increase in folic acid supplement use; and OR = 0·76, 95 % CI 0·62, 0·93 for use during pregnancy only, with OR = 0·968, 95 % CI 0·941, 0·994 per 2-week increase) and no significant association was observed among women who took supplements before conception only. We did not observe significant associations between folic acid supplement use and SGA at <37 weeks of gestational age. Nor did we observe any significant associations of SGA at ≥37 weeks of gestational age and SGA at <37 weeks of gestational age with dietary folate intake. In addition, we assessed the joint effect of folic acid supplementation and dietary folate intake on SGA at ≥37 weeks of gestational age and SGA at <37 weeks of gestational age; there was no significant interaction on SGA at ≥37 weeks of gestational age (P interaction = 0·698) or on SGA at <37 weeks of gestational age (P interaction = 0·085).
Table 3 Associations of folic acid supplementation and dietary folate intake with the risk of small for gestational age (SGA) at ≥37 weeks of gestational age and SGA at <37 weeks of gestational age among women (n 8758) and their children enrolled in the 2010–2012 Gansu Provincial Maternity and Child Care Hospital birth cohort, Lanzhou, China
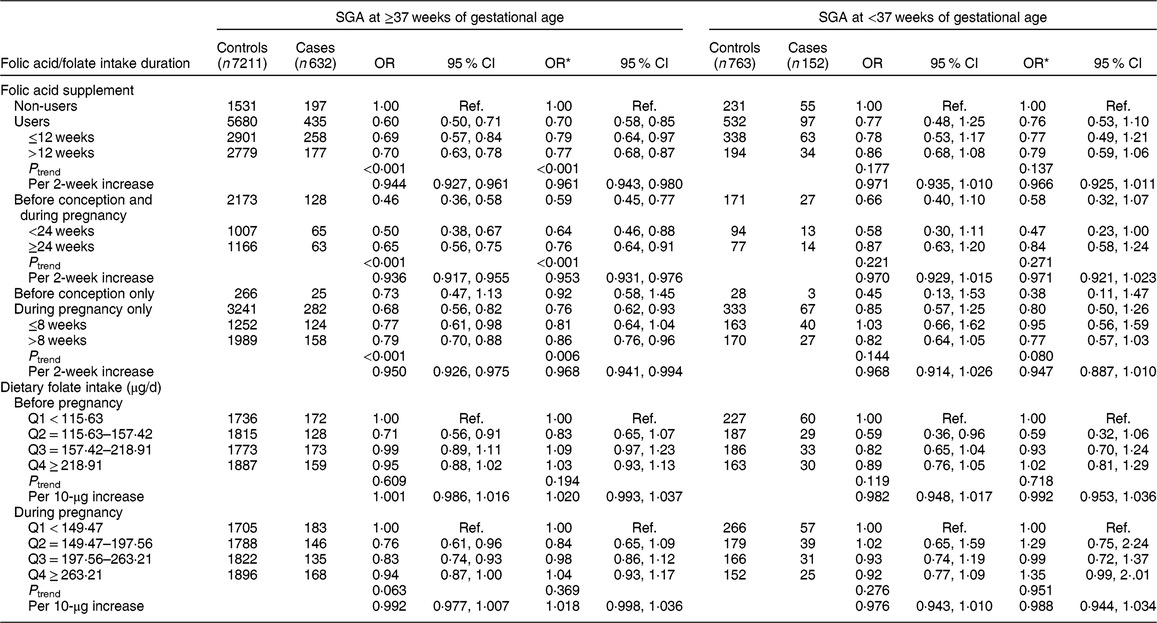
Q, quartile; ref., reference category.
Multiplicative interaction on SGA at ≥37 weeks of gestational age: OR = 1·04 (95 % CI 0·84, 1·29), P = 0·698.
Multiplicative interaction on SGA at <37 weeks of gestational age: OR = 0·64 (95 % CI 0·38, 1·06), P = 0·085.
* OR adjusted for maternal age, monthly income per capita, education level, smoking, employment, pre-pregnancy BMI, weight gain during pregnancy, pre-eclampsia, parity, caesarean section, height of child’s father, history of preterm birth, total energy intake, dietary folate intake or folic acid supplement.
We further stratified the analysis by parity (Table 4). A significant protective effect on nulliparous SGA was observed among folic acid supplementation users (OR = 0·67, 95 % CI 0·54, 0·84), but folic acid supplementation was not related to multiparous SGA (OR = 0·80, 95 % CI 0·61, 1·06). The significant protective effect on nulliparous SGA was observed for those who took supplements before conception and during pregnancy (OR = 0·62, 95 % CI 0·46, 0·83, P trend = 0·002; OR = 0·960, 95 % CI 0·937, 0·984 per 2-week increase in folic acid supplement use) and during pregnancy only (OR = 0·71, 95 % CI 0·56, 0·90, P trend = 0·002; OR = 0·969, 95 % CI 0·941, 0·998 per 2-week increase), but not before conception only (OR = 0·85, 95 % CI 0·51, 1·41). We did not observe significant associations of nulliparous SGA and multiparous SGA with dietary folate intake. In addition, we assessed the joint effect of folic acid supplementation and dietary folate intake on nulliparous SGA and multiparous SGA, and found no significant interaction (nulliparous SGA: P interaction = 0·228, multiparous SGA: P interaction = 0·174).
Table 4 Associations of folic acid supplementation and dietary folate intake with the risk of nulliparous small for gestational age (SGA) and multiparous SGA among women (n 8758) and their children enrolled in the 2010–2012 Gansu Provincial Maternity and Child Care Hospital birth cohort, Lanzhou, China
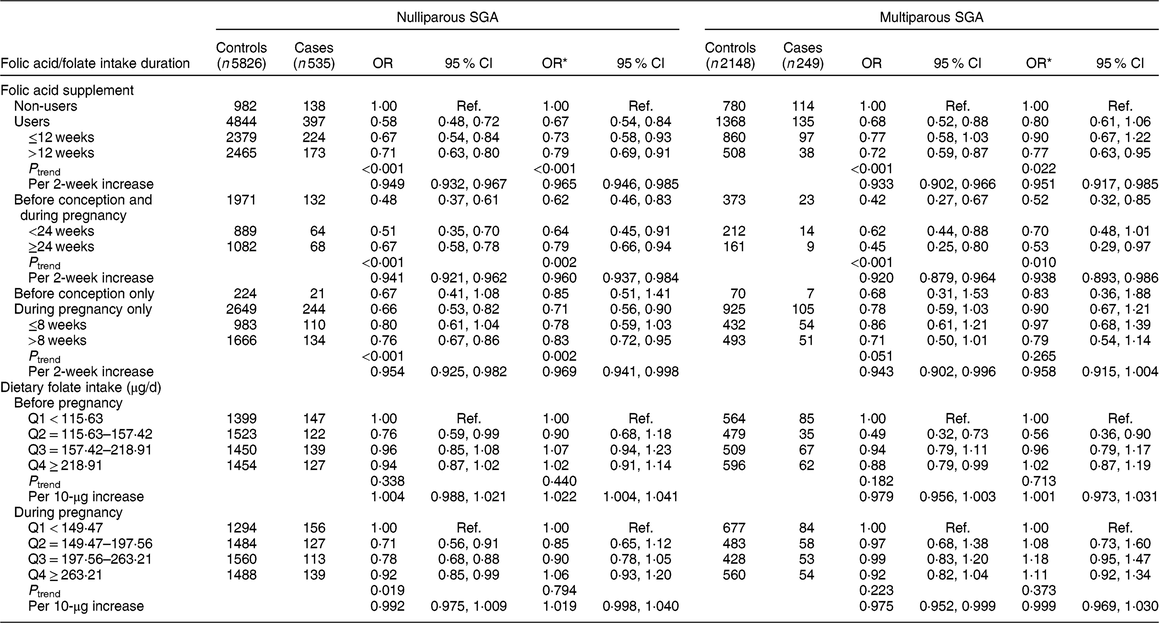
Q, quartile; ref., reference category.
Multiplicative interaction on nulliparous SGA: OR = 0·88 (95 % CI 0·72, 1·08), P = 0·228.
Multiplicative interaction on multiparous-SGA: OR = 0·79 (95 % CI 0·56, 1·11), P = 0·174.
* OR adjusted for maternal age, monthly income per capita, education level, smoking, employment, pre-pregnancy BMI, weight gain during pregnancy, pre-eclampsia, caesarean section, height of child’s father, history of preterm, total energy intake, dietary folate intake or folic acid supplement.
Discussion
To our knowledge, our study represents the first one to concurrently examine folic acid supplementation and dietary folate intake in relation to the risk of SGA. Our study found that folic acid supplementation was associated with a reduced risk of SGA and the protective association was seen mainly for term births and births from nulliparous mothers. No significant interaction between folic acid supplementation and dietary folate intake was found for SGA, SGA at <37 weeks of gestational age, SGA at ≥37 weeks of gestational age, nulliparous SGA and multiparous SGA.
Earlier studies investigating the associations of folic acid supplementation and dietary folate intake with SGA provided conflicting results(Reference Baker, Mackie and Lean12–Reference Li, Li and Ye22,Reference Nilsen, Vollset and Monsen25–Reference Timmermans, Jaddoe and Hofman30) . Variations in dosage of folic acid used, time periods and duration of folic acid supplementation, definition of SGA, and lack of consideration of effects of preterm birth and parity on SGA among different populations might partially contribute to the inconsistent results.
Our study found that folic acid supplementation was associated with a reduced risk of SGA overall. However, when data were stratified by time periods of folic acid supplementation, significant associations were observed for those who took supplements before conception and during pregnancy or during pregnancy only, but not for those taking supplements before conception only, which was consistent with some previous studies(Reference Bergen, Jaddoe and Timmermans13,Reference Dwarkanath, Barzilay and Thomas17–Reference Li, Li and Ye22,Reference Papadopoulou, Stratakis and Roumeliotaki26,Reference Rolschau, Kristoffersen and Ulrich28,Reference Timmermans, Jaddoe and Hofman30,Reference Yang, Gu and Wei33) . The magnitude of the protective effect of folic acid supplementation seemed greater for taking supplements before conception and during pregnancy compared with taking supplements during pregnancy only. While the strengthened association could be due to longer duration of intake, there are possible biological mechanisms that could explain the observed association. Folate is an essential ingredient in the synthesis of methionine and subsequent S-adenosylmethionine, which plays a key role in DNA methylation(Reference Tamura and Picciano52). The epigenome is particularly susceptible during the early stages of embryogenesis(Reference Timmermans, Jaddoe and Silva53). Abnormal folate concentration may cause epigenetic modifications and subsequently result in altered placental and fetal growth patterns(Reference Bailey and Gregory54–Reference Steegers-Theunissen and Steegers58). Furthermore, folate plays a critical role in protein and DNA synthesis(Reference Kloosterman36,Reference Bailey and Gregory54) . Sufficient folate intake promotes a cell-rich placenta(Reference Rolschau59), which is beneficial later in the quantitatively important growth phase of the fetus, and insufficient folate intake during pregnancy will lead concentrations of folate in maternal plasma and erythrocytes to decrease from the fifth month of pregnancy onwards(Reference Cikot, Steegers-Theunissen and Thomas60). Significant fetal growth takes place during the later second trimester and the third trimester, therefore a sufficient supply of folate during pregnancy is critical to maintain normal fetal growth(Reference Thaler61–Reference Scholl, Hediger and Schall63).
Our study found that folic acid supplementation was associated with SGA at ≥37 weeks of gestational age but not with SGA at <37 weeks of gestational age, suggesting that SGA at ≥37 weeks of gestational age and SGA at <37 weeks of gestational age are two different types of SGA and might have different aetiological profiles. To our knowledge, the current study is the first investigating the relationship between SGA at ≥37 weeks of gestational age and folic acid supplementation, and the second study investigating the relationship between SGA at <37 weeks of gestational age and folic acid supplementation, but Chen et al. found that taking folic acid supplementation more than 3 months before pregnancy was associated with a significant reduction in incidence of preterm SGA(Reference Chen, Zhu and Zhu16). So, the biological processes should be studied further.
Our study also found a significant protective effect of folic acid supplementation on nulliparous SGA but not multiparous SGA. It has been suggested that multiparous women offer a more favourable environment for placental and fetal growth by remodelling of the maternal vascular structure in former pregnancies(Reference Kloosterman36,Reference Bleker, Buimer and van der Post55) , which may attenuate the effect of folic acid supplementation on fetal growth. Actually, the biological processes should also be further studied.
Limitations should be considered when interpreting the study results. Information on folic acid supplementation and dietary folate intake was based on self-report, thus potential recall bias was unavoidable. Since the benefit of folic acid on SGA was not well established, there was unlikely differential recall bias associated with SGA. A strong correlation between self-reported folate intake and serum folate concentrations during pregnancy has been suggested(Reference Scholl, Hediger and Schall63). While we collected detailed information on potential confounding variables, residual confounding resulting from unknown sources cannot be ruled out. Although our study had relatively large sample size, limited statistical power was presented for stratified analysis, particularly for SGA at <37 weeks of gestational age. In addition, the representation of the sample in the study was limited because the participation rate was 74 %.
Conclusion
In conclusion, our study suggested a protective effect of folic acid supplementation on risk of SGA and the protective effect varied by preterm status and parity. Future studies are warranted to replicate the findings.
Acknowledgements
Acknowledgements: The authors thank all the study personnel from the GPMCCH for their exceptional efforts on study subject recruitment. Financial support: This study was financially supported by internal funding from the GPMCCH and the National Institutes of Health (grant numbers K02HD70324 and R01ES019587). The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: Y.Z. and J.Q. designed the birth cohort study; H.G., B.M. and M.W. analysed data and wrote the paper; H.G., B.M., M.W., Q.L., L.Y., Y.X., Y.W., X.H., H.C., X.L., L.L., M.Z. and X.X. conducted this research; Y.Z. and H.G. had primary responsibility for the final content. All authors read and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the human investigation committees at the GPMCCH and Yale University. Written informed consent was obtained from all subjects.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980019003331






