Iodine is biochemically involved in the production of thyroid hormones, such as thyroxine (T4) and triiodothyronine (T3), which play important roles in neurological development, metabolism, growth and reproduction(Reference Bath1,Reference Zimmermann2) . Optimal thyroid hormone production may be particularly relevant during pregnancy, as maternal T4 activates genes that regulate fetal brain development(Reference Abel, Caspersen and Meltzer3). Suboptimal iodine levels during gestation also contribute to oxidative stress, with consequent disturbances in placental redox balance, which is central to optimal fetal development, including the brain(Reference Dominguez-Perles, Gil-Izquierdo and Ferreres4). It is thus recommended that pregnant women consume sufficient levels of iodine to accommodate the increased need for both mother and fetus(Reference Zimmermann2,Reference Murcia, Rebagliato and Iñiguez5) .
The impact of severe iodine deficiency in pregnancy on fetal brain development is well established(Reference Bath1), emerging epidemiological studies, largely outside of the USA, document consequences of mild-to-moderate deficiencies. Indeed, inadequate iodine intake is a growing public health issue in the USA and other industrialised countries, in part due to changing dietary patterns and food manufacturing practices(Reference Hatch-McChesney and Lieberman6). For example, changes to commercial processing techniques for milk and bread(Reference Kerver, Pearce and Ma7) have led to a decrease in iodine intake(Reference Abel, Caspersen and Meltzer3,Reference Abel, Ystrom and Caspersen8) . Moreover, surveillance data from the US documents a worrisome trend among pregnant women. Data from the US National Health and Nutrition Examination Survey (NHANES) 2005–2010 reported a median urinary iodine concentration (UIC) of 129 μg/l (95 % CI 101, 173) in pregnant women(Reference Caldwell, Pan and Mortensen9), significantly lower than in 2001–2006 (153 μg/l, 95 % CI 105, 196)(Reference Perrine, Herrick and Serdula10,Reference Perrine, Herrick and Gupta11) . Non-Hispanic Black pregnant women were particularly likely to have iodine levels below 150 µg/l, the median UIC for pregnant women recommended by the WHO(12).
A recent scoping review summarised epidemiological studies from Europe, India, Japan and Scandinavian countries that have examined associations between gestational iodine intake and measured levels in relation to child neurodevelopmental outcomes and reported mixed results(Reference Griebel-Thompson, Sands and Chollet-Hinton13). The Norwegian Mother and Child Cohort Study using data on 33 047 mother–child pairs found that children born to women with inadequate iodine intake levels during pregnancy were more likely to score lower on tests of verbal IQ, reading accuracy, reading comprehension and spelling(Reference Abel, Caspersen and Meltzer3). Specifically, iodine intake below the dietary reference intakes estimated average requirement (EAR) of 160 µg/d of iodine for pregnant women was associated with language delay, behaviour problems and reduced fine motor skills at the age of 3 years(Reference Abel, Caspersen and Meltzer3). In this same population, investigators also linked low dietary iodine intake during pregnancy with higher attention-deficit hyperactivity disorder (ADHD) symptom scores among 8-year-olds(Reference Abel, Ystrom and Caspersen8). Studies examining associations between prenatal iodine status and child neurodevelopmental outcomes in US samples are sparse, particularly among racially/ethnically diverse women and children. Moreover, much of this literature considers outcomes in older children. Research on early outcomes in infancy and preschool-aged children is needed to identify risk as early in development as possible.
Temperament, measurable beginning in infancy, encompasses individual variations in behavioural tendencies of emotional responses and reactions to internal, social and environmental stimuli and comprises various domains that reflect relatively stable traits over an individual’s lifespan(Reference Rothbart and Ahadi14). Specifically, temperament has been characterised as involving two reactive dimensions (i.e. negative affectivity and extraversion/surgency) and one regulatory dimension (i.e. orienting/regulation in infancy, effortful control in childhood). Infant temperament is associated with later personality and social development and risk for emotional and behavioural problems(Reference Gartstein and Rothbart15). For example, orienting/regulation has been positively associated with more optimal executive control and executive function in preschool and later childhood periods(Reference Kraybill, Kim-Spoon and Bell16). Increased negative affectivity has been linked to persistent difficulties, including internalising problems(Reference Enlow, Devick and Brunst17) in adolescence and later life(Reference Klein, Dyson, Kujawa, Zentner and Shiner18), highlighting the need to explicate potentially modifiable environmental risk factors that contribute to early-life behavioural domains so that interventions can be applied early to promote optimal development.
Although the association between infant temperament difficulties and later psychopathology is robust, the magnitude of effects can be variable(Reference Abulizi, Pryor and Michel19,Reference Kostyrka-Allchorne, Wass and Sonuga-Barke20) . This may be due, in part, to how children respond to similar environmental challenges in divergent ways, with individual characteristics of the child, including sex, influencing pathways to adaptive or maladaptive outcomes. A few early developmental studies have associated prenatal dietary patterns or specific micronutrient (e.g. folate, B12) and macronutrient (dietary fat) with changes in temperament(Reference Lipton, Brunst and Kannan21,Reference Gustafsson, Kuzava and Werner22) . However, to date, no published study has looked at the associations between prenatal iodine and temperament in infancy. Moreover, many neurodevelopmental outcomes including temperament present with a sex or gender bias(Reference Khan and Avan23). Given the focus herein on prenatal neuro-programming, we conceptualise influences of biological sex rather than the social construct of gender(Reference Mauvais-Jarvis, Bairey Merz and Barnes24). In early development, differing structure, neuronal morphology and synaptic connections lead to sexually dimorphic brain circuitry(Reference Cosgrove, Mazure and Staley25). Sex steroids differentially influence kinetics and toxicity of chemicals and micronutrients(Reference Morris, Jordan and Breedlove26). Sex differences in antioxidant defence and placental responses also play a role(Reference Minghetti, Greco and Zanardo27). Although data are sparse in relation to prenatal iodine intake, recent findings from the preschool years suggests that associations may differ early in infancy based on child sex(Reference Goodman, Hall and Green28).
We leveraged an ethnically diverse pregnancy cohort in the Northeastern USA to examine relations among prenatal iodine intake (dietary and supplemental) and infant temperament. Given the potential for sexual dimorphism in neurodevelopment in relation to maternal nutrition(Reference Bale, Baram and Brown29,Reference Ceasrine, Devlin and Bolton30) , sex-specific effects were also examined.
Methods
Study participants
Participants were from the PRogramming of Intergenerational Stress Mechanisms (PRISM) study, an ongoing longitudinal pregnancy cohort aimed at exploring relationships among prenatal social, nutritional and chemical environmental factors and child developmental outcomes. PRISM recruited 1691 women receiving prenatal care from the Beth Israel Deaconess Medical Center and East Boston Neighborhood Health Center in Boston, MA (March 2011–December 2013) and The Mount Sinai Hospital in New York City, NY (April 2013–April 2020). Eligibility criteria included age ≥ 18 years, English- or Spanish-speaking, and singleton pregnancy. Participants were excluded if they consumed ≥ 7 alcoholic drinks/week prior to pregnancy recognition or any alcohol consumption during pregnancy, had a positive HIV status, or congenital abnormalities that could impact participation. The analytic sample for the current study includes 892 mother–child participants with data on prenatal dietary and supplemental iodine intake.
Maternal iodine levels
Dietary and supplemental iodine
The Block98 FFQ has been validated to evaluate dietary nutrient consumption among pregnant US women, including among ethnic minorities(Reference Brunst, Kannan and Ni31). Maternal dietary iodine intake and supplement use over the past 3 months were assessed during the second trimester through the modified Block98 (version 98.2; NutritionQuest, Berkeley, CA, USA) FFQ (Block 2006_Bodnar FFQ), which consists of approximately 120 food/beverage items(Reference Brunst, Kannan and Ni31) The Block98 FFQ accounts for American national consumption data and was designed based on the dietary questionnaire used by the third National Health and Nutrition Examination Survey (NHANES III)(Reference Brunst, Kannan and Ni31,Reference Wirfält, Jeffery and Elmer32) . The FFQ contains items about typical food and beverage intakes, including how often (rarely or never, daily, weekly, monthly) and how much (small, medium or large serving with portion size pictures provided) on average they were consumed in the prior 3 months(Reference Cade, Thompson and Burley33). Additional items accounted for the type and frequency of dietary supplements used throughout the prenatal period. FFQs were administered in English or Spanish by bilingual research assistants.
To compute iodine micronutrient values in food, beverages and supplements, we used the most recent US Department of Agriculture (USDA), Food and Drug Administration (FDA), the National Institutes of Health (NIH) Office of Dietary Supplements (ODS), NIH Database for the Iodine Content of Common Foods(Reference Roseland, Spungen and Patterson34–Reference Pehrsson, Roseland and Patterson36) and other scientific resources(Reference Ershow, Skeaff and Merkel37). Supplement data were linked to NHANES dietary supplement files. For specific iodine-containing supplement use analysis, we identified supplements by their inclusion of iodine based on their ingredient identification codes in the Dietary Supplement Database (DSD) file. After matching the reported dietary supplement product with the ingredient information from the NHANES database, we categorised study participants as user or non-user of dietary supplements with iodine and matched the compositional quantity of iodine in the respective supplement. Estimates of iodine intake from dietary sources were generated through linkage to dietary intake tools and to the data generated by the NHANES. To estimate updated iodine intake data from reported foods, the database developed by USDA version 3.0(Reference Roseland, Spungen and Patterson34), the FDA Center for Food Safety and Applied Nutrition (CFSAN) and the NIH-ODS were utilised. We examined the correlation between food groups and dietary iodine intake finding that intake was most positively correlated with dairy products, seafood, meat and poultry, fruit and vegetable juices (canned with salt), and nuts, seeds, sweets and salty snacks. As in prior reports(Reference Lee, Shin and Cho38), dairy product consumption had the largest positive correlation coefficient with dietary iodine intake (r = 0·90) compared to the other food groups where correlation ranged from r = 0·26 to r = 0·37 (P < 0·05 for all). Women were classified as having met or not met the EAR for iodine for pregnant women of 160 µg/d.
Infant temperament
Women completed the Infant Behavior Questionnaire-Revised (IBQ-R) when infants were approximately 6 months old(Reference Gartstein and Rothbart15,Reference Bosquet Enlow, White and Hails39) . The IBQ-R has been validated in a culturally and sociodemographically diverse sample in the USA among infants 3–12 months old(Reference Gartstein and Rothbart15,Reference Bosquet Enlow, White and Hails39) , with good internal consistency. Frequency of engagement in and reactions to specific day-to-day behaviours over the prior 1 to 2 weeks were reported by mothers, with response options ranging on a seven-item Likert scale from 1 = never to 7 = always. The IBQ-R has fourteen individual scales including Distress to Limitations, Fear, Duration of Orienting, Smiling and Laughter, High Intensity Pleasure, Soothability, Falling Reactivity/Rate of Recovery from Distress, Cuddliness, Perceptual Sensitivity, Sadness, Approach, Vocal Reactivity, Activity Level and Low Intensity Pleasure. The scales were used to calculate three overall infant temperament dimensions: Surgency/Extraversion, Negative Affectivity, and Orienting/Regulation, consistent with prior work(Reference Gartstein and Rothbart15) as well as in our sample based on the results of a confirmatory and exploratory factor analysis in the PRISM cohort(Reference Bosquet Enlow, White and Hails39). Surgency/Extraversion included scales of Vocal Reactivity, High Intensity Pleasure, Activity Level, Perceptual Sensitivity and Smiling and Laughter; Negative Affectivity included scales of Distress to Limitations, Sadness, Fear, and Falling Reactivity/Rate of Recovery from Distress; and Orienting/Regulation included scales of Duration of Orienting, and Low Intensity Pleasure. Research has now shown this factor structure converges in multiple cultures(Reference Shiner, Buss and McClowry40). Surgency/Extraversion, Negative Affectivity and Orienting/Regulation scores were derived by computing the mean of the corresponding subscale scores with higher scores indicating greater levels of that temperament domain.
Covariates
Maternal age at birth in years, parity, maternal education, annual household income, previous treatment for a thyroid disease (yes or no), child sex and child age at temperament (IBQ-R) assessment were considered as potential covariates. Maternal age at birth was scored continuously. Parity was scored as nulliparous, primiparous or multiparous. Maternal educational attainment was categorised as completed high school/general equivalency diploma or less or completed more than high school (associate degree or some college, college, or graduate degree). Annual household income was categorised as less than $25 000/year, $25 000–50 000/year or more than $50 000/year. Treatment for thyroid disorder was based on self-report of treatment for thyroid disease in the past and from abstraction from medical records. Child sex was recorded as male and female. Child age at temperament assessment was recorded in months.
Statistical analysis
Data analysis included mother–child participants with no missing food iodine intake (n 892). Missing data for infant temperament scores (33 %) and covariates (0·5–5 %) were imputed by chained equations(Reference White, Royston and Wood41). A total of forty datasets were imputed using 500 between imputation iterations. Imputation diagnostic plots are presented in online Supplementary Figures 1 and 2. Descriptive statistics of frequencies and relative frequencies and means and standard deviations were used to summarise categorical and continuous variables, respectively. Next, dietary iodine and dietary/supplemental iodine levels were summarised according to maternal and child factors to assess for any differences in intake. Similarly, mean child infant temperament scores for Surgency/Extraversion, Negative Affectivity and Orienting/Regulation were summarised according to maternal and child sociodemographic factors and prenatal dietary iodine intake levels. Child infant temperament scores were normally distributed; therefore, linear regression was used to determine if prenatal total dietary iodine intake alone and diet plus supplements intake were associated with infant temperament scores, with analyses conducted separately by child sex after testing for multiplicative interaction between prenatal iodine and child sex, setting the threshold for significant interaction to P < 0·2. Because thirty mothers had more than one child represented in the study, regression models accounted for clustering of children within mothers. Analyses were performed in SAS v. 9.4 (SAS Institute) and R 4·3 (R Foundation for Statistical Computing).
Results
Sample characteristics
The sample was ethnically diverse, with 44 % of the women identifying as Black/Hispanic Black and 35 % as non-Black Hispanic. Nearly half (46 %) reported an annual household income < $25 000/year, and 40 % reported having less than a high school education. Six percent noted prior treatment for thyroid disease. For the sample overall, the median (IQR) prenatal dietary iodine intake was 161 µg/d (IQR: 110, 279). Nearly half of the women had intakes less than the EAR for pregnant women of < 160 µg/d, whether based on dietary intake (n 443, 49 %) or when food and supplements were considered together (n 379, 43 %). Table 1 summarises median iodine intake, based on both dietary intake and dietary plus supplemental intake, according to maternal and child characteristics. There were no significant differences in median iodine intake across maternal and child characteristics (Table 1).
Table 1 Distribution of sociodemographic characteristics according to dietary and supplemental iodine intake, PRISM birth cohort

PRISM, PRogramming of Intergenerational Stress Mechanisms; IQR, interquartile range; IBQ-R – Infant Behavior Questionnaire-Revised.
* sd.
† IQR.
‡ P-value for Wilcoxon–Mann–Whitney rank sum test.
§ IBQ-R.
|| Treatment for a thyroid disease was based on self-report and medical record extraction.
Table 2 summarises infant temperament scores across covariates. Infant temperament scores differed by maternal age at birth, race/ethnicity and income. Specifically, children of younger mothers at birth had significantly higher Extraversion/Surgency (mean = 5·36; sd: 0·65) and Negative Affectivity (mean = 3·21; sd: 0·77) scores than children of older mothers. Children of non-Black Hispanic mothers had higher Surgency/Extraversion (mean = 5·34; sd: 0·70), Negative Affectivity (mean = 3·24; sd: 0·77) and Orienting/Regulation (mean = 5·40; sd: 0·56) scores than the respective overall mean scores and higher than the scores in children of women from the other racial/ethnic groups. Children of women in the $25 000–$50 000/year income category had the highest scores for Surgency/Extraversion (mean = 5·34; sd: 0·70), Negative Affectivity (mean = 3·24; sd: 0·77) and Orientating/Regulation (mean = 5·40; sd: 0·56) when compared to the other income groups (Table 2).
Table 2 Infant temperament scores according to maternal characteristics and dietary iodine intake
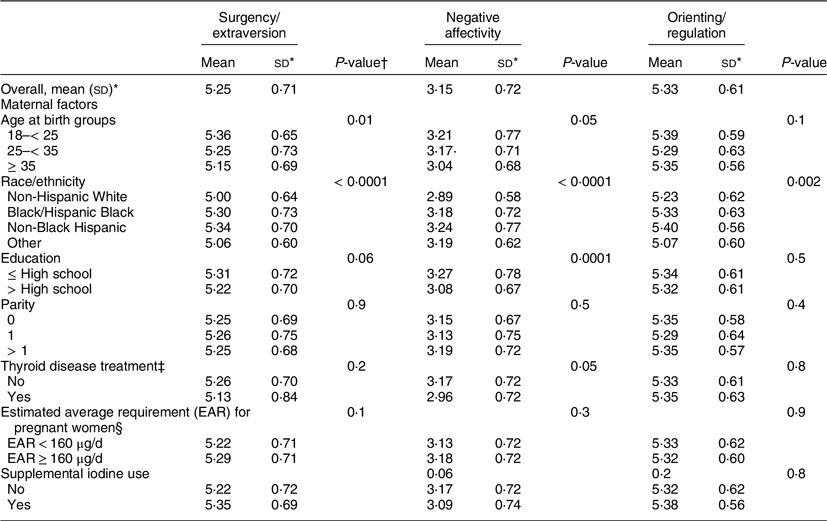
* sd.
† P-value for ANOVA.
‡ Treatment for a thyroid disease was based on self-report and medical record extraction.
§ EAR for prenatal dietary and supplemental iodine intake.
Table 3 shows infant temperament dimension scores by prenatal iodine intake (dietary and supplements) and prior treatment for thyroid disease for boys and girls. There were no meaningful differences across groups. Similarly, results considering dietary intake of iodine alone were substantively like those using dietary plus supplemental iodine intake (data not shown).
Table 3 Iodine intake parameters by infant temperament scores for boys and girls
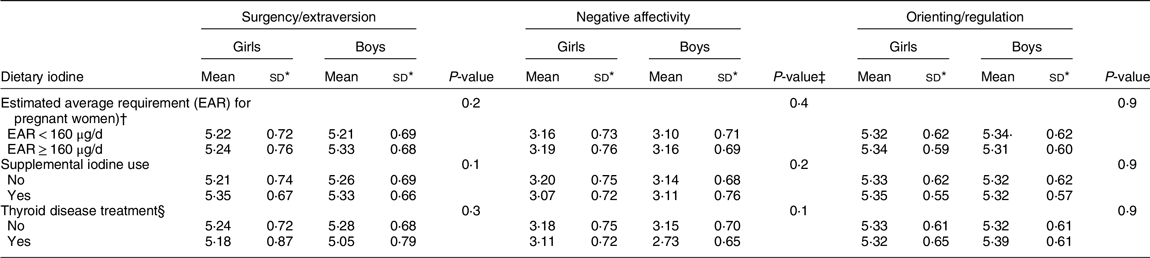
* sd.
† EAR for prenatal dietary and supplemental iodine intake.
‡ P-value for ANOVA.
§ Treatment for a thyroid disease was based on self-report and medical record extraction.
Table 4 summarises the results of linear regression models examining associations between prenatal iodine intake and infant temperament dimension scores among girls and boys separately. Effect estimates of dietary iodine and dietary and supplemental iodine intake (EAR ≥ 160 µg/d compared to the referent group of EAR < 160 µg/d) were considered in separate models. The direction of effect estimates was generally similar in girls and boys, whereby higher iodine intake in pregnancy was associated with higher Surgency/Extraversion, lower Negative Affectivity and higher Orienting/Regulation scores. After adjusting for covariates, only the association between dietary plus supplemental iodine intake EAR ≥ 160 µg/d and lower Negative Affectivity scores among boys was meaningfully different (β = –1·66 (–1·97, –1·35)). Both prenatal dietary and dietary/supplemental iodine intake at an EAR ≥ 160 µg/d was associated with a higher Surgency/Extraversion score for girls in unadjusted models β = 0·23 (95 % CI = 0·13, 0·32); when adjusted for covariates, the association became less precise, β = 0·05 (95 % CI = –0·08, 0·10). The prenatal iodine intake by child sex interaction term was significant at P < 0·2 level only for Negative Affectivity when dietary/supplemental iodine intake was considered.
Table 4 Associations between dietary iodine with and without supplemental iodine intake and infant temperament: overall sample and by child sex strata

EAR, estimated average requirement; IOM, Institute of Medicine.
Bold font delineates significant results.
* Food Iodine and Food and Supplemental Iodine levels based on the IOM, EAR for pregnant women ≥ 160 µg/d compared to referent group EAR < 160 µg/d. Models were adjusted for maternal age at birth, education, parity and previous treatment for a thyroid disease.
Discussion
This study examined associations between iodine intake during pregnancy and infant temperament in an urban sample of women and children in the Northeastern USA. We found that nearly half of these women reported iodine intake during pregnancy below the EAR of ≥ 160 µg/d whether based on dietary intake (49 %) or when food and supplements were considered together (43 %), similar to recent estimates reported in another US cohort of pregnant women finding that 41 % remained below the EAR even after supplementation(Reference Griebel-Thompson, Sands and Chollet-Hinton42). Prenatal iodine intake from diet plus supplements at or exceeding an EAR ≥ 160 µg/d was associated with lower negative affectivity scores after adjusting for covariates, particularly among boys.
Infant temperament may have long-term consequences for development, including influencing later personality and social skills development and risk for emotional and behavioural problems(Reference Gartstein and Rothbart15). It is notable that we found a benefit of adequate iodine intake in pregnant women on reduced negative affectivity in 6-month-old boys. It has been suggested that features of negative affectivity (fear, sadness, distress reactivity and recovery) may have import for developmental outcomes. Measures of infant negative affectivity show similarity to measures of negative affectivity in older children and to the adult personality factor of neuroticism(Reference Gartstein and Rothbart15), suggesting that negative affectivity may be relatively stable across the life course. A meta-analysis(Reference Kostyrka-Allchorne, Wass and Sonuga-Barke20) found negative affectivity before age 24 months to be positively associated with subsequent psychopathology, including ADHD and autism spectrum disorder, and internalising and externalising problems, assessed prior to 18 years old. Further, they found that higher self-regulation was associated with lower risk of later psychopathology, including internalising problems and ADHD, but not autism spectrum disorder. Thus, explicating factors that shape negative affectivity may inform our understanding of the earliest origins of mental health risk, resilience and other developmental outcomes.
Although we found associations between more optimal iodine intake when considering dietary and supplemental use together and reduced infant negative affectivity specifically in boys, research has been mixed on the benefits of iodine supplementation during pregnancy. Some studies have indicated evidence of no protective effect of supplemental iodine use during pregnancy on neurodevelopment and psychopathological outcomes examined in older children(Reference Abel, Caspersen and Meltzer3). The Norwegian Mother and Child Cohort Study reported no beneficial effects of maternal use of iodine-containing supplementation on child ADHD diagnosis or symptom score(Reference Abel, Ystrom and Caspersen8). Further, initiation of iodine supplementation among iodine-deficient mothers in the first trimester of pregnancy was associated with an increased risk of ADHD in this Norwegian sample(Reference Abel, Ystrom and Caspersen8). Murcia et al. (2011) found that maternal intake of iodine-containing supplements was associated with lower psychomotor achievement in infants, supporting the controversial nature of iodine supplementation(Reference Murcia, Rebagliato and Iñiguez5). A systematic review of randomised controlled trials in populations with severe iodine deficiency found that iodine supplementation prior to or early in pregnancy reduced the risk of congenital hypothyroidism and improved motor function, but no effects on cognition were reported in preschool-aged children(Reference Zhou, Anderson and Gibson43). Likewise, a randomised controlled trial in mild-to-moderately deficient populations also reported no differences in preschool child behaviour or cognition (verbal and performance IQ) scores between the iodine supplementation group and placebo(Reference Gowachirapant, Jaiswal and Melse-Boonstra44). Therefore, our findings of significantly lower adjusted negative affectivity score among boys warrant further exploration.
Such discrepancies may in part be explained by findings that associations between maternal iodine intake in pregnancy and neurodevelopmental outcomes in children are complex and may be nonlinear(Reference Sullivan, Best and Gould45). Furthermore, our finding may have been due to over-adjustment or non-adjustment for unmeasured confounders. Additional research is needed to elucidate these inconsistencies before clinical recommendations can be made based on child neurodevelopmental outcomes.
Strengths and limitations
Our study has several strengths and makes a unique contribution to this literature. The examined associations have been understudied in general and specifically among ethnically mixed US samples. Indeed, few studies have examined this association because prior to 2020, there was no US national database of the iodine content of common food items because of the high variability in iodine content of food items. We assessed both dietary and supplemental intakes to characterise prenatal iodine intake and to the best of our knowledge, this is the first study to examine prenatal iodine intake in relation to infant temperament. The focus on behavioural domains could identify children early in development who may be at risk for longer-term behavioural and psychological disorders and thus is a strength, as research on the neurodevelopmental effects of prenatal iodine intake have largely focused on outcomes in later childhood.
We also consider some limitations. Food and supplemental intake estimates were based on participant recall of usual intake over the previous 3 months and therefore may be subject to potential measurement error. For example, food items and choices available in an FFQ may not be as precise as dietary data collected by food records or 24-h dietary recalls where specific brand name products can be reported. Although the high daily variation in iodine intake may limit reliance on a 24-h dietary recall, it is useful when used in combination with FFQ data. Indeed, research has shown that FFQ are a useful and less expensive option for obtaining estimates of usual intake over time (e.g. months), including during pregnancy, with less participant burden(Reference Blumfield, Hure and Macdonald-Wicks46). Also, FFQ can better capture day-to-day variation as they assess food items that may be less frequently or episodically consumed. Moreover, misclassification of nutrient intake is likely random and therefore would be expected to result in an underestimation of associations. Nevertheless, future research could be enhanced through validation with food records and/or 24-h dietary recalls or the addition of biomarkers (e.g. UIC). Notably UIC is not without its limitations, for instance, analysis of a single spot urine only reflects iodine content of recently consumed food, whereas recommendations for considering repeated UIC over gestation is likely to overcome this limitation. In addition, measures of thyroid hormones will be useful to consider in future studies for a more accurate assessment of habitual iodine intake. Considerations need to be made for major food groups of iodine (e.g. seafood, eggs and dairy products) in future studies. Although we had fifteen iodine food groups, we were unable to adjust for them individually due to our limited sample size. Lack of adjustment for iodine food groups may have resulted in measurement error. Although the recently created USDA Database of iodine in food items account for iodised salt in cooked and processed food, it is difficult to accurately estimate iodine intake from iodised salt used in cooking, food processing and at the table(Reference Skeaff47). As effects of iodine on infant temperament might be modest, future research in larger samples may detect meaningful associations beyond those found in our sample as well as provide greater power to examine sex-specific effects suggested in these results. Infant temperament was ascertained through maternal report. Maternal report of infant temperament leverages the mother’s ability to observe her infant’s behaviour over a range of contexts, which is an advantage; however, maternal factors (e.g. personality and psychopathology) may influence reporting accuracy. The IBQ-R was designed to reduce the influence of such biases by inquiring about multiple examples of concrete infant behaviours for each domain rather than asking for abstract judgements(Reference Gartstein and Rothbart15). Furthermore, maternal ratings of infant negative affectivity on the IBQ-R correlate with laboratory observations(Reference Gartstein and Marmion48), although others report discrepancies(Reference Stifter, Willoughby and Towe-Goodman49). Although parental perception of temperament may reveal subtle differences according to cultural background(Reference Gartstein, Gonzalez and Carranza50), we have previously documented construct validity in this ethnically diverse sample(Reference Bosquet Enlow, White and Hails39). Future studies should also consider relative contribution of other important factors contributing to neurobehavioural outcomes (e.g. genetics) along with nutritional factors such as iodine as well as gene x nutrition interactions.
Conclusions
A significant proportion of the ethnically diverse women in this US sample reported suboptimal iodine intake in pregnancy, consistent with concerning trends from national surveillance data(Reference Caldwell, Pan and Mortensen9) and recent findings reported in another US cohort of pregnant women(Reference Griebel-Thompson, Sands and Chollet-Hinton42). Suboptimal iodine intake in pregnancy may have implications for child neurodevelopment, evident as early as infancy, as shown in this study. Identifying risk and protective factors that are readily amenable to intervention such as optimising prenatal nutritional intake of essential micronutrients such as iodine can inform prevention strategies.
Acknowledgements
The authors thank the participating families whose generous donation of time made this study possible.
Financial support
This work was supported by the National Institutes of Health (grant numbers: R01 HL095606, R01 HL114396, R21ES035169, and UG3 OD023337 supporting the cohort follow-up and data collection and statistical support through P30 ES023515 and UL1 TR001419. During the preparation of this manuscript, AAA was supported by NIEHS, 5K12ES033594. None of the funding sources had any role in the study design, collection, analysis, or interpretation of the data, manuscript writing, or in the decision to submit the manuscript for publication.
Conflicts of interest
There are no conflicts of interest.
Authorship
A. A. A., J. D. and R. J. W.: conceptualisation, methodology, investigation and writing – original draft; A. A. A.: statistical analysis; S. K., T. J. H., J. L., M. B. E., X. Z. and R. O. W.: methodology and writing – review and editing; J. L. and S. K.: exposure estimation; R. J. W.: supervision, project administration and funding acquisition. All authors have read and approved the final manuscript.
Ethics of human subject participation
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the human studies committee at the Brigham and Women’s Hospital and Icahn School of Medicine at Mount Sinai. All participants provided written consents in their primary language.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980024001575







