The metabolic syndrome (MetS) refers to the clustering of cardiovascular risk factors driven by peripheral insulin resistance. Data from recent studies involving a population of children and adolescents indicate that the prevalence of MetS varies between 3 % and 12 %( Reference Tailor, Peeters and Norat 1 ). Suggestions for future research include establishing which individual components of the MetS cluster confer the greatest risk on future morbidity in children. There is no universally accepted definition of MetS in childhood( Reference Weiss 2 ) but for investigating associations between cardiorespiratory fitness (CRF) and metabolic risk, a total risk score has been calculated in various studies as the sum of Z-scores for the single risk factors defining MetS( Reference Steele, Brage and Corder 3 ).
However, the International Diabetes Federation consensus defines MetS in children and adolescents as central obesity plus any two of the following classical parameters: (i) raised TAG levels, (ii) reduced HDL cholesterol (HDL-C) levels, (iii) hypertension and (iv) elevated fasting plasma glucose( Reference Zimmet, Alberti and Kaufman 4 ). Moreover, some non-classical factors such as plasma uric acid elevation have been associated with features of insulin resistance; indeed, it has been suggested that uric acid could be a key link for the diagnosis of MetS in obese prepubertal children( Reference Gil-Campos, Aguilera and Cañete 5 ). The homeostasis model assessment–insulin resistance index (HOMA-IR) and levels of total cholesterol, LDL cholesterol (LDL-C), apo-A1 and apo-B( Reference Gil-Campos, Aguilera and Cañete 5 – Reference Sellers, Singh and Sayers 7 ), transaminases( Reference Bouglé, Zunquin and Sesbouë 8 ) and C-reactive protein (CRP)( Reference Mauras, Delgiorno and Kollman 9 ) are also altered in children with MetS, but they are considered non-traditional features of MetS.
Going from moderate to high levels of CRF has consistently been associated with a lower risk of developing MetS, CVD or type 2 diabetes( Reference Kriemler, Manser-Wenger and Zahner 10 ). CRF involves a set of health or skill-related attributes with a strong genetic component that remains relatively static, needing some time to change, and it is assessed using a battery of field tests. Nevertheless, variability in CRF is known to influence metabolic status( Reference Steele, Brage and Corder 3 ). Physical activity (PA) is often used interchangeably with energy expenditure and physical fitness and has been defined as any bodily movement produced by skeletal muscles which results in energy expenditure. Some authors suggest that CRF and PA may affect metabolic risk but the relationship is weak and they may act through different pathways( Reference Ekelund, Anderssen and Froberg 11 , Reference Froberg and Andersen 12 ).
In children, some studies have shown that CRF is independently associated with clustered metabolic risk factors( Reference Ekelund, Anderssen and Froberg 11 , Reference Ruiz, Ortega and Rizzo 13 ). Non-traditional factors have been also researched in children in relation to CRF( Reference Bouglé, Zunquin and Sesbouë 8 , Reference Invitti, Maffeis and Gilardini 14 ). Nevertheless, further research is needed to ascertain whether changes in fitness in the general population of children predict changes in cardiovascular risk profile and whether fitness and fatness independently influence metabolic risk( Reference Bouglé, Zunquin and Sesbouë 8 , Reference Suriano, Curran and Byrne 15 ). Thus the aim of the present study was to assess classical and non-classical metabolic risk biomarkers, including insulin resistance, plasma lipid profile, uric acid, liver transaminases and CRP, in prepubertal healthy children with different levels of CRF.
Experimental methods
Participants and design
A group of 141 healthy children, eighty-eight boys (≤10 years, n 52; >10 years, n 36) and fifty-three girls (all ≤10 years), at prepubertal stage was selected from local elementary schools in Córdoba, Spain. Children were asked to perform a 20 m shuttle run test (20-mSRT) in order to evaluate their CRF.
Inclusion criteria were age 7–12 years and prepubertal stage (Tanner stage I), as validated by appropriate plasma sex hormone levels. Exclusion criteria were as follows: presence of pubertal development, disease, long periods of rest after illness, use of any medication that alters blood pressure or glucose or lipid metabolism, consumption of any special diet and failure to get the same record reached in the first attempt in the 20-mSRT( Reference Hasselstrøm, Hansen and Froberg 16 ). Written informed consent was obtained from parents or legal guardians and the study was approved by an institutional ethics committee at the University Reina Sofia Hospital.
The validated scale developed by Olds et al.( Reference Olds, Tomkinson and Léger 17 ) was used to measure CRF after the 20-mSRT. The test performances are expressed as mean and standard deviation relative to all children of similar age and sex from all countries. In the present study, children were split into two groups according to the methodology used in previous studies( Reference García-Artero, Ortega and Ruiz 18 , Reference McGavock, Torrance and McGuire 19 ): (i) the children recording a score equal to or greater than the average reference value (seventy-five participants) were assigned to the group designated ‘equal or higher cardiovascular fitness’ (EHCF); and (ii) those with less-than-average scores (sixty-six participants) were assigned to the group ‘low cardiovascular fitness’ (LCF).
Furthermore, children were divided into quintiles after estimating their VO2max obtained by cross-referencing the final level and shuttle number completed, using the formula developed by Leger et al.(
Reference Léger, Mercier and Gadoury
20
) (Y, ml/kg body weight per min), from the speed (X, km/h) corresponding to that stage (speed = 8+0·5 stage no.) and age (A, years): ![]() . VO2max correlates oxygen consumption with exercise duration, and estimated relative VO2max and extrapolated absolute VO2max are used to determine fitness. Thus top and bottom groups were compared.
. VO2max correlates oxygen consumption with exercise duration, and estimated relative VO2max and extrapolated absolute VO2max are used to determine fitness. Thus top and bottom groups were compared.
Physical examination and measurements
Anamnesis and a physical examination were assessed by paediatricians despite illness in all of the children. Sexual maturity was observed by physical examination according to the Tanner five-stage scale( Reference Tanner 21 ).
Weight and height were measured by standard techniques, using a beam balance and a precision stadiometer (SC700; Seca, Hamburg, Germany), with participants lightly dressed and barefooted. BMI was calculated as weight (kg)/[height (m)]2. Waist circumference was measured in duplicate with an inelastic tape according to standardized methods. These anthropometric measurements were compared with Spanish reference standards( Reference Sobradillo, Aguirre and Aresti 22 , Reference Moreno, Pineda and Rodríguez 23 ) and with the age- and sex-specific cut-off points proposed by Cole et al.( Reference Cole, Bellizzi and Flegal 24 ) to define obesity. Thus we ensured that most of the children of the study were healthy.
Systolic and diastolic blood pressures (BP) were measured in the right arm in a sitting position, using a random-zero sphygmomanometer (Dinamap V-100; GE Healthcare, Spain) after the participants had rested without changing position for at least 5 min.
Children were observed while engaged in an after-school programme at least three times weekly for at least 1 year to estimate PA. Additionally, a short test based on the validated questionnaire of the National Institute of Child Health and Human Development( Reference Pianta 25 ) was used for both groups to obtain information about PA practice and sedentary habits.
Evaluation of cardiorespiratory fitness
Standardized Eurofit battery tests( Reference Léger, Mercier and Gadoury 20 , 26 ) were performed to evaluate fitness; the 20-mSRT was used to assess CRF, and upper and lower body strength were also evaluated.
The 20-mSRT required participants to run back and forth between two lines set 20 m apart. Running speed started at 8·5 km/h and increased by 0·5 km/h each minute, reaching 18·0 km/h at minute 20. Running speed cues were indicated by signals emitted by a commercially available CD-ROM. Participants were allowed to voluntarily withdraw from the test after being verbally encouraged to maximally perform during each assessment. The test finished when the participant failed to reach the finishing lines concurrent with the audio signals on two consecutive occasions( Reference Vale, Santos and Soares-Miranda 27 ).
Upper-body muscular strength was assessed by means of the handgrip strength and the bent arm hang tests with a digital hand dynamometer (Takei TKK Q4-5110, precision 0·1 kg and range 5 to 100kg; Takei Scientific Instruments Co., Ltd, Niigata, Japan) and another test evaluating the number of abdominals performed within 30 s. Lower-body muscular strength was assessed by using the standing broad jump test.
Sampling
Baseline fasting blood samples were obtained from all children using an indwelling venous line to draw a 3 ml sample for the measurement of plasma glucose and insulin levels, lipid profiles and uric acid levels.
Biochemical analysis
Fasting gonadotrophins and sex hormones – follicle stimulating hormone (CV = 3·6 %), luteinizing hormone (CV = 3·1 %), testosterone (CV = 2 %) and oestradiol (CV = 1·8 %) – were measured by chemiluminescence using an automatic analyser (Architect I4000; Abbott Laboratories, Abbott Park, IL, USA) to validate that the children selected by clinical signs and Tanner stage were truly prepubertal.
Glucose was analysed using the glucose oxidase method in an automatic analyser (CV = 1 %). Plasma TAG (CV = 1·5 %), total cholesterol (CV = 0·9 %), HDL-C (CV = 0·8 %), LDL-C (CV = 1·5 %), apo-A1 (CV = 1·7 %), apo-B (CV = 2·6 %), uric acid (CV = 1·9 %), aspartate aminotransferase (CV = 1·7 %), alanine aminotransferase (CV = 3 %) and CRP were also measured using an automatic analyser (Accelerator APS system, Architect-c16000; Abbott Laboratories). CRP (CV = 4 %) was measured by particle-enhanced turbidimetric immunoassay (Dade Behring Inc., Deerfield, IL, USA). Plasma insulin was analysed by RIA with an automatic analyser for microparticles (Axsym; Abbott Laboratories). Insulin resistance was calculated by means of HOMA-IR, defined by the equation: HOMA-IR = [fasting glucose (mmol/l) × fasting insulin (μU/ml)]/22·5.
The criteria using classical features agreed by the International Diabetes Federation in 2007( Reference Zimmet, Alberti and Kaufman 4 ) were used in the present study to estimate the presence of MetS in the sample of prepubertal children.
Statistical analysis
Data were expressed as means and standard deviations. Normal distribution of data was assessed by the Shapiro–Wilk test. Homogeneity of variances was estimated using the Levene test. Comparison of means between groups for continuous variables with normal distribution was done using Student's t test for unpaired samples and for those with an asymmetric distribution by the Mann–Whitney U test. The χ 2 test was applied for comparison of proportions. Comparisons between the two groups of children after tests were performed using analysis of covariance after adjusting for age, sex and BMI. Correlations between variables were assessed using Pearson's test.
Cohen typified differences (d) in the studied parameters between children in the top and bottom quintiles of fitness and effect size correlation were calculated in order to quantify the magnitude of the difference (effect size).
The statistical software package PASW® Statistics 18 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
Anthropometric and BP measurements and PA performance between groups of children with different levels of CRF are shown in Table 1. BMI and waist circumference values were significantly higher in the LCF group than in the EHCF group but none of these children were obese. There were no differences in BP levels between both groups.
Table 1 Anthropometric parameters, blood pressure and physical activity as a function of cardiorespiratory fitness level in healthy prepubertal children (n 141) aged 7–12 years, Córdoba, Spain

EHCF, equal or higher cardiovascular fitness; LCF, low cardiovascular fitness; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; 20-mSRT, 20 m shuttle run test; abdominals, abdominal exercises; HJ, horizontal jump; RHD, right hand dynamometry; LHD, left hand dynamometry; PPA, performed physical activity.
*Statistical significance after application of a Student t test to data expressed as mean and sd.
†Statistical significance after application of a χ 2 test to data expressed as a percentage.
For the Eurofit physical test battery, the EHCF group displayed significantly higher results in the abdominals test and the horizontal jump test; no between-group differences were observed in the dynamometry test. Most children (72 %) in the EHCF group practised PA regularly in an after-school programme (Table 1). A positive significant correlation was observed between CRF and PA (r = 0·346; P < 0·001).
Results for classical MetS biochemical parameters were as follows: the LCF group displayed significantly higher TAG and lower HDL-C levels, as well as significantly lower values for the non-traditional lipid marker apo-A1 (Fig. 1). The LCF group also displayed significantly higher plasma glucose (Fig. 2(a)) and insulin (Fig. 2(b)) and HOMA-IR scores (Fig. 2(c)).
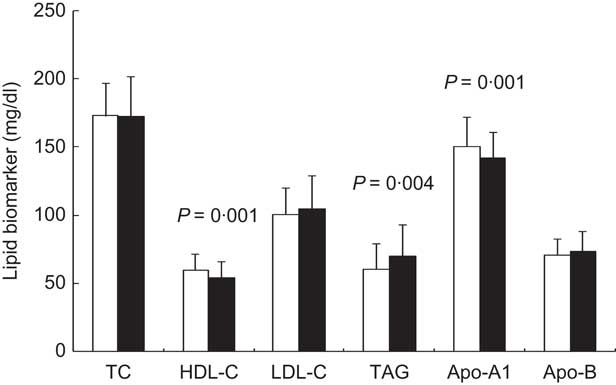
Fig. 1 Lipid biomarkers (TC, total cholesterol; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol) in healthy prepubertal children (n 141) aged 7–12 years, Córdoba, Spain, according to cardiorespiratory fitness (![]() , equal or higher cardiovascular fitness (EHCF) group;
, equal or higher cardiovascular fitness (EHCF) group; ![]() , low cardiovascular fitness (LCF) group). Values are means and standard deviations represented by vertical bars; P values indicate statistical significance after application of a Student t test to the data
, low cardiovascular fitness (LCF) group). Values are means and standard deviations represented by vertical bars; P values indicate statistical significance after application of a Student t test to the data
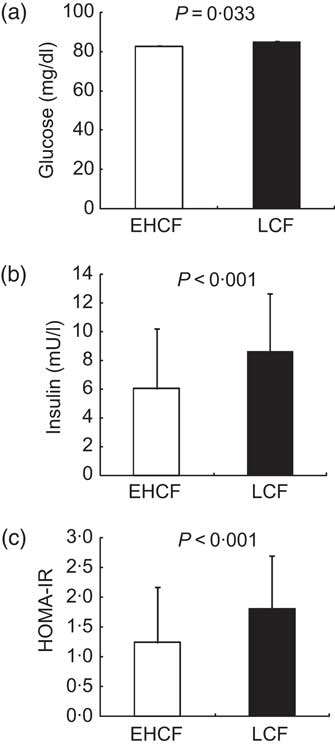
Fig. 2 Insulin resistance biomarkers in healthy prepubertal children (n 141) aged 7–12 years, Córdoba, Spain, according to cardiorespiratory fitness (![]() , equal or higher cardiovascular fitness (EHCF) group;
, equal or higher cardiovascular fitness (EHCF) group; ![]() , low cardiovascular fitness (LCF) group): (a) plasma glucose; (b) plasma insulin; (c) homeostasis model assessment–insulin resistance index (HOMA-IR). Values are means and standard deviations represented by vertical bars; P values indicate statistical significance after application of a Student t test to the data
, low cardiovascular fitness (LCF) group): (a) plasma glucose; (b) plasma insulin; (c) homeostasis model assessment–insulin resistance index (HOMA-IR). Values are means and standard deviations represented by vertical bars; P values indicate statistical significance after application of a Student t test to the data
With regard to non-classical MetS biomarkers, the EHCF group had significantly higher aspartate aminotransferase levels (Fig. 3(a)), and CRF was associated with this transaminase (r = 0·266; P = 0·002). Plasma uric acid (Fig. 3(b)) and CRP levels (Fig. 3(c)) were significantly higher in the LCF group than in the EHCF group.

Fig. 3 Non-traditional biomarkers in healthy prepubertal children (n 141) aged 7–12 years, Córdoba, Spain, according to cardiorespiratory fitness (![]() , equal or higher cardiovascular fitness (EHCF) group;
, equal or higher cardiovascular fitness (EHCF) group; ![]() , low cardiovascular fitness (LCF) group): (a) aspartate aminotransferase (AST); (b) uric acid; (c) C-reactive protein (CRP). Values are means and standard deviations represented by vertical bars; P values indicate statistical significance after application of a Student t test to the data
, low cardiovascular fitness (LCF) group): (a) aspartate aminotransferase (AST); (b) uric acid; (c) C-reactive protein (CRP). Values are means and standard deviations represented by vertical bars; P values indicate statistical significance after application of a Student t test to the data
After adjustment for BMI, age and sex, no significant differences were found between groups for the biomarkers analysed. However, using the criteria suggested by Zimmet et al.( Reference Zimmet, Alberti and Kaufman 4 ), 4·25 % of the children were diagnosed as having MetS and all of them belonged to the LCF group. In the correlation analysis, CRF was inversely associated with HOMA-IR (r = −0·240; P = 0·05), TAG (r = −0·196; P = 0·023), LDL-C (r = −0·227; P = 0·008) and uric acid (r = −0·178; P = 0·042).
Moreover, when we compared the top and the bottom quintiles after estimating VO2max data, we found differences between these groups regarding age, height and systolic and diastolic BP. Children included in the bottom quintile were older and taller and showed higher BP levels than the children in the top quintile, who presented better CRF levels. Apart from the results obtained in relation to EHCF and LCF groups, we also found higher LDL-C and apo-B levels in the lower quintile (Table 2).
Table 2 Comparison between the top and bottom quintiles of the studied parameters after estimating VO2max: healthy prepubertal children (n 141) aged 7–12 years, Córdoba, Spain

Quintile 5, top quintile with the best fitness; quintile 1, bottom quintile with the worst fitness; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; HOMA-IR, homeostasis model assessment–insulin resistance index; CRP, C-reactive protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
*Statistical significance after application of the Student t test or the Mann–Whitney U test to data expressed as mean and sd; Cohen d and effect size analysis has been done for these groups.
As far as the top and bottom quintiles are concerned, we did not find relevant differences between groups due to the effect size when Cohen's d and effect size correlations were applied. Most of these values were between 0·2 and 0·8 (Table 2), which indicates a moderate effect size.
Discussion
In the present study, the major finding was that prepubertal children in the LCF group (with low CRF) displayed higher levels for some MetS risk factors such as lipid profile and insulin resistance and for certain non-traditional markers such as uric acid and CRP than did children in the EHCF group. After adjustment for BMI, age and sex, there were no significant between-group differences, suggesting a probable influence of body mass on fitness condition. Moreover, the correlations between traditional and non-traditional risk factors of MetS suggest that individual components of MetS might be influenced by CRF.
Paediatric data are inconsistent regarding whether fitness or fatness is most relevant to health outcomes in children. Only recently, a small number of prospective studies systematically examined the independent and joint associations of CRF and body mass with health outcomes( Reference LaMonte and Blair 28 ). Some research in children suggests that CRF has a protective influence on metabolic health( Reference Ekelund, Anderssen and Froberg 11 , Reference Allen, Nemeth and Clark 29 ) whereas other studies report that the effects of fitness may be indirect, mediated through its relationship to fatness( Reference Allen, Nemeth and Clark 29 , Reference Thomas, Cooper and Williams 30 ); fatness appears to be more predictive of cardiovascular risk than CRF( Reference Froberg and Andersen 12 , Reference Ruiz, Ortega and Rizzo 13 ).
In the present study, CRF was assessed using the 20-mSRT( Reference Vale, Santos and Soares-Miranda 27 ); since this is a weight-bearing activity, the test might conceivably prompt overestimation of the relationship between CRF and both fatness and MetS risk factors. In other studies, however, measures were not modified by weight status( Reference Kriemler, Manser-Wenger and Zahner 10 ) and others support the validity of using clinical measurements of physical fitness to predict insulin resistance. Low CRF is strongly associated with high BMI and recent investigations suggest that further research is required to explore more fully this relationship( Reference Suriano, Curran and Byrne 15 , Reference Vale, Santos and Soares-Miranda 27 , Reference Aires, Silva and Silva 31 ). The results obtained in the current study provide additional information about non-traditional metabolic factors in prepubertal healthy children in relation to different levels of CRF and the influence of fatness.
In the present work, children in the EHCF group had lower BMI and a better metabolic profile (lower levels of TAG and higher levels of HDL-C and apo-A1, and lower levels of insulin and HOMA-IR) than did children from the LCF group, suggesting that children with good CRF seem to have better weight status and enjoy better metabolic health. Moreover, this group had lower uric acid and CRP levels. Similarly to our study, higher cardiovascular fitness has been associated with a favourable metabolic profile( Reference Froberg and Andersen 12 ).
The inverse association seems to be consistent with some findings in adolescent studies( Reference Twisk, Kemper and van Mechelen 32 – Reference Kelishadi, Cook and Amra 34 ) although others do not find these associations with insulin resistance markers( Reference Shaibi, Ball and Cruz 35 ). The clinical value of fasting glucose as a risk biomarker has been questioned, although in our study there were significant differences between CRF groups. In the LCF group, glucose, insulin and HOMA-IR were higher than in the EHCF group, although neither group reached pathological levels (Fig. 2(c)). After adjustment for BMI, however, there were no significant between-group differences, suggesting an influence of body mass on glucose metabolism. In a sample of 1140 children, Ruiz et al.( Reference Ruiz, Ortega and Rizzo 13 ) found a positive association between these parameters and body fat after adjusting for CRF, and a negative association between both HOMA-IR and fasting insulin and CRF. This inverse association appears to be consistent with findings in adolescent research( Reference Twisk, Kemper and van Mechelen 32 – Reference Kelishadi, Cook and Amra 34 ) although these associations with insulin resistance markers are not reported in other studies( Reference Katzmarzyk, Church and Blair 36 ). Indeed, HOMA-IR has been independently associated with lower CRF in children( Reference Suriano, Curran and Byrne 15 , Reference Telford, Cunningham and Shaw 37 , Reference Puder, Schindler and Zahner 38 ) and an inverse correlation between HOMA-IR and PA has been also reported( Reference Twisk, Kemper and van Mechelen 32 ).
High CRF is associated with low levels of TAG, suggesting that CRF more than PA influences the lipid profile( Reference Bouglé, Zunquin and Sesbouë 8 , Reference Suriano, Curran and Byrne 15 ). Another remarkable result in the present study was that 72 % of the children who performed PA were in the EHCF group. PA has been inversely associated with metabolic risk factors( Reference Ostojic and Stojanovic 39 ) independently of CRF and adiposity( Reference Tailor, Peeters and Norat 1 , Reference Zimmet, Alberti and Kaufman 4 , Reference Ekelund, Anderssen and Froberg 11 ). However, in other studies, its relationship was attenuated( Reference Rizzo, Ruiz and Hurtig-Wennlöf 40 ) or unchanged( Reference Brage, Wedderkopp and Ekelund 41 ) even after further adjustment according to adiposity( Reference Ruiz, Ortega and Rizzo 13 ) and CRF. Research suggests that exercise training improves glucose metabolism( Reference Pedersen and Saltin 42 ), inflammation( Reference Parrett, Valentine and Arngrímsson 43 ) and both lipid and lipoprotein profiles, thus highlighting the impact of PA on cardiovascular risk( Reference Ring-Dimitriou, von Duvillard and Paulweber 44 ). In our results, the positive significant correlation between PA and CRF may indicate the influence of the different pathways involved in either PA or CRF. PA may thus exert an independent beneficial effect on health regardless of fatness( Reference Ekelund, Anderssen and Froberg 11 ).
To study these relationships, several studies have used mixed populations of children and adolescents( Reference Anderssen, Cooper and Riddoch 45 , Reference Kwon, Burns and Janz 46 ), thus ignoring to evaluate the pubertal state. Puberty is associated with a decrease in insulin sensitivity( Reference Hannon, Janosky and Arslanian 47 ); due to this, working only with prepubertal children can reduce this effect. Nevertheless, little research has been done with homogeneous sub-populations( Reference Kriemler, Manser-Wenger and Zahner 10 , Reference Ekelund, Anderssen and Froberg 11 , Reference Telford, Cunningham and Shaw 37 ). In fact, the low significance in the results of the present study may partly be due to the early age of our groups.
With regard to non-traditional markers, a decline in plasma apo-A1 levels has been associated with low levels of HDL-C, and thus with increased cardiovascular risk( Reference Sellers, Singh and Sayers 7 ). In the present study, the LCF group displayed lower levels for these lipid parameters than the EHCF group (Fig. 1). Moreover, although uric acid has been put forward as a new biomarker for metabolic risk in childhood obesity( Reference Gil-Campos, Aguilera and Cañete 5 , Reference Choi and Ford 48 ), its relationship to CRF or PA has not been analysed. Only a study in patients with type 2 diabetes mellitus showed that uric acid was negatively associated with CRF( Reference Kadoglou, Iliadis and Angelopoulou 49 ). Children from the LCF group of the present study had higher uric acid levels than EHCF children (Fig. 3(b)), suggesting that CRF may improve this metabolic alteration; a significant negative correlation was also noted between uric acid and CRF. Nevertheless, aspartate aminotransferase was higher in the EHCF group (Fig. 3(a)). The increase in liver transaminases has usually been associated with other components of MetS, particularly insulin resistance; some authors have found a relationship between alanine aminotransferase and CRF or PA in youth( Reference Bouglé, Zunquin and Sesbouë 8 , Reference Kelishadi, Cook and Amra 34 ) despite no changes being reported by others( Reference Lee, Song and Kim 50 ).
An increase in pro-inflammatory cytokines and acute-phase reactants has been associated with cardiovascular risk factors. CRP is an indicator of a range of inflammatory processes of both vascular and non-vascular origin; elevated CRP may reflect a pro-atherogenic metabolic state that predisposes to atherothrombotic events( Reference Wijnstok, Twisk and Young 51 ). Recent studies in children have shown strong evidence for the association between CRP increase and various markers for MetS( Reference Ekelund, Anderssen and Froberg 11 , Reference Martos, Valle and Morales 52 ), diabetes mellitus and CVD( Reference Shaibi, Ball and Cruz 35 ), and it has been reported that other potential factors and processes such as reduced CRF could contribute to low-grade inflammation in apparently healthy individuals. Here, the EHCF group had lower CRP levels than the LCF group (Fig. 3(c)). In prepubescent children, CRF has been inversely associated with CRP( Reference Parrett, Valentine and Arngrímsson 43 ).
Concerning effect size results, there was little or moderate effect size in the present study. Moreover, none of the P values for the parameters compared between top and bottom quintiles groups were near significance. So, perhaps effect size did not have had an important role.
Finally, the International Diabetes Federation consensus report was used to evaluate associations of CRF with MetS, leaving aside the summarized metabolic risk score due to some limitations( Reference Zimmet, Alberti and Kaufman 4 ). In a longitudinal study by McMurray et al.( Reference McMurray, Bangdiwala and Harrell 53 ), children with MetS (4·6 %) had lower PA levels and CRF outcomes than the non-MetS group. Some 4·25 % of prepubertal children in the current study presented MetS; these children belonged to the LCF group and did not perform PA. A similar percentage has been reported in another study of children( Reference Tailor, Peeters and Norat 1 ). The classical features for MetS appear to be present even in our general prepubertal paediatric sample and to be related to low CRF. It is therefore important to clarify the impact of CRF and PA on non-traditional risk factors in children.
In relation to overall public health, new research is needed to evaluate metabolic risk as well as the factors related to it in children with low fitness measured by VO2max, in different stages and ages. This would contribute to establish the groups with health risk in childhood that could benefit from scheduled PA programmes.
Conclusions
The metabolic health profile of prepubertal children displaying high levels of CRF is characterized by low TAG, HOMA-IR, uric acid and CRP levels, and higher levels of HDL-C and apo-A1, compared with children with low CRF. The present study provides new information to understand the role not only of weight status but also of the level of CRF on the metabolic health profile in prepubertal children in relation to traditional and non-traditional metabolic risk factors.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The authors have no conflict of interest. The contribution of each author to the manuscript was as follows: F.J.L.-C., J.d.D.B.-S. and M.G.-d.C. coordinated the fitness and physical activity tests; J.L.P.-N. and M.G.-C. performed the paediatric evaluations; F.J.L.-C. and M.G.-C. processed the biochemical samples; M.C.M.-V. carried out the statistical analyses. All authors contributed to the evaluation of results and writing of the manuscript. Each author has seen and approved the contents of the submitted manuscript.







