Vitamin B12 is an essential micronutrient for human development and health. Vitamin B12 deficiency can lead to a range of diseases, such as anaemia, birth defects and neurological damage.
Low serum vitamin B12 concentrations have been reported in approximately 10 % of the elderly population in industrialized countries and this prevalence increases with age, from approximately 5 % at age 65 years to 20 % at age 85 years(Reference Hin, Clarke and Sherliker1, Reference Clarke, Refsum and Birks2).
In clinical practice, vitamin B12 deficiency is commonly manifested as macrocytic anaemia and through neurological signs; the latter may occur without the presence of anaemia in 20 % of cases(Reference Cuskelly, Mooney and Young3). Even when macrocytosis is considered during diagnosis, increased mean cell volume (MCV) values are, in most cases, related to other conditions such as drug and alcohol abuse. Furthermore, cobalamin and folate deficiencies have been shown to be responsible for macrocytosis in only 6 % of the cases(Reference Cuskelly, Mooney and Young3).
Cobalamin deficiency may be caused by pernicious anaemia, which is an autoimmune disease characterized by gastric mucosa destruction through a primarily cell-mediated process(Reference Masnou, Domenech and Navarro-Llavat4). The factors that contribute to the malabsorption of food cobalamin include chronic presence of Helicobacter pylori, achlorhydria and intestinal microbial proliferation, which can be brought on by antibiotic treatment(Reference Cuskelly, Mooney and Young3, Reference Solomon5).
In patients with pernicious anaemia, studies of methylmalonic acid (MMA) and homocysteine values compared with vitamin B12 levels have determined that metabolites are elevated prior to the development of any clinical abnormalities and often prior to abnormal serum vitamin B12 concentrations(Reference Lewerin, Matousek and Steen6).
A correct diagnosis of vitamin B12 deficiency is complicated due to the poor sensitivity and specificity of conventional kit assays(Reference Stabler and Allen7). The advent of novel assays for holotranscobalamin II (holoTC II), the active fraction of vitamin B12, could improve the detection of vitamin B12 deficiency; however there are many conflicting studies(Reference Clarke, Sherliker and Hin8).
Throughout the deficiency process, the earliest marker of a negative vitamin B12 balance is probably a low holoTC II concentration (<40 pg/ml). As the deficiency progresses, at least one of the metabolic markers (most commonly MMA or less often homocysteine) also increases as vitamin B12 becomes unavailable for the metabolism of these compounds(Reference Raman, Tatsioni and Chung9). Further progression may lead to an elevated MCV and, eventually, outright anaemia.
Importantly, the stage at which neurological damage first appears during decreasing vitamin B12 status is not yet clear. Therefore, exactly when vitamin B12 deficiency becomes clinically important, or, alternatively, when the damage caused by this deficiency first occurs, has not yet been established(Reference Cuskelly, Mooney and Young3).
There are few studies regarding vitamin B12 deficiency in developing countries. A study in Jordan with 216 healthy adult volunteers described a deficiency rate of 48·1 %. In this subgroup, 36·5 % presented with low MCV and 7·7 % with folate deficiency(Reference Fora and Mohammad10). A study with female teenagers (12 to 16 years old) in Maiduguri, Nigeria, showed that 2·4 % presented serum folate concentrations <6.8 nmol/l and 9·0 % had serum vitamin B12 concentrations ≤134 pmol/l(Reference VanderJagt, Spelman and Ambe11). Curiously, most physicians still believe that the most prevalent deficiencies in these populations are Fe and folate deficiencies, neglecting vitamin B12 deficiency.
In Brazil, a late diagnosis of vitamin B12 deficiency is common. This diagnosis is, in most patients, confused with other diagnoses, often leading to a progression of severe and irreversible neurological damage, not to mention the increased cost of misdiagnosis due to unnecessary examinations. Thus, the aim of the present study was to verify the frequency of vitamin B12 deficiency in two Brazilian populations (elderly and adults) and to compare different methods of vitamin B12 deficiency detection.
Methods
Participants
Five hundred participants from health centres of the city of Campinas, São Paulo State, who were submitted to routine check-ups during blood collection, were invited to participate in the study. The 500 individuals agreed to participate, providing written informed consent. The Ethics Committee of the University of Campinas approved the study protocol (CAAE 0349.0.146.000-06).
Protocol
All subjects responded to an FFQ with questions regarding foods of animal origin. As vitamin B12 deficiency is more frequent in elderly individuals than in young subjects, the participants were separated into two groups: 250 participants aged 60 years or more (mean 69 (sd 7) years) and 250 participants from 30 to 59 years of age (mean 40 (sd 8) years).
Venous blood samples were collected and refrigerated until the serum separated at the Haematology Centre within 2 h after blood collection. Serum was separated and stored at −20°C for subsequent measurement of vitamin B12 and creatinine and at −80°C for subsequent MMA measurements; both were protected from light. MMA quantification was carried out only in those participants suspected of having deficiency (one or two vitamin B12 results >200 pmol/l) or with discrepant results according to the two vitamin B12 measurement methods described below. All procedures, refrigeration, storage, thawing and manipulation of the samples, were carried out by one single person. For full blood counts, blood was drawn into Vacutainers containing EDTA.
Those subjects suspected of having B12 deficiency or with discrepant results were asked to attend a medical consultation at the Haematology and Transfusion Centre (Haematology Centre – UNICAMP) and were submitted to gastric endoscopy with biopsy, H. pylori detection and quantification of serum creatinine performed in Modular PP Roche (Indianapolis, IN, USA) equipment (with kit lot 11875418 216) using the modified Jaffé method.
Vibratory sense was tested using a C 128 tuning fork, struck with moderate force against the examiner’s thenar eminence to produce vibration and then applied to the subject’s medial malleoli. Intact vibratory sensation was defined as a perception of vibration, buzzing or tingling. Ataxia was assessed using a timed 50ft walk. The Romberg test was performed and considered positive when the subject swayed or fell while their eyes were closed.
Laboratory methods
Vitamin B12 and folate concentrations were measured at the Department of Clinical Pathology, University of Campinas Hospital, using a Roche Elecys electrochemiluminescence immunoassay (ECI) performed on Elecsys 2010/I and II equipment (with kit lot 173049/174222/176146) and also a DCP (Diagnostic Products Corporation, Los Angeles, CA, USA) MedLab RIA performed on COBAS equipment (with kit lot KDS P1905), with a normality range of 200–900 pmol/l for vitamin B12 and 3–17 ng/ml for folate.
In participants suspected of having B12 deficiency or with discrepant results, MMA was measured by LC coupled to tandem MS with a sensitivity of 0.08–200 μmol/l and a normality range of 0·08–0·25 μmol/l.
Full blood counts were carried out at the Haematology Centre in Campinas using standard methods (reagents: Sheth rinse, Perox I, Perox II, Perox III, Ez Kleen, HGB, Perox Sheath and RBC/PCT) of ADvia 120 (Roche, Dublin, Ireland).
Statistical methods
The results of vitamin B12 quantification were separated into the following ranges: <200, 200–300, 200–400 and >400, for both test methods.
The Wilcoxon test was used to compare the results from the two age groups. The correlation of ECI, RIA and MMA was performed by the Spearman test. Differences were considered significant at P < 0·05.
Results
Participants
None of the participants was vegetarian and all ingested some type of animal-origin food at least once a day, according to the interview and the FFQ completed by them beforehand. Furthermore, the minimum vitamin B12 intake of the studied population was estimated as 1·8 μg/d (Table 1).
Table 1 Characteristics of the total population studied, including the results of vitamin B12 quantification, socio-economic status and food intake frequency obtained from the FFQ: adult and elderly participants, south-east Brazil (n 500)
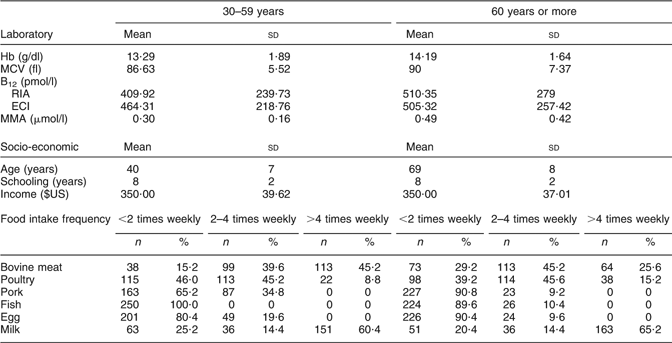
MCV, mean cell volume; ECI, electrochemiluminescence immunoassay; MMA, methylmalonic acid.
Laboratory quantifications
Folate
None of the participants presented folate deficiency, despite the fact that none of them had previously received vitamin B12 or folic acid supplementation.
Blood count
All participants, including those with cobalamin levels <200 pmol/l, presented normal blood count results and MCV <99 fl.
Vitamin B12
Using the lower cut-off laboratory reference range of <200 pmol/l for both vitamin B12 assays, low vitamin B12 concentrations by at least one of the two methods were identified in 7·2 % of the participants aged 60 years or more and in 6·4 % of the participants aged 30–59 years. Thus, thirty-six participants aged 60 years or more and forty-six participants aged 30–59 years were grouped because they were suspected of having B12 deficiency as their results for vitamin B12 were >200 pmol/l or because they had vitamin B12 results >400 pmol/l with a discrepancy of >100 pmol/l between the two cobalamin methods.
The proportion of the results for each group is shown in Fig. 1.
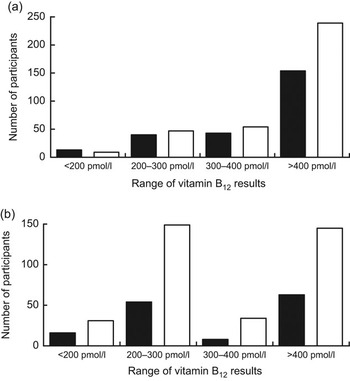
Fig. 1 Vitamin B12 levels (pmol/l) obtained by electrochemiluminescence immunoassay (□) and RIA (▪), with the results separated into the respective ranges (<200 pmol/l, 200–300 pmol/l, 300–400 pmol/l and >400 pmol/l), among participants from south-east Brazil aged (a) 30–59 years (n 250) and (b) 60 years or more (n 250)
Differences between the test methods
Vitamin B12 quantification resulted in the following: (i) for participants aged 60 years or more, six were considered deficient by both methods, ten by RIA only and two by ECI only; and (ii) for participants aged 30–59 years, four were considered deficient by both methods, seven by RIA only and five by ECI only.
A trend to lower vitamin B12 levels in the younger subjects was detected by ECI. However, no difference in vitamin B12 levels measured by RIA was detected between groups (Table 2).
Table 2 Results of vitamin B12 and MMA quantificationFootnote * for the studied population: elderly and adult participants, south-east Brazil

ECI, electrochemiluminescence immunoassay; MMA, methylmalonic acid.
* Vitamin B12 quantification was performed in all participants (n 500); MMA quantification was performed only in participants with a suspicion for vitamin B12 deficiency (n 82).
The correlation of vitamin B12 results (ECI v. RIA) of all participants (n 500) was extremely high (Spearman’s ρ = 0·84, P < 0·0001), as demonstrated in Fig. 2(a). As the categories drew near deficiency, the correlation was reduced. Considering just those participants with vitamin B12 <400 pmol/l (n 242) the correlation was still high (Spearman’s ρ = 0·49, P < 0·001) as shown in Fig. 2(b); however, when considering just those participants with vitamin B12 results <300 pmol/l (n 129, Fig. 2(c)) and <200 pmol/l (n 34, Fig. 2(d)) the correlation decreased (Spearman’s ρ = 0·29, P < 0·001 and Spearman’s ρ = −0·12, P = 0·50 respectively).

Fig. 2 Correlation between vitamin B12 levels (pmol/l) obtained by electrochemiluminescence immunoassay (ECI) and RIA among adult and elderly participants from south-east Brazil: (a) all participants (n 500, Spearman’s ρ = 0·84, P < 0·0001); (b) considering values <400 pmol/l for both tests (n 242, Spearman’s ρ = 0·49, P < 0·001); (c) considering values <300 pmol/l for both tests (n 129, Spearman’s ρ = 0·29, P < 0·001); (d) considering values <200 pmol/l for both tests (n 34, Spearman’s ρ = –0·12, P = 0·50)
Quantification of methylmalonic acid and creatinine
These tests were carried out only in the participants with a suspicion for vitamin B12 deficiency or with discrepant results (thirty-six participants aged 60 years or more, forty-six participants aged 30–59 years).
MMA levels were significantly higher in the older participants (w = 1090·5, P = 0·007) than in the younger ones (Table 2). A much higher correlation of MMA v. RIA was observed compared with the correlation of MMA v. ECI (Spearman’s ρ = −0·34, P = 0·014 v. Spearman’s ρ = −0·27, P = 0·0017; Figs 3(a) and (b), respectively).

Fig. 3 Correlation between methylmalonic acid (MMA) levels (μmol/l) and vitamin B12 levels (pmol/l) among adult and elderly participants from south-east Brazil: (a) MMA v. cobalamin by RIA (n 82, Spearman’s ρ = −0·34, P = 0·0017); (b) MMA v. cobalamin by electrochemiluminescence immunoassay (ECI; n 82, Spearman’s ρ = −0·27, P = 0·014)
Importantly, higher results of MMA quantification do not always correlate with vitamin B12 deficiency. According to MMA levels, cobalamin deficiency was estimated as being present in over 11 % of both groups studied (Table 3).
Table 3 Vitamin B12 levels according to MMA quantification in elderly and adult participantsFootnote *, south-east Brazil

MMA, methylmalonic acid; ECI, electrochemiluminescence immunoassay.
* Each participant has been considered only once, distributed among the different ranges totalling eighty-two participants (thirty-six aged 60 years old or more and forty-six aged 30–59 years).
All of these participants presented normal levels of creatinine, excluding the hypothesis of increased serum MMA due to renal dysfunction.
Microbiological and neurological tests
These tests were carried out only in the participants with a suspicion for vitamin B12 deficiency or with discrepant vitamin B12 results and who returned to medical consultation (thirty-two participants aged 60 years or more and twenty-eight participants aged 30–59 years).
A positive test for H. pylori was found in 35·7 % of the older participants and 64·3 % of the younger individuals. In 78·6 % of these participants, MMA was above 0·25 μmol/l as shown in Table 4.
Table 4 Relationship of neurological tests and MMA quantification in elderly and adult participants, south-east Brazil

MMA, methylmalonic acid.
Regarding neurological tests, 12·5 % of participants aged 60 years or more v. 3·6 % of participants aged 30–59 years presented with ataxia; correspondingly 18·7 % v. 7·1 % presented with positive Romberg signal and decreased vibration sense.
Discussion
The present study showed that vitamin B12 deficiency is frequent among Brazilian adults, and can reach over 6 % when using an immunological assay or at least 11 % if MMA quantification is used. Folate deficiency was not detected in any of the 500 participants of our studied population; however, Brazilian flour is supplemented with 250 μg folic acid/100 g flour.
The fact that none of the participants presented macrocytosis or anaemia may corroborate the hypothesis that folic acid fortification masks vitamin B12 deficiency. Therefore, the results of our study showed that neurological damage is present in populations where vitamin B12 deficiency is frequent whereas no folic acid deficiency was detected. This may have important public health implications. Actions may be need to be taken to prevent irreversible neurological damage by lowering the present folic acid supplementation level, or by fortifying flour with folic acid and vitamin B12, and/or by early detection of neurological damage in the population.
Thus, our study showed that, in reality, vitamin B12 deficiency and not folate deficiency is a public health issue in the studied population. This finding is similar to that of VanderJagt et al.’s study in Maiduguri, Nigeria, where they concluded that the teenage females studied had a greater risk for vitamin B12 deficiency than for folate deficiency(Reference VanderJagt, Spelman and Ambe11).
The vitamin B12 deficiency detected here was not related to inadequate intake or nutritional deficiency, as all participants ingested at least 1·8μg cobalamin/d. In addition, most of the subjects with suspected vitamin B12 deficiency had positive results for H. pylori, which is known to sometimes cause a reduction in absorption due to gastric mucosal atrophy. This leads us to propose that vitamin B12 deficiency in our population is caused by malabsorption of food cobalamin. This result is in accordance with Cuskelly et al., who described that nutritional deficiency was rare among healthy adults, even when considering elderly adults (<5 %)(Reference Cuskelly, Mooney and Young3).
We believe that one of the strong points of our study lies in the reliability of the procedures, as rigorous criteria were used for handling the samples in order to minimize factors which could render discrepancy in the results. Furthermore, the samples were representative of the young and elderly population in a city with a million inhabitants in the most populated state of Brazil, with a great migratory flow and a great miscegenation of European, African, Indigenous and Asian descendants, with people coming from all over the country. However, a limitation of the study was the difficulty of arriving at a correct diagnosis for vitamin B12 deficiency due to the discrepancy of some results complicated by the poor sensitivity and specificity of conventional kit assays, as mentioned by Stabler and Allen(Reference Stabler and Allen7). In fact, the discrepancy observed between RIA and ECI, especially as they tend to lower cobalamin levels, confirms that there is no gold standard method for vitamin B12 measurement yet, as per Green’s comment relating to Solomon’s study(Reference Green12).
In the present study, cobalamin levels measured by RIA presented a greater correlation with MMA levels, suggesting that this method is more reliable than the ECI method. The variance we found between the methodologies reinforces the results found by Kumar et al., who measured vitamin B12 in 300 Indian subjects using three different techniques and detected deficiency based on one methodology though not on the others(Reference Kumar, Ghosh and Das13).
Our results have shown that the frequency of high serum MMA in elderly individuals is higher than in younger individuals, corroborating the finding of Raman et al. who reported that elevated MMA precedes low serum vitamin B12 levels or that MMA quantification could be more sensitive(Reference Raman, Tatsioni and Chung9). However, this is an expensive assay and few laboratories provide this methodology. In addition, MMA values are difficult to interpret, as we may see false positive elevated MMA and false negative elevated MMA. In addition, clinical deficiencies associated with vitamin B12 deficiency could be multifactorial. The quantification of this metabolite may be misinterpreted and a cut-off is difficult to establish. In our study, the cut-off value that best correlated with low levels of vitamin B12 was 0·25 μmol/l (Table 3). According to our study, abnormalities in neurological data (ataxia, Romberg and vibration sense) showed a mean and median of MMA quantification of 0·35 μmol/l, indicating that subjects with values below 0·35 μmol/l should be considered as cobalamin-deficient. Therefore, a cut-off of 0·25 μmol/l comprised a higher number of patients with abnormalities in neurological tests than would a cut-off of 0·4 μmol/l (Table 4).
An early diagnosis of vitamin B12 deficiency remains difficult. Solomon suggested that there are three types of patients who respond to cobalamin therapy: (i) patients with vitamin B12 deficiency due to decreased dietary intake and absorption, showing low cobalamin and high MMA; (ii) those who have impaired transport, impaired coenzyme formation, abnormal apoenzymes and cobalamin inhibitors, showing normal cobalamin and high MMA; and (iii) those who, due to pharmacological effects on cobalamin-related processes and cobalamin-unrelated process, present normal cobalamin and normal MMA(Reference Solomon5).
Therapeutic trial is a practical diagnostic test which still has a role in internal medicine, and is quite important in order to detect the epidemiology of vitamin B12 deficiency in populations and to indicate, as quickly as possible, the correct treatment. This is the first study that shows that vitamin B12 deficiency is frequent among Brazilian adults.
Acknowledgements
The study was supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo; grant number 06/57665-0), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior). There are no conflicts of interest regarding the present manuscript. J.M.A.B. and F.F.C. co-oriented the present work. S.T.O.S. oriented the present work. We are grateful to Mr Roberto Zulli for performing the statistical analysis, to Ms Raquel S Foglio for the English revision, and to Mr Gilberto Fernandes, Ms Laurione Oliveira and Ms Ana Claudia Alessio for technical support.









