An optimal sleep pattern has important implications for health maintenance and health promotion( Reference Amlaner and Fuller 1 ). However, cross-sectional studies have suggested that between 14 and 40 % of the general population has impaired sleep, including insufficient sleep duration, long sleep-onset latency, frequent and long nocturnal awakenings, and other sleep disturbances( Reference Cho, Shin and Yun 2 – Reference Wong and Fielding 6 ). Sleep deprivation and sleep impairment can affect cognitive performance in children( Reference Liu, Zhou and Wang 7 ) and adults( Reference Dinges and Baynard 8 ). Over time, impaired sleep patterns have been linked to depression( Reference Riemann, Berger and Voderholzer 9 ), obesity( Reference Liu, Zhang and Li 10 ), metabolic( Reference Vgontzas, Liao and Pejovic 11 ) and cardiovascular diseases( Reference Cappuccio, Cooper and D’Elia 12 ), cancer( Reference Qin, Zhou and Zhang 13 ) and increased risk of mortality( Reference Gallicchio and Kalesan 14 ). The high prevalence and consequent negative impact of sleep impairment highlight the importance of understanding potentially modifiable factors.
As the relationship between insufficient sleep, weight gain and obesity has been observed in both children( Reference Liu, Zhang and Li 10 ) and adults( Reference Canuto, Pattussi and Macagnan 15 ), increasing attention has been given to the potential links between sleep, dietary intake and nutrition. Whereas current findings consistently support the impact of sleep deprivation and sleep problems on dietary intake and metabolic outcomes( Reference Canuto, Pattussi and Macagnan 15 – Reference Burt, Dube and Thibault 17 ), recent studies have revealed a reverse relationship between dietary or serum nutrient levels and sleep problems( Reference Afaghi, O’Connor and Chow 18 – Reference Bertisch, Sillau and de Boer 20 ). Researchers have found that macronutrients, such as carbohydrates and amino acids (specifically tryptophan), can involve and influence the levels of neurotransmitters in the intrinsic sleep processes and affect sleep patterns( Reference Peuhkuri, Sihvola and Korpela 21 , Reference Ursin 22 ). For example, low proportion of carbohydrate intake was found to increase the percentage of slow-wave sleep (SWS; or deep sleep) and reduce the percentage of rapid-eye-movement (REM) sleep among healthy good sleepers( Reference Afaghi, O’Connor and Chow 18 ).
Micronutrient intake has not received as much attention as macronutrients as a modifiable factor for sleep deprivation and sleep problems. However, experimental studies indicate that micronutrients may impact important nerve-signalling chemicals or neurotransmitters of sleep regulation, including serotonin( Reference Ursin 22 ), N-methyl-d-aspartate (NDMA) glutamate( Reference Sowa-Kućma, Legutko and Szewczyk 23 ) and melatonin secretion( Reference Honma, Kohsaka and Fukuda 24 ). Several epidemiological studies and clinical trials have also provided evidence to support the relationship between micronutrient intake and sleep patterns. For example, randomized controlled trials in infants found longer night-time and total sleep duration in those receiving supplemental Zn or Fe compared with the placebo group( Reference Kordas, Siegel and Olney 25 ). To date, however, no study has critically reviewed the current literature on the association between micronutrients and sleep in a developmental perspective for sleep patterns.
The aim of the current review was twofold: (i) to examine empirical research on the relationship of dietary or biological micronutrient levels with sleep patterns; and (ii) to identify issues surrounding implications for future research and public health practice. As micronutrient deficiency and poor sleep are of particular concern in both developed and developing countries( Reference Diaz, De las Cagigas and Rodriguez 26 – Reference Qin, Melse-Boonstra and Shi 29 ), understanding the possible roles of micronutrients in sleep will inform future prevention and intervention programmes for the multifaceted and interrelated public health issues of nutrition and sleep; and shed light on the full extent of their consequences on health.
Method
The current review was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement checklist( Reference Moher, Liberati and Tetzlaff 30 ). We searched articles in PubMed, Embase and Scopus through January 2016. PubMed was searched using MeSH to identify articles with the medical subject headings ‘micronutrients’, ‘minerals’, ‘iron compounds’, ‘zinc’, ‘copper’, ‘cobalt’, ‘potassium’, ‘magnesium’, ‘calcium’, ‘sodium’, ‘phosphorus’, ‘manganese’ and ‘sleep’, and the keywords ‘vitamin*’, which yielded 153 articles. Embase was searched using EMTREE with ‘trace element’, ‘vitamin’, ‘mineral intake’, ‘mineral deficiency’, ‘mineral blood level’, ‘trace metal blood level’, ‘zinc deficiency’, ‘vitamin deficiency’, ‘sodium deficiency’, ‘selenium deficiency’, ‘potassium deficiency’, ‘phosphate deficiency’, ‘calcium deficiency’, ‘copper deficiency’, ‘iron deficiency’, ‘magnesium deficiency’, ‘cobalt’ and ‘sleep’, yielding 100 articles. Additionally, Scopus was searched using the keywords ‘micronutrient*’, ‘vitamin*’, ‘trace element’, ‘sleep’, ‘sleep pattern’ and ‘sleep quality’, identifying 538 results. As shown in Fig. 1 (PRISMA flow diagram of the article selection process), duplicates were identified and deleted in Refworks, yielding 749 potentially relevant articles. Twenty-three articles were identified for additional scrutiny after reviewing titles and abstracts. The reference lists of the twenty-three articles were then manually searched. Only articles published in English and Chinese and with full text available were considered. No year restriction was set in the literature search.

Fig. 1 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of the article selection process (OSA, obstructive sleep apnoea; RLS, restless-leg syndrome)
Inclusion and exclusion criteria
Studies relevant to the research question were expected to focus on micronutrient intake or biological micronutrient levels and sleep patterns in human subjects. More specifically, the term ‘micronutrients’ refers to three types of nutrients: (i) vitamins; (ii) trace elements, such as Fe, Zn, Cu, Co, Se and Mn; and (iii) minerals, such as Ca, Mg and K( Reference Katz 31 ). The term ‘sleep pattern’ is used to denote frequently examined parameters in subjective and objective sleep studies, including sleep duration (the time one spends sleeping), sleep-onset latency (the amount of time from lights out, or bedtime, to the commencement of sleep), night awakenings (number of times waking up in the middle of the night), sleep stages (REM and four stages of non-REM (NREM) sleep scored according to standard polysomnographic criteria), as well as sleep phases in circadian sleep rhythm, indicated by habitual bedtime and wake time( Reference Cortese, Konofal and Yateman 32 ). A total of seventy articles met the inclusion criteria. To examine the direct correlation between micronutrients and sleep patterns, articles were excluded if they met any of the following criteria: (i) the study focused on secondary sleep impairment due to comorbidities such as mood disorders, pain and treatment effects (n 3); (ii) the study investigated the relationship of micronutrient status with physiological sleep disorders, such as restless-leg syndrome or obstructive sleep apnoea (n 25); (iii) the study primarily examined the molecular or genetic pathways linking micronutrients and sleep patterns using animal models (n 9); (iv) the study was a narrative literature review, expert opinion or case study (n 9); (v) the study examined the effect of multiple treatments with micronutrient supplementation as one of the therapeutic elements (n 1).
Data extraction and analysis
Extracted data included research design, subjects (age, sex and sample size), the covariates that were adjusted for in observational studies, study site, micronutrient measurement, sleep measurement and key findings (see Tables 1 and 2). The methodological strengths and limitations of each study were also summarized in Tables 1 and 2. The research quality of each study was then further evaluated using the validity questions of the American Dietetic Association’s Quality Criteria Checklist for primary research( 33 ). Each study was classified as positive, neutral or negative according to the rating criteria of the Quality Criteria Checklist.
Table 1 Observational studies on micronutrients and sleep patterns in human subjects
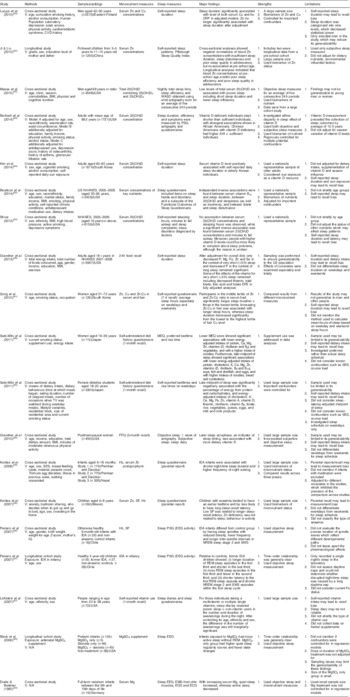
V, variables adjusted for; TV, television; SES, socio-economic status; IQ, intelligence quotient; IDA, Fe-deficiency anaemia; N/A, not applicable; NHANES, National Health and Nutrition Examination Survey; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D2, 25-hydroxyergocalciferol; 25(OH)D3, 25-hydroxycholecalciferol; SF, serum ferritin; WASO, wake time after sleep onset; PSG, polysomnography; MEQ, Morningness–Eveningness Questionnaire; EEG, electroencephalography; EOG, electro-oculogram; ECG, electrocardiography; hs-CRP, high-sensitivity C-reactive protein; AHI, apnaea–hypopnoea index; DFE, dietary folate equivalents; NREM, non-rapid eye movement; SWS, slow-wave sleep; REM, rapid eye movement.
Table 2 Clinical trials on micronutrients and sleep patterns in human subjects

RCT, randomized controlled trial; G, group; FA, folic acid; DSPS, delayed sleep phase syndrome; N/A, not available; EEG, electroencephalography; SWS, slow-wave sleep; WASO, wake after sleep onset.
Results
A final sample of twenty-six articles assessing the association between micronutrients and sleep was identified for review, including nineteen observational studies (Table 1) and seven clinical trials (Table 2). The reviewed articles covered a range of non-institutionalized samples from infants to older adults across several different countries. However, adolescents, who may experience significant developmental sleep alteration and metabolic changes, were rarely reported in the articles reviewed. Micronutrients studied in the existing literature included Fe, Zn, Cu and Mg, as well as vitamins D and B12. Studies with an observational design measured micronutrient status by 24 h dietary recall (one day only; n 1), generic FFQ (n 3), micronutrient intake recall (n 2) or laboratory indices of serum/hair concentrations (n 13). The seven clinical trials specifically examined the sleep effect of dietary supplements with various treatment periods, from 1 week to 12 months. The body of literature on sleep pattern variables associated with micronutrients is broadly categorized into sleep stage, sleep duration, sleep-onset latency, waking after sleep onset and circadian rhythm of sleep. Along with retrospective questionnaires and prospective sleep logs, objective measurements of actigraphy and polysomnography have been used to collect sleep data. Table 3 shows the scientific validity of each study.
Table 3 Research validity assessment using the American Dietetic Association’s Quality Criteria Checklist( 33 )

Y, yes; N, no; unclear, not clearly reported.
* Randomized controlled trials, baseline comparison between groups; observational studies: were pre-existing differences accounted for by using appropriate adjustments?
† Positive (+): if most (six or more) of the answers to the above validity questions are ‘Yes’ (including criteria 2, 3, 6 and 7), the report should be designated with a plus symbol (+) on the Evidence Worksheet. Neutral (Ø): if the answers to validity criteria questions 2, 3, 6 and 7 are ‘Yes’ but several other criteria indicate study weaknesses, the report should be designated with a neutral (Ø) symbol on the Evidence Worksheet. Negative (–): if most (six or more) of the answers to the above validity questions are ‘No’, the report should be designated with a minus (–) symbol on the Evidence Worksheet.
Trace elements and sleep patterns
Fe
The association between Fe and sleep duration has been consistently reported in infants and the general adult population. Based on results from a cross-sectional study and a clinical trial, iron-deficiency anaemia (IDA) may be associated with more night waking and shorter total sleep duration compared with better-nourished infants( Reference Kordas, Siegel and Olney 34 ), whereas Fe supplementation can decrease the length of daytime naps among IDA infants, and increase night-time and total sleep duration in infants regardless of their IDA status at baseline( Reference Kordas, Siegel and Olney 25 ). A recent observational study extended such associations to adults, showing an association between decreased Fe intake and very short sleep (<5 h) after controlling for overall diet( Reference Grandner, Jackson and Gerstner 35 ). Fe deficiency is also associated with altered characteristics of sleep stages. Compared with non-IDA infants, IDA infants showed more awake times, shorter quiet-sleep duration and delayed sleep-spindle patterns in NREM sleep at night( Reference Peirano, Algarín and Garrido 36 ). Such alterations in the temporal organization of sleep architecture may be long lasting. A longitudinal study followed up children with and without IDA in infancy, indicating that former IDA infants exhibited altered distribution of NREM and REM sleep at 4 years old relative to the non-IDA controls( Reference Peirano, Algarin and Garrido 37 ).
Zn and Cu
Although the association between Zn and sleep phase in circadian rhythm has been suggested in several studies( Reference Golub, Takeuchi and Keen 38 , Reference Kordas, Casavantes and Mendoza 39 ), no consensus has been reached on this effect. In a sample of children aged 6–8 years, researchers found no significant association between low serum Zn and bedtime or wake-up time( Reference Kordas, Casavantes and Mendoza 39 , Reference Sato-Mito, Sasaki and Murakami 40 ). In contrast, the study by Sato-Mito et al., which classified participants into quintiles (Q) by the midpoint of sleep (Q1/earliest=2:32am, Q2 =3:10 am, Q3=3:37 am, Q4=4:11 am, Q5/latest=5:31 am), reported a significant reduction of energy-adjusted Zn intake in women who had the latest midpoint of sleep( Reference Sato-Mito, Sasaki and Murakami 40 ).
With regard to sleep duration, five studies provided evidence to support the association of Zn and Cu with sleep duration among different populations. The randomized controlled trials, using maternal reports of sleep patterns, found longer night-time and total sleep duration in infants who received supplemental Zn than in the placebo group( Reference Kordas, Siegel and Olney 25 ). This finding agrees with an observational study that found an association between decreased Zn and very short sleep in a general adult population( Reference Grandner, Jackson and Gerstner 35 ). In terms of nutritional biomarkers, researchers found that shorter sleep duration or increased odds of sleep insufficiency were associated with lower serum Zn levels in women( Reference Song, Kim and Jung 41 ) and children in early adolescence( Reference Ji and Liu 42 ). Cu levels showed mixed effects in prior research. Whereas researchers reported a negative relationship between hair Cu levels and sleep duration in 126 women aged 21–72 years( Reference Song, Kim and Jung 41 ), a study of 2570 men aged 42–60 years showed highest average levels of serum Cu in the group with the longest sleep duration( Reference Luojus, Lehto and Tolmunen 43 ).
Minerals and sleep patterns
Mg
The involvement of Mg in sleep patterns has been investigated in infants and older adults. Researchers found that increased serum Mg was associated with increased quiet sleep and decreased active sleep in full-term infants( Reference Dralle and Bodeker 44 ). In a longitudinal study, Black et al.( Reference Black, Holditch-Davis and Schwartz 45 ) reported that preterm infants whose mothers received prenatal MgSO4 treatment (whether or not they also received steroids) had more active sleep without REM, whereas the MgSO4-only group showed higher quiet-sleep regularity and fewer state changes. In older adults, a clinical trial also suggested that oral Mg supplementation may increase SWS delta power and sigma power measured by polysomnography( Reference Held, Antonijevic and Künzel 46 ).
K
Using sleep log and actigraph data, the randomized controlled trial by Drennan et al.( Reference Drennan, Kripke and Klemfuss 47 ) reported reduced sleep duration and wakefulness after sleep onset in young males following K supplements compared with controls. These researchers also identified a later bedtime after K supplementation, which is contrary to an observational study on female students aged 18–20 years that found a negative association between the midpoint of sleep and dietary K intake( Reference Sato-Mito, Shibata and Sasaki 48 ).
Vitamins and sleep patterns
Vitamin B12
The results showed mixed effects of vitamin B12 on sleep patterns. No significant or definitive effect of vitamin B12 on sleep phase and sleep duration at night was reported in early human studies( Reference Honma, Kohsaka and Fukuda 24 , Reference Okawa, Mishima and Nanami 49 , Reference Takahashi, Okawa and Matsumoto 50 ), except the clinical trial by Mayer et al.( Reference Mayer, Kroger and Meier-Ewert 51 ) that reported an alerting effect of vitamin B12 supplementation with a decrease in sleep duration. A recent study consistently found an independent inverse relationship between serum vitamin B12 concentrations and sleep duration in adults( Reference Beydoun, Gamaldo and Canas 52 ). Additionally, one study investigated the relationship between vitamin B12 and sleep timing, measured as the midpoint of sleep. That study showed that young women with lower intakes of vitamin B12 were more likely to have a later sleep period( Reference Sato-Mito, Shibata and Sasaki 48 ).
Vitamin D
Several studies have indicated a potential protective effect of vitamin D on sleep. Analyses of data from the National Health and Nutrition Examination Surveys (NHANES) 2005–2006 and found inverse correlations of serum vitamin D with sleep latency (minutes to fall asleep)( Reference Shiue 53 ) and daytime sleepiness( Reference Beydoun, Gamaldo and Canas 52 ), but not sleep duration( Reference Shiue 53 ), in adults. While these relationships were found from heterogeneous populations including both young adults and older adults, studies specific to community-dwelling older adults reported a positive association between vitamin D levels in serum and sleep duration( Reference Bertisch, Sillau and de Boer 20 , Reference Kim, Chang and Kim 54 , Reference Massa, Stone and Wei 55 ). Current studies also examined the link between vitamin D and sleep phase but showed inconsistent findings. In a study on postmenopausal women, participants with higher intake of dietary vitamin D showed a later sleep acrophase (which refers to the peak of a fitted 24 h cosine wave that was an indicator of sleep timing measured by actigraphy; this is an indicator of mathematically modelled curve peak)( Reference Grandner, Kripke and Naidoo 56 ). In contrast, an inverse relationship was reported in another study conducted with female students aged 18–20 years in Japan( Reference Sato-Mito, Sasaki and Murakami 40 ).
Possible interactions of micronutrients on sleep patterns
While the main effect of each micronutrient on sleep patterns has been reported in current research, such sleep effect may change depending on the level of another micronutrient, suggesting an interaction among micronutrients. For example, although Fe and Zn supplements alone reduced the length of naps as well as increased the duration of night-time sleep and total sleep in infants, infants receiving Fe together with Zn supplements did not exhibit such sleep effect( Reference Kordas, Siegel and Olney 25 ). Zn:Cu in serum and hair also significantly predicted sleep duration in women, with longer sleep duration associated with a medium tertile of Zn:Cu in serum or a high tertile of Zn:Cu in hair( Reference Song, Kim and Jung 41 ). In combining effects of vitamins, researchers of a cross-sectional study reported negative effects from a multivitamin or multiple single-vitamin use on sleep patterns in the number and duration of night-time awakenings compared with non-vitamin users( Reference Lichstein, Payne and Soeffing 57 ). Considering the significant effects of vitamins mentioned, this finding suggested possible antagonistic effects between vitamins on sleep outcomes.
Discussion
Sleep stages in relation to micronutrient status
The reviewed studies consistently supported the hypothesis that micronutrient levels, particularly Fe and Mg, can predict the organization of sleep stages. However, researchers should interpret these findings from a developmental perspective for sleep patterns across the life span. Two distinct sleep states are defined on the basis of polysomnography: REM and NREM sleep, which are called active sleep and quiet sleep in infants, respectively( Reference Amlaner and Fuller 1 ). Given that one of the main alterations in infant sleep is the transition from predominantly active sleep to increased quiet sleep by about 4 months of age( Reference Peirano, Algarín and Garrido 36 ), decreased quiet sleep and delayed spindle patterns in NREM sleep from IDA( Reference Peirano, Algarín and Garrido 36 ) indicate that Fe may be essential for the normal development of quiet sleep/NREM sleep in infants.
Prenatal exposure to Mg in preterm infants( Reference Black, Holditch-Davis and Schwartz 45 ) and postnatal serum Mg concentrations in full-term infants( Reference Dralle and Bodeker 44 ) have consistently been associated with an increase in the duration of quiet sleep, suggesting a potentiating role of Mg in the maturity of quiet sleep during infancy. In contrast, preterm birth with prenatal exposure to MgSO4 was also associated with more active sleep without REM( Reference Black, Holditch-Davis and Schwartz 45 ). REM sleep in infants has been linked to the development of the neuromuscular and sensory system, as well as brain function( Reference Holditch-Davis 58 ). As the amount of active sleep without REM often decreases over the preterm period( Reference Holditch-Davis, Scher and Schwartz 59 ), increased active sleep without REM suggests delayed development of active sleep and a possible influence on brain development, thus raising a concern over Mg treatment for pregnant women and infants. The effects of Mg on infant sleep need to be interpreted cautiously due to the coexistence of acceleration in the development of quiet sleep and a delay in the maturity of active sleep.
The benefit of Mg supplements on increased SWS in the elderly is of particular interest. Whereas the most prominent alterations in elderly sleep are known to be reductions of SWS, REM sleep and sleep efficiency( Reference Amlaner and Fuller 1 ), Mg may have beneficial effects on sleep patterns due to its action on reversing age-related sleep changes. Additionally, given that the delta power of SWS in electroencephalography reflects the restorative property of sleep( Reference Knyazev 60 ), Mg may improve the physiological function of sleep in older adults, which warrants future research on the clinical application of Mg supplementation for older adults.
Sleep duration in relation to micronutrient status
Most trace elements and minerals in the present review correlated significantly with sleep duration in different populations, despite the discrepancy in the direction of these relationships across micronutrients. Specifically, Fe, Zn and Mg may positively associate with sleep duration, whereas the relationship of sleep duration is inverse to the level of hair Cu, as well as K and vitamin B12 supplements. The relationship between vitamin D and sleep duration is controversial. Considering the negative correlation between the number of waking episodes and sleep duration at night( Reference Kordas, Siegel and Olney 34 ), together with associated sleep-onset latency( Reference Kordas, Casavantes and Mendoza 39 ), the effect of Fe, Zn and Mg on sleep duration might be moderated or mediated by the number of awakenings after sleep onset and sleep latency at night. This hypothesis may not be supported by the study on K due to the coexistence of reduced sleep duration and wakefulness after sleep onset( Reference Drennan, Kripke and Klemfuss 47 ). However, delayed sleep time reported by sleep logs in that study could explain such paradoxical findings.
Although micronutrient status could be a modifiable factor to improve sleep duration, micronutrient levels may not show a linear association with sleep, with the longest sleep duration found in the middle tertile level of Zn and Cu in women( Reference Song, Kim and Jung 41 ). This finding, together with evidence that both short- and long-sleep duration have been documented to increase all-cause mortality (cardiovascular-related, cancer-related and all)( Reference Gallicchio and Kalesan 14 ), indicates that optimal rather than high or low micronutrient levels are essential for healthy sleep.
Sleep phase in relation to micronutrient status
The published literature supported the presence of an association between sleep phase and micronutrient variables of Zn, K and vitamin D. However, the limited evidence and lack of consensus on such associations preclude the conclusion of causal links or concrete clinical recommendations. The different results could be attributed to the large night-to-night variation of the indicators for sleep phase: sleeping time and wake-up time. Sleep habits in human subjects vary greatly between weekdays and weekends( Reference Liu, Zhao and Jia 61 ), and human chronotype is known to correlate better with the midpoint of sleep on free days than on work days in a non-experimental environment( Reference Roenneberg, Kuehnle and Juda 62 ). However, studies in the current review did not differentiate weekdays from weekends for bedtime and wake-up time, thus leading to substantial measurement error and biased findings.
The inconsistent results of micronutrients and sleep phase may also result from the variability of micronutrient and sleep measurements. Dietary intake may be positively associated with biological micronutrient levels in red blood cells and serum( Reference Kordas, Siegel and Olney 25 , Reference Mayer, Kroger and Meier-Ewert 51 , Reference Chollet, Franken and Raffin 63 ). However, metabolic characteristics enable some micronutrients, such as vitamin B12, to maintain a normal range under a short period of dietary deficiency( Reference Katz 31 ), suggesting a possible discrepancy between dietary intake and biological levels of micronutrients. Similarly, sleep data were collected by different questionnaires, and thus it is possible that inconsistent patterns of statistical significance reflect different psychometrical properties rather than true differences in the associations. Besides, subjective sleep measurements do not highly correlate with estimates based on physiological measures( Reference Kurina, McClintock and Chen 64 ) and may lead to different results as compared with objective measurements. Therefore, standardized objective measures of micronutrient status and sleep should be incorporated into future studies.
Neurobiological mechanisms of current findings
Although the possible neurobiological mechanisms underlying the main effects of micronutrients on sleep patterns are not fully understood, researchers have suggested a number of causal pathways linking micronutrients with sleep in experimental studies. For example, micronutrients may be essential in the synthesis and transportation of neurotransmitters that are related to sleep homeostasis. Whereas Fe, Zn, Cu and Mg may be associated with antagonists of excitatory transmissions, including the NDMA receptor( Reference Marchetti, Baranowska-Bosiacka and Gavazzo 65 , Reference Takeda, Minami and Seki 66 ) and dopaminergic neurons( Reference Beard, Erikson and Jones 67 , Reference Panossian and Veasey 68 ), micronutrients can also potentiate inhibitory transmissions, such as γ-aminobutyric acid (GABAA) receptors( Reference Schwartz, Wagner and Yu 69 , Reference Turgeon and Albin 70 ). In addition to the neurobiological pathways, researchers have documented that retinoic acid, a metabolite of vitamin A, significantly up-regulates the expression of clock/bmal (circadian locomotor output cycles kaput/brain and muscle arylhydrocarbon receptor nuclear translator)-dependent circadian genes, thus modulating the circadian-sleep regulatory process and affecting sleep phase, sleep duration, as well as the organization of sleep stages( Reference Navigatore-Fonzo, Delgado and Golini 71 ). The mechanisms underlying the association between micronutrients and sleep regulation warrant future examination of the effect of micronutrient supplementation on sleep patterns.
The long-term effect of suboptimal levels of micronutrients, such as Fe deficiency, may be due to an irreversible impact on the brain. Several studies on rats have shown that Fe deficiency causes disturbances and damage to brain Fe distribution and sleep-related neurotransmitter systems, and such disturbances could not be completely normalized by Fe replenishment( Reference Beard 72 ). Current literature has also supported the interactions between micronutrients on sleep patterns, which possibly result from the antagonism between micronutrients in either metabolic absorption or the binding to receptors of neurotransmitters( Reference Singh 73 , Reference Sharonova, Vorobjev and Haas 74 ). Specifically, a high intake of Zn may interfere with absorption of Fe and Cu( Reference Singh 73 ). Additionally, under physiological conditions, Zn ion would liberate Cu ion from the GABAA receptor and inhibit the effect of Cu on sleep regulation( Reference Sharonova, Vorobjev and Haas 74 ). These interactions have made it hard to disentangle the direct effect of a single micronutrient on sleep outcomes from current findings.
Implications for future nutrition–sleep research
The results from the current review have important implications for future research. Due to the possibility of concentration-dependent effect and intertwined actions of micronutrients, further studies demonstrating the nature of the effects of micronutrient levels on sleep are warranted. In terms of studied populations, whereas articles involving human subjects have primarily focused on infants, young children, general adults, postmenopausal women and older adults, very few studies have specifically investigated children in adolescence when substantial developmental changes in sleep pattern occur. Additionally, many of the current studies were heterogeneous in age, including all young, middle-aged and older adults( Reference Grandner, Jackson and Gerstner 35 , Reference Beydoun, Gamaldo and Canas 52 ). Given that the metabolic characteristics of micronutrients and the biological regulation of sleep may vary across the life span, researchers should take into account the developmental effect when interpreting results.
More than half of the human studies are observational studies and the degree of control for confounders varies across studies. Confounders that were typically adjusted for included age, sex, education, family income, BMI, energy intake, smoking status and race/ethnicity. There are only four studies involving covariates of depressive symptoms or antidepressant use( Reference Bertisch, Sillau and de Boer 20 , Reference Luojus, Lehto and Tolmunen 43 , Reference Beydoun, Gamaldo and Canas 52 , Reference Shiue 53 ). Future research should take into account important covariates, such as a medical history of psychiatric disorder or medication usage known to affect micronutrient absorption or sleep, to uncover the nature of the associations. Furthermore, a majority of reviewed studies were cross-sectional or focused on the short-term effects of micronutrient supplements. Research with a longitudinal design is needed to investigate how micronutrient status early in life predicts later sleep patterns and how the trajectory of micronutrient profile over the parts of the life span are associated with age-related changes in sleep patterns.
Implications for public health practice
Both micronutrient deficiency and poor sleep are significant public health issues worldwide. Although the clinical relevance of micronutrients on sleep patterns needs further examination, health-care providers, particularly those practising in primary-care settings, should take into account several findings from the current literature in health-care practice for individuals and communities. First, health-care providers should be attentive about micronutrient levels in infants due to the irreversible influence on brain function and sleep organization, Fe status in particular. Second, when using Mg in prenatal settings, particular attention should be given to mixed effects on the development of active sleep and quiet sleep in infants. However, Mg may be beneficial for elderly sleep and brain function. Third, despite the indefinite recommendation of optimal doses at this moment, it is important to note that optimal intake rather than high or low intake of micronutrients is needed to maintain normal sleep patterns.
Limitations of the current review
The current review has several potential limitations. First, only articles focusing on a direct relationship between micronutrient variables and sleep pattern variables are included. Studies on physiological sleep disorders such as restless-leg syndrome and obstructive sleep apnoea, and health complaints such as pain, which were not reviewed here, may uncover other missed relationships between micronutrients and sleep patterns. Second, the review did not stratify findings by gender, race and cultural context due to the unavailability of relevant data, and thus it cannot disentangle the interplays between social factors and micronutrients on sleep patterns.
Conclusion
Although no definite clinical recommendations can be made at this point due to the limited evidence, current studies have linked trace elements, minerals and vitamins to sleep patterns in human subjects. In the articles reviewed, researchers have observed a beneficial effect of adequate serum Fe on the development of sleep stages in infants, and of Mg supplements on inhibiting age-related sleep changes in older adults. Published literature also supported an association between sleep duration and micronutrients, with sleep duration positively associated with Fe, Zn and Mg, and negatively associated with Cu, K and vitamin B12. However, the results of the associations between micronutrients and sleep phase were insufficient and inconsistent. Future research is needed to investigate the concentration-dependent as well as the longitudinal relationships between micronutrient levels and human sleep across populations, test the interactions among micronutrients on sleep outcomes, and ultimately examine the clinical relevance of micronutrients on sleep health.
Acknowledgements
Acknowledgements: The authors would like to thank Drs Maureen George, Jinyoung Kim and Tanja Kral for their helpful comments on the initial draft of this paper. Financial support: This study was funded by the Office of Nursing Research grant award issued by the School of Nursing, University of Pennsylvania. The funder had no role in the design, analysis or writing of this article. Conflict of interest: None of the authors declare any conflict of interest that may be relevant to the materials presented in this paper. Authorship: X.J. developed the idea for the study, performed the systematic searches and the article retrieval, analysed the data, and wrote and revised the article. J.L. mentored the review process, including conceptualizing the research question, the literature search and the interpretation of results, and critically revised the article. M.A.G. contributed to the conceptualization of the research question, the initial outline of the manuscript and article revision. Ethics of human subject participation: Not applicable.







