Heart disease is a public health problem with serious consequences for the individual and for society as a whole. It is not possible to solve this health problem solely through treatment, and to prevent heart disease it is necessary to understand causal relationships between lifestyle factors, including dietary habits, and risk of heart disease. A logical consequence of official recommendations in most high-income countries to decrease intake of dietary fat is an increase in the intake of carbohydrate-rich foods. This increases the relevance of exploring the effects of different types of carbohydrate on risk of heart disease. The glycaemic index (GI) provides a basis for ranking carbohydrates according to their potential to raise blood glucose level. Overall daily GI is the weighted average of the GI values of all carbohydrate-containing foods eaten by a subject over one day, and expresses the carbohydrate quality of the diet (in relation to blood glucose-raising potential) as a single number. Glycaemic load (GL) quantifies the hypothetical overall glycaemic effect of a portion of food or a diet(Reference Salmeron, Ascherio and Rimm1, Reference Salmeron, Manson and Stampfer2). GL is a measure that incorporates both the amount and quality of carbohydrates.
It has been proposed that eating high-GI carbohydrates is associated with an increased risk of CVD because of postprandial hyperglycaemia and hyperinsulinaemia(Reference Ludwig3). Postprandial hyperglycaemia may lead to oxidation of membrane lipids, protein, lipoproteins and DNA. The hyperglycaemia may also activate inflammation. Hyperinsulinaemia may increase risk of CVD by affecting blood pressure, serum lipids, coagulation factors, inflammatory mediators and endothelial function, in the absence of any insulin resistance syndrome(Reference Brand-Miller, Nantel and Slama4). At present two prospective longitudinal studies in men and three in women are published examining the effect of overall GI and/or GL on risk of CVD or CHD(Reference Hare-Bruun, Nielsen and Grau5). One case–control study in men and women has examined GI and GL in relation to acute myocardial infarction (AMI)(Reference Tavani, Bosetti and Negri6). There seems to be a protective effect of low GI and GL on heart disease, but no significant effect has been found among men. On this background we decided to test the hypothesis that overall GI and GL are positively associated with risk of CVD morbidity, CVD mortality and CHD morbidity, among 3959 adult men and women.
Materials and methods
Study population
The study population initially included 3959 adult Danes aged 30–70 years at baseline (see Fig. 1). The sample is a pool of four observational cohorts from the Research Centre for Prevention and Health (the former Glostrup Population Studies): (i) a cohort of individuals born in 1914 (n 531); (ii) a cohort of individuals born in 1936 (n 636); (iii) the MONICA1 cohort (n 2103) including individuals born in 1922, 1932, 1942 and 1952; and (iv) the MONICA3 cohort (n 689) including individuals born in 1921, 1931, 1941, 1951 and 1961. MONICA1 and MONICA3 are both part of the WHO MONICA Project – a multi-centre study that monitors deaths and occurrence of CHD and its risk factors(Reference Bothig7). The characteristics of the cohorts and the general differences between participants and non-participants have been described in detail previously(Reference Haveman-Nies, Bokje and Ocké8–Reference Schroll11). The participation rates were 70–88 %. To be included in the present study, subjects had to have given information on dietary habits at baseline. As the study population comprises subjects from four different cohorts, and as new participants have continuously been enrolled in the cohorts, baseline is at eight different points of entry: in 1974, 1976, 1981, 1982, 1984, 1987, 1991 and 1993. Subjects were followed until December 1999, at which time they were 38–85 years of age, if still alive. All surveys mentioned were conducted in accordance with the Helsinki Declaration of 1975 as revised in 1983, and approved by the Ethics Committee for Copenhagen County.
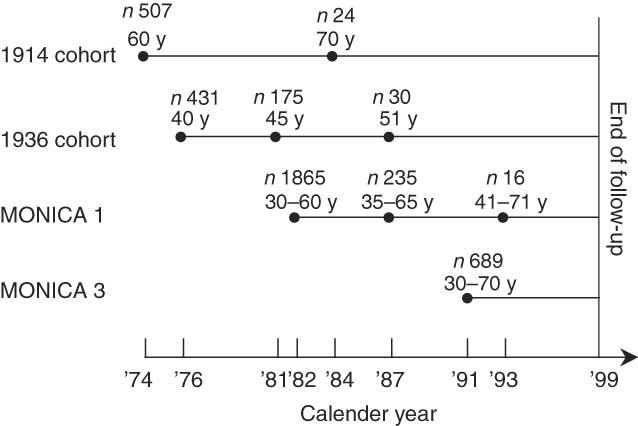
Fig. 1 Overview of study sample comprising four cohorts and 3972 subjects during 1974–1999, indicating number of subjects (n) and age in years (y) at each point of entry (●)
Exposures and covariates
Information on socio-economic and lifestyle factors was retrieved through a detailed self-administered questionnaire. Anthropometrics were measured in accordance with WHO standards(12). For each subject only one measurement of food intake, at baseline, was used. The dietary information was retrieved through either 7 d diet records (1914 cohort, 1936 cohort, MONICA1 in 1982 and MONICA3) or diet history interviews (MONICA1 in 1987 and 1993). Energy and macronutrient intakes collected under comparable conditions by either a 7 d diet record or a diet history interview can be collapsed and analysed independent of the underlying diet method(Reference Høidrup, Andreasen and Osler13). In the present study the data were collapsed after calculating overall GI and GL. Overall GI was calculated in each subgroup by multiplying the GI value of each carbohydrate-containing food variable by its proportion of total available carbohydrate and summing up these values(Reference Brand-Miller, Nantel and Slama4, Reference Liu, Manson and Buring14–Reference Wolever, Jenkins and Jenkins17):
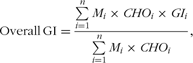
Mi being the amount of the food i in g/d, CHOi the amount of available carbohydrate per gram of food i, and GIi the glycaemic index value of the food variable i. The numerator is dietary GL and the denominator is total intake of available carbohydrate. An overall GI of 100 indicates that the average carbohydrate of the diet has the blood glucose-raising potential of white bread. After dividing the numerator by 100, a GL of 10 units is the theoretical equivalent of the rise in blood glucose produced by a portion of white bread containing 10 g available carbohydrate, which is approximately 20 g white bread or a little less than an average slice. The GI values of the individual food variables included in the calculations of overall GI and GL were based on values found in the International Table of Glycemic Index and Glycemic Load Values: 2002 (Reference Foster-Powell, Holt and Brand-Miller18). GI values depend upon differences in methodology and within-individual variations(Reference Foster-Powell, Holt and Brand-Miller18–Reference Wolever20). To minimize such non-food-related variations overall GI and GL were calculated on the basis of means of GI values from different studies measuring GI values of similar foods. The following criteria were used to select GI values from the International Table of Glycemic Index and Glycemic Load Values: 2002, upon which calculations of overall GI and GL were based:
1. The GI value was measured over 2 h if subjects were healthy or over 3 h if subjects were type I or type II diabetic(Reference Wolever20–Reference Jenkins, Wolever and Taylor22).
2. The reference food originally used to measure the GI value was either glucose or white bread (when glucose was reference food, the GI value was multiplied by 1·43 to obtain a GI value corresponding to having used white bread as reference).
3. The reference food and test food portions used for measurement both contained (with few exceptions) 50 g available carbohydrate(Reference Jenkins, Wolever and Taylor22).
4. The GI value was (with few exceptions) measured on more than five subjects(23).
Most vegetables apart from root vegetables and legumes were not included in the calculations of overall GI and GL since their GI values have not been measured. However, most of these vegetables contain an amount of carbohydrates too small to affect overall GI and GL appreciably whether they have high or low GI(Reference Wolever24).
Exclusions
Two different sets of exclusion criteria were used when examining associations between overall GI, GL and CVD morbidity, CVD mortality and CHD morbidity. First, associations were analysed using exclusion of subjects previously diagnosed with CVD or CHD, only, depending on end point. For 507 men and women entering the study in 1974, information on previous CVD diagnoses was not available, and instead previous diagnosis of CHD was used as exclusion criterion for this subgroup in analyses of CVD morbidity and mortality. For all end points we further repeated the analyses after excluding subjects with previous diagnosis of larger types of cancer (not including skin cancer), subjects reporting suffering from diabetes or taking antihypertensive medication, and hypertensive subjects. The blood pressure threshold to indicate hypertension at baseline was ≥140/≥90 mmHg according to guidelines of the WHO/International Society of Hypertension(25). These more comprehensive exclusion criteria were chosen to avoid subjects with increased risk of CVD and CHD due to a previous diagnosis and to avoid exposure misclassification in subjects who changed their diet due to a diagnosis. A number of subjects had missing values in one or more of the covariates or the variables used for exclusion. These subjects were left out of all analyses. Finally, for both sexes, subjects reporting energy intakes more than three standard deviations from the mean intake were excluded from all analyses (men with energy intakes below 4433 kJ or above 24 935 kJ (n 15) and women with energy intakes below 2844 kJ or above 21 367 kJ (n 9)).
Outcomes
The end points were: (i) CVD morbidity, International Classification of Diseases 8th revision (ICD-8) codes 390–458/International Classification of Diseases 10th revision (ICD-10) codes I00–I52 and I60–I99 including deaths and hospitalizations due to CVD; (ii) CVD mortality including deaths due to CVD; and (iii) CHD morbidity, ICD-8 codes 410–414/ICD-10 codes I20–I25 including deaths and hospitalizations due to CHD. By means of participants’ civil registration number, cause of death was identified retrospectively in the National Register of Cause of Death and hospitalizations were identified in the National Register of Patients. When analyses were conducted the registers contained information on all deaths and hospital discharges up until 31 December 1999.
Statistical analyses
Pearson’s correlation coefficients were used to identify the carbohydrate-containing food variables associated with high and low overall GI, respectively. Since food variables varied over time and with method of diet assessment, correlations were calculated in subgroups.
Overall GI and GL were used as exposure variables. Age, sex, BMI, smoking habits, level of education, physical activity, total energy intake and intakes of carbohydrate, fat, protein and dietary fibre were a priori hypothesized to be potential confounders of an effect of overall GI on heart disease risk, and were adjusted for in the models describing the relationship between overall GI and each of the end points. Macronutrient and fibre intakes were not included in models describing effects of GL since carbohydrate intake then is held constant and such models only describe the part of the variations in GL that is caused by variation in GI. Except for macronutrients and dietary fibre, the covariates included in the GL models were the same as for overall GI.
Cox proportional hazards models were used to assess relationships between exposures and outcomes. Age was used as the time scale in all time-to-event analyses, and all analyses were stratified by sex. The relationships between exposures and outcomes were explored by modelling overall GI and GL, which are continuous variables, as restricted cubic splines. Thus fewer implicit assumptions about the shape of any relationship between GI or GL and the heart disease outcomes are made than if comparing quintiles. The knots were chosen automatically using the macro for fitting Stone and Koo’s additive spline constraints(26–Reference Harrell28). To adjust GI, GL, macronutrient intake and fibre intake for total energy intake, the residuals model was used(Reference Willett29). It was a priori hypothesized that BMI, smoking status and physical activity could possibly modify a potential effect of GL and that overall GI additionally could be modified by the macronutrients. A Wald test was used to assess the significance of interaction terms. Crude models adjusted for age and total energy only. Full models additionally adjusted for the confounder variables described above. Extra analyses controlled for reported family history of AMI. Statistical analyses were conducted with use of the SAS statistical software package release 11 (SAS Institute Inc., Cary, NC, USA).
Results
Foods for which daily intakes were correlated with high and low overall GI, respectively, did not differ with method of diet assessment. Food items correlating with high overall GI were white bread and potatoes. Intakes of milk and fruit, i.e. low-GI simple sugars, were correlated with low overall GI (Table 1).
Table 1 Food variables correlating with overall GI with a coefficient ≥±0·2 in a subgroup of the study population (n 2790)

GI, glycaemic index; GL, glycaemic load.
*Based on means of GI values of food items in International Table of Glycemic Index and Glycemic Load Values: 2002 (Reference Foster-Powell, Holt and Brand-Miller18).
†A GI value has not been ascribed to the food variable and the food variable is not included in calculations of overall GI and GL.
Baseline characteristics – after exclusion of subjects previously diagnosed with CHD – of male and female subjects in the first, third and fifth quintile of energy-adjusted overall GI and energy-adjusted GL, respectively, are given in Table 2. Characteristics were essentially similar whether none, those with CHD, those with CVD, or those with CVD, cancer, diabetes and/or hypertension were excluded (data not shown). In men, overall GI varied from 75 to 91 between the lowest and highest quintiles (white bread is reference with a GI of 100). Women had slightly lower overall GI values than men, varying from 72 in the first to 89 in the fifth quintile. Male subjects in the highest overall GI quintile were significantly older than men in lower quintiles. In women, there was no age difference between quintiles. Men and women with high overall GI were less active and poorer educated. A greater part of subjects with high overall GI smoked at the time of entry. Also, they received a greater part of total energy from fat, at the expense of protein and carbohydrate, and thus also had a lower intake of dietary fibre. Finally, men with high GI reported larger alcohol intakes. Generally, for both sexes the presence of risk factors for heart disease increased with increasing quintile of overall GI. In men, mean GL varied from 102 to 220 between lowest and highest quintiles of residuals of logarithm-transformed GL (each GL unit resembles 1 g available carbohydrate from white bread). Mean GL was lower and varied less (from 84 in the first to 166 in the fifth quintile) in women. Mean BMI was significantly lower in the fifth quintile than in the first and third quintiles among men, whereas in women there was no difference in BMI between quintiles. Subjects with low energy-adjusted GL more often smoked at baseline compared with subjects with high energy-adjusted GL. Percentage of energy from carbohydrate and intake of dietary fibre increased with increasing quintile of energy-adjusted GL in both men and women. Other variables contributing to total energy decreased with increasing quintile. Generally, the presence of risk factors for heart disease seemed to decrease with increasing quintile of energy-adjusted GL for both men and women.
Table 2 Baseline characteristics (mean or percentage) in the first, third and fifth quintile of energy-adjusted overall GIFootnote * and energy-adjusted GLFootnote †, respectively, among 3774 DanesFootnote ‡ participating in four observational cohorts from the Research Centre for Prevention and Health, 1974–1999

GI, glycaemic index; GL, glycaemic load.
* Residual of overall GI conditional on total energy.
† Residual of logarithm-transformed GL conditional on total energy.
‡ Subjects previously diagnosed with CHD are excluded.
§ White bread is reference with a GI value of 100.
|| Each GL unit resembles 1 g available carbohydrate from white bread.
Analyses in men
Analyses adjusted for energy, age, macronutrients, BMI, level of education, level of physical activity, smoking status and cohort showed significant inverse associations between overall GI and CVD and CHD morbidity. The association between overall GI and CVD mortality was similar, but borderline significant only. The associations between overall GI and CVD morbidity and mortality for men, when excluding subjects with previous CVD, are illustrated Table 3. Table 4 gives details on the associations between overall GI and CHD morbidity among men in crude and adjusted analyses, when only excluding men with previous CHD and when additionally excluding men with previous CVD, diabetes and hypertension. Risk functions of overall GI and heart disease among men were generally weaker before than after adjustment for covariates, as illustrated by the association between overall GI and CHD morbidity (Table 4). Results were essentially similar whether minimal or comprehensive exclusion criteria were applied as illustrated by the associations between GI and CHD morbidity (Table 4). Furthermore, the decrease in hazard ratio for male subjects with a higher overall GI than average was greater for CVD mortality and the more homogeneous and specific CHD diagnoses than for CVD morbidity comprising CVD deaths as well as hospitalizations due to CVD (Tables 3 and 4).
Table 3 HRFootnote * with 95 % CI of CVD morbidity and CVD mortality in men at residuals of overall GI conditional on total energy corresponding the 5, 10, 25, 50, 75, 90 and 95 percentiles; four observational cohorts from the Research Centre for Prevention and Health, Denmark, 1974–1999 (n 1819)Footnote †

HR, hazard ratio; GI, glycaemic index.
* Adjusted for age, total energy intake, BMI, energy-adjusted carbohydrate intake, energy-adjusted fat intake, energy-adjusted protein intake, energy-adjusted fibre intake, cohort, level of education (0–7 years, 8–11 years, 12+ years), level of physical activity (sedentary, walk a lot, exercising) and smoking status (current smoker, current non-smoking). Additional adjustment for alcohol and saturated fat produced about similar estimates.
† Subjects previously diagnosed with CVD are excluded.
‡ Residual 0 is reference and is the average overall GI for a given energy intake. Residual 10 is an overall GI 10 units higher than average for a given energy intake.
Table 4 HR with 95 % CI of CHD morbidity in men at residuals of overall GI conditional on total energy corresponding the 5, 10, 25, 50, 75, 90 and 95 percentiles using minimal and comprehensive exclusion criterion, respectively; four observational cohorts from the Research Centre for Prevention and Health, Denmark, 1974–1999

HR, hazard ratio; GI, glycaemic index.
*Residual 0 is reference and is the average overall GI at a given energy intake and residual 10 is an overall GI 10 units higher than average for a given energy intake.
†Subjects previously diagnosed with CHD are excluded.
‡Subjects previously diagnosed with CVD or cancer, subjects reporting suffering from diabetes, and hypertensive subjects are excluded.
§Adjusted for age and total energy intake.
||Adjusted for age, total energy intake, BMI, energy-adjusted carbohydrate intake, energy-adjusted fat intake, energy-adjusted protein intake, energy-adjusted fibre intake, cohort, level of education (0–7 years, 8–11 years, 12+ years), level of physical activity (sedentary, walk a lot, exercising) and smoking status (current smoker, current non-smoking). Additional adjustment for alcohol and saturated fat produced about similar estimates.
Analyses of GL in relation to heart disease indicated no association between the two (Table 5).
Table 5 HRFootnote * with 95 % CI of CVD morbidity, CVD mortality and CHD morbidity in men at residuals of logarithm-transformed GL conditional on total energy corresponding the 5, 10, 25, 50, 75, 90 and 95 percentiles; four observational cohorts from the Research Centre for Prevention and Health, Denmark, 1974–1999

HR, hazard ratio; GL, glycaemic load.
* Adjusted for age, total energy intake, BMI, cohort, level of education (0–7 years, 8–11 years, 12+ years), level of physical activity (sedentary, walk a lot, exercising) and smoking status (current smoker, current non-smoking). Additional adjustment for alcohol and saturated fat produced about similar estimates.
† Residual 0 is reference and is average GL for given energy intake. Residuals −0·7/0·7 are GL 2 units lower/higher than average for total energy intake.
‡ Subjects previously diagnosed with CVD are excluded.
§ Subjects previously diagnosed with CHD are excluded.
Analyses in women
Crude analyses showed positive relationships between overall GI and CVD and CHD morbidity (data not shown). These relationships disappeared, however, after adjustment for potential confounders (Table 6). In neither crude nor adjusted analyses did we find any relationship between overall GI and CVD mortality (P crude = 0·1). Results from adjusted analysis are shown in Table 6. A positive, non-linear association was found between GL and heart disease (Table 7). The association was highly significant for both CVD morbidity (P = 0·0002) and CHD morbidity (P < 0·0001), but not for CVD mortality (P = 0·2). For low or average GL there seemed to be no relationship between GL and heart disease. At high GL risk of CVD and CHD morbidity increased with increasing GL (Table 7).
Table 6 HRFootnote * with 95 % CI of CVD morbidity, CVD mortality and CHD morbidity in women at residuals of overall GI conditional on total energy corresponding the 5, 10, 25, 50, 75, 90 and 95 percentiles; four observational cohorts from the Research Centre for Prevention and Health, Denmark, 1974–1999

HR, hazard ratio; GI, glycaemic index.
* Adjusted for age, total energy intake, BMI, energy-adjusted carbohydrate intake, energy-adjusted fat intake, energy-adjusted protein intake, energy-adjusted fibre intake, cohort, level of education (0–7 years, 8–11 years, 12+ years), level of physical activity (sedentary, walk a lot, exercising) and smoking status (current smoker, current non-smoking). Additional adjustment for alcohol and saturated fat produced about similar estimates.
† Residual 0 is reference and is the average overall GI at a given energy intake. Residual 10 is an overall GI 10 units higher than average for a given energy intake.
‡ Subjects previously diagnosed with CVD are excluded.
§ Subjects previously diagnosed with CHD are excluded.
Table 7 HRFootnote * with 95 % CI of CVD morbidity, CVD mortality and CHD morbidity in women at residuals of logarithm-transformed GL conditional on total energy corresponding the 5, 10, 25, 50, 75, 90, 95 and 99 percentiles; four observational cohorts from the Research Centre for Prevention and Health, Denmark, 1974–1999

HR, hazard ratio; GL, glycaemic load.
* Adjusted for age, total energy intake, BMI, cohort, level of education (0–7 years, 8–11 years, 12+ years), level of physical activity (sedentary, walk a lot, exercising) and smoking status (current smoker, current non-smoking). Additional adjustment for alcohol and saturated fat produced about similar estimates.
† Residual 0 is reference and is average GL for given energy intake. Residuals −0·7/0·7 are GL 2 units lower/higher than average for total energy intake.
‡ Subjects previously diagnosed with CVD are excluded.
§ Subjects previously diagnosed with CHD are excluded.
For men as well as for women, similar estimates were obtained when effects of overall GI and GL on heart disease were additionally adjusted for alcohol, saturated fat and family history of AMI (data not shown).
Discussion
In this prospective observational study of 3959 men and women we found a significant inverse association between overall GI of the habitual diet and risk of CVD morbidity and CHD morbidity, and a borderline significant inverse association between overall GI and CVD mortality for men. The puzzling inverse association found for men – but not for women – became significant only after control for competing risk factors of heart disease, thus limiting the probability that the inverse association could be attributed to confounding. Furthermore, the observed inverse relationship was remarkably stable; it persisted when subjects with pre-existing disease were excluded and grew stronger when the outcome measure changed from CVD to the more specific CHD.
This inverse association between overall GI and CVD and CHD found among men is surprising but not inconsistent with previous studies. Only two previous studies have examined overall GI in relation to prospective development of heart disease in men, and neither of the two studies found evidence of an effect of overall GI on CHD(Reference Levitan, Mittleman and Hakansson30, Reference van Dam, Visscher and Feskens31).
For women we found no relationship between overall GI and heart disease. Previous studies (including the Nurses’ Health Study) of the effect of overall GI on CVD or CHD risk found positive associations between overall GI and CVD or CHD(Reference Beulens, de Bruijne and Stolk32–Reference Liu, Willett and Stampfer34). Since we had only 108 female CVD deaths and 114 female cases of CHD, one could argue that our failure to identify a positive association between overall GI and CVD mortality or CHD morbidity among women could be due to insufficient statistical power. However, the crude analyses of the relationship between overall GI and CVD mortality and CVD morbidity, respectively, and the adjusted analysis of the relationship between overall GI and CVD morbidity were all sufficiently powered to detect associations, if present, but did not show any evidence of a positive association.
Our finding of a positive, but non-linear association in women between GL and heart disease is in partial agreement with the previous finding of a positive association between GL and CVD or CHD(Reference Beulens, de Bruijne and Stolk32–Reference Liu, Willett and Stampfer34). In men we could not replicate this positive association.
Meal and snack frequency may influence blood glucose response and thus the effect of overall GI and GL on risk of heart disease. Information on meal frequency was available in a sub-sample of 2655 subjects. However, inclusion of meal and snack frequency in our statistical models gave essentially similar associations between overall GI, and GL, and CVD morbidity, CVD mortality and CHD morbidity, and hence does not explain the discrepancies between our and other studies (data not shown).
Overall GI and GL were calculated using the same principles as in the Nurses’ Health Study and although the level was slightly higher in the present study, the variation in overall GI was similar to that found in previous studies(Reference Wolever, Nguyen and Chiasson16, Reference Liu, Willett and Stampfer34, Reference Buyken, Toeller and Heitkamp35). Hence, differences in GI variations between this and Nurses’ Health Study are not a likely explanation for the discrepant findings. On the other hand, the inconsistency between the results may be caused by differences in the foods determining the dietary GI. Such differences in other dietary aspects than overall GI and GL could also explain why our findings did not reflect the positive effects of low-GI diets on serum lipids and glucose metabolism that have been found in most published clinical intervention studies(Reference Brand, Colagiuri and Crossman36–Reference Wolever, Jenkins and Vuksan51). In clinical intervention studies overall GI in the low-GI intervention group is most often reduced by replacing high-GI carbohydrates with low-GI starchy foods(Reference Brand, Colagiuri and Crossman36, Reference Collier, Giudici and Kalmusky38, Reference Fontvieille, Rizkalla and Penfornis39, Reference Järvi, Karlström and Granfeldt48). By contrast, low overall GI in our study was associated with intake of low-GI simple sugars, which may have different metabolic effects compared with slowly absorbed starchy foods(Reference Wolever, Nguyen and Chiasson16). Further, the difference in the association between overall GI and GL and heart disease between men and women can also be explained by differences in the food patterns determining overall GI. For instance, while food variables associated with low overall GI in our study are similar for men and women, there is a difference in foods associated with high overall GI. In addition to foods associated with high GI in men (different types of refined bread and potatoes), Danish pastry, butter and soft drinks were characteristic of a high-GI diet in women.
Since heart disease is different in men and women, it is of course possible that the sex difference we have found in the relationships between overall GI and GL and heart disease would be present even if the dietary patterns behind overall GI and GL were the same for men and women. In fact, if the effect of low overall GI and GL depends on which low-GI carbohydrates are in the diet then the concepts of GI and GL would seem of limited use.
It is important to understand whether the effects of low-GI diets on risk factors of heart disease found in some metabolic and observational studies may be limited to subgroups with disturbed metabolism. More observational studies of healthy populations with different dietary traditions need to be carried out before conclusions can be drawn on the effects of overall GI and GL on development of heart disease. For GL, it should be investigated further whether possible health effects of GL can be attributed to the carbohydrate intake or to overall GI.
In conclusion, our findings do not support that diets with high-GI carbohydrates are associated with greater risk for heart disease than low-GI diets with identical macronutrient composition. Rather, a strong, independent and stable inverse relationship between overall GI and heart disease was seen in men, indicating that among men – but not among women – those with a habitual diet with low overall GI are at greater risk of developing CVD, and especially CHD, than those with a habitual diet with high overall GI. The hypothesis that high-GL diets increase risk of heart disease was partially supported by our finding of a non-linear direct association in women, but it was not supported by our findings for men.
Acknowledgements
Source of funding: This study was supported by Apotekerfondet. Conflicts of interest: There were no conflicts of interest. Author contributions: B.L.H. was responsible for conception and design of the study, data harmonization and obtaining of funding. B.L.H., I.T. and K.G. were responsible for analysis and interpretation of data and drafting of the manuscript. B.L.H., I.T., K.S.B. and K.G. all revised the manuscript for important intellectual content. B.L.H., I.T. and K.S.B. supervised. Acknowledgements: The authors thank the Research Department for Prevention and Health for making data available. The establishment of the Research Unit for Dietary Studies was financed by the FREJA (Female REsearchers in Joint Action) programme from the Danish Medical Research Council. The authors also thank Peder Frederiksen for supervision on statistical analyses.










