The Mediterranean diet is considered one of the healthiest dietary models, and potential health benefits of its adoption during childhood and adolescence have been suggested(Reference Iaccarino, Scalfi and Valerio1–Reference Garcia Cabrera, Herrera Fernandez and Rodriguez Hernandez3). Higher adherence to the Mediterranean diet may reduce the negative effect of overweight and obesity and decrease different chronic non-communicable diseases such as CVD(Reference Iaccarino, Scalfi and Valerio1). Likewise, greater adherence to the Mediterranean diet could reduce the burden of malnutrition (overnutrition and micronutrient deficiency)(Reference Peng, Berry and Goldsmith4).
Valid dietary indices adapted for children and adolescents are needed to assess the adherence to dietary guidelines among this young population group. Throughout the past decades, numerous diet quality indices or scores measuring adherence to the Mediterranean dietary guidelines have been developed(Reference Vyncke, Cruz Fernandez and Fajo-Pascual5), especially for adults. Mediterranean dietary scores specifically designed for children and adolescents have been applied based on specific recommendations for these age groups, such as the Mediterranean Diet Quality Index for children and adolescents (KIDMED)(Reference Serra-Majem, Ribas and Ngo6) and several revised versions of the original Mediterranean Diet Score (MDS), among others(Reference Feskanich, Rockett and Colditz7–Reference Handeland, Kjellevold and Wik-Markhus13). Nevertheless, there are few studies investigating the use and validity of these indices in a European adolescent population(Reference Darnton-Hill, Nishida and James14). The cross-sectional Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study provides data about nutrient and food intakes and nutritional biomarkers(Reference Castro-Quezada, Roman-Vinas and Serra-Majem15) which could be used to compare and validate dietary indices for adolescents using an evidence-based approach.
Therefore, the aim of the present study was to investigate whether adherence to the adapted Mediterranean Diet Score for Adolescents (MDS_A) and the adapted KIDMED for Adolescents (KIDMED_A) is associated with better food and nutrient intakes as well as nutritional biomarker serum profiles in European adolescents. Different variants of these two scores have been examined, considering the guidelines from the original score, although also considering specific dietary needs (e.g. calcium and vitamin D for attaining peak bone mass) and restrictions (e.g. alcohol not recommended) for this young population group.
Methods and materials
Study design
The HELENA study is a population-based multi-centre investigation of the nutritional and lifestyle status among European adolescents. Data were collected from October 2006 to December 2007 and fieldwork took place in ten European cities of nine countries: Vienna (Austria), Ghent (Belgium), Lille (France), Dortmund (Germany), Athens (Greece), Heraklion (Greece), Pécs (Hungary), Rome (Italy), Zaragoza (Spain) and Stockholm (Sweden). The purpose of the study was to obtain standardized, reliable and comparable data from a random sample of European adolescents on a broad battery of relevant nutrition and health-related parameters. This included dietary intake, food choices and preferences, blood indicators of lipid metabolism and glucose metabolism, vitamin and mineral status, anthropometry, physical activity, fitness and genetic markers. The entire description of the HELENA study design and sampling procedure has been published previously(Reference Moreno, González-Gross and Kersting16,Reference Beghin, Castera and Manios17) .
Participants
The study population included 3528 adolescents (52·3 % females) aged 12·5–17·5 years. Adolescents were excluded from the database a posteriori if they met one or more of the following exclusion criteria: age <12·5 or ≥17·5 years; no measurement of weight and/or height; completion of <75 % of the tests; participating simultaneously in another clinical trial; or contracting an acute infection during the week prior to the examination(Reference Moreno, González-Gross and Kersting16). As a result of logistical problems (limited staff capacity for collecting and analysing 24 h dietary recall data), there was no nutrient intake information from Heraklion or Pècs participants, for which case they were excluded.
For one purpose of the present study, we analysed the association between the adapted MDS and food and nutrient intakes without exclusion of under-reporters, resulting in 2330 adolescents (53·8 % females), and with exclusion of under-reporters, resulting in 1804 adolescents (52·6 % females). Under-reporting was considered when the individual ratio of energy intake divided by the estimated BMR was lower than 0·96(Reference Black18). The group of under-reporters consisted of a higher percentage of females (57·8 % v. 52·6 % plausible reports; P = 0·036) and had higher median (minimum, maximum) BMI values (22·5 (15·0, 45·6) kg/m2 v. 20·1 (14·1, 40·8) kg/m2 in the plausible reports; P < 0·001).
Additionally, to investigate the relationship between different indices and nutritional biomarkers, blood samples were collected in a randomly selected sub-sample of the total HELENA study population (1089 adolescents) of whom 697 had two 24 h dietary recalls. Following the same criteria, we analysed with exclusion of under-reporters resulting in 552 adolescents (52·3 % females).
For the second aim of the present study, we examined the association between the adapted KIDMED and food and nutrient intakes. For this index, under-reporters were not excluded as the adapted KIDMED index was partly based on the dietary questionnaire data and not only on 24 h recall data. The power for the adapted KIDMED score was already reduced since several individuals were excluded because they did not complete the FFQ or the Food Choices and Preferences (FCP) questionnaire. The total sub-sample for this index was 1114 adolescents (56·5 % females). To analyse the association between adapted KIDMED and nutritional biomarkers, 552 adolescents were included (52·3 % females).
The study was conducted following the ethical guidelines of the Declaration of Helsinki, the Good Clinical Practice rules, and the legislation about clinical research in human subjects in each of the participating countries. Written informed consent was obtained from all adolescents, their parents or guardians, and the protocol was approved by the Human Research Review Committees of the centres involved(Reference Beghin, Castera and Manios17).
Dietary intake assessment
Dietary intake was obtained by means of 24 h dietary recalls(Reference Biró, Hulshof and Ovesen19), an FFQ and the FCP questionnaire(Reference Gilbert, Hegyi and Sanchez20,Reference Gilbert, Hall, Hegyi, Gonzalez-Gross, De Henauw and Gottrand21) . The 24 h dietary recalls were collected via the HELENA-DIAT (Dietary Assessment Tool) software on two non-consecutive days within a period of 2 weeks. Adolescents completed the 24 h dietary recall autonomously in the computer classroom during school time and dietary intake was referred to the day before the interview; therefore, no information on Fridays and Saturdays was available.
This tool was adapted from a previous version developed and validated for Flemish adolescents(Reference Vereecken, Covents and Matthys22). This assessment tool is based on six meal occasions (breakfast, morning snacks, lunch, afternoon snacks, evening meal, evening snacks) referring to the previous day. Trained dietitians assisted the adolescents to complete the 24 h dietary recalls when needed. Adolescents autonomously selected all the consumed foods and beverages from a standardized food list in the HELENA-DIAT(Reference Vereecken, Covents and Sichert-Hellert23). Items not available in the list could be added by the participant at any moment. Foods were translated to nutrients by use of the German Food Code and Nutrient Data Base (Bundeslebensmittelschlüssel (BLS) Version II.3.1)(Reference Dehne, Klemm and Henseler24,Reference Julian-Almarcegui, Bel-Serrat and Kersting25) . The Multiple Source Method was used to estimate the usual dietary intakes of nutrients and foods(Reference Haubrock, Harttig and Souverein26,27) . This statistical modelling technique takes account of within-person and between-person variability and calculates usual intakes corrected for age, sex and study centre.
The FFQ was designed to estimate adolescents’ habitual food intake(Reference Vereecken, Dohogne and Covents28) and was based on the Health Behaviour in School-aged Children (HBSC) FFQ, which was validated in a Belgian youth population(Reference Vereecken and Maes29). In the current study, three questions have been selected from the FFQ: fruits, vegetables and sweets. There were seven answer categories ranging from ‘never’ up to ‘more than once a day’. The answer ‘never’ was taken to count as 0 points and ‘once a day, every day’ and ‘more than once a day’ were merged together and categorized as 1 point.
The FCP questionnaire aimed to explore attitudes and issues of concern among adolescents regarding food choices, preferences, healthy eating and lifestyle(Reference Gilbert, Hegyi and Sanchez20,Reference Gilbert, Hall, Hegyi, Gonzalez-Gross, De Henauw and Gottrand21) . From this questionnaire, ‘usually skipping breakfast’ was collected with five answer categories ranging from ‘strongly disagree’ up to ‘strongly agree’. When participants answered ‘moderately agree’ or ‘strongly agree’, the score was 1 point. Additionally, ‘How often do you consume fast food?’ ranging from ‘never’ up to ‘each day’ was considered in the index score. The score was 1 point if participants answered from ‘often’ up to ‘each day’.
Adapted Mediterranean diet score for adolescents
Adherence to the Mediterranean dietary pattern was assessed by an adapted version of the traditional MDS developed for adults as reported by Trichopoulou et al.(Reference Trichopoulou, Costacou and Bamia30). The traditional MDS for adults includes nine components (vegetables; fruits and nuts; legumes; cereals; fish; monounsaturated fat:saturated fat ratio; dairy products; meat and poultry; and wine); each component (except for alcohol intake) is assigned a score of 0 or 1 using the sex-specific medians as cut-off values (below and above, respectively). Dairy products and meat (including poultry), as detrimental components, are reversely scored. In addition, 1 point is given for alcohol intake within a specified range: 5–25 g/d for women and 10–50 g/d for men; intakes above score as ‘0’.
In our proposal, we modified the MDS for use in 12·5–17·5-year-old adolescents (MDS_A). We used age- and sex-specific median intakes of the study participants as a cut-off value for each component. Alcohol intake was scored as a detrimental component since ethanol consumption is not recommended for children and adolescents, while dairy products were scored as a beneficial component because dairy is recommended in growing age, namely childhood and adolescence(Reference Serra-Majem, Ribas and Ngo6). However, an MDS variant including dairy products as a detrimental component had also been calculated and validated(Reference Trichopoulou, Costacou and Bamia30). As such, four different versions of the adapted adolescent MDS were created as categorical variables (see Table 1 for more details):
1. MDS_A_7P_2N, with seven positive components (fruits, vegetables, pulses, cereals, fish and seafood, monounsaturated fat:saturated fat ratio and dairy products) and two negative components (meat and alcohol);
2. MDS_A_NA_7P_1N, with seven positive components (fruits, vegetables, pulses, cereals, fish and seafood, monounsaturated fat:saturated fat ratio and dairy products) and one negative component (meat), excluding alcohol (‘NA’ stands for ‘no alcohol’);
3. MDS_A_NA_6P_2N, with six positive components (fruits, vegetables, pulses, cereals, fish and seafood, and monounsaturated fat:saturated fat ratio) and two negative components (dairy and meat), excluding alcohol; and
4. MDS_A_6P_3N, with six positive components (fruits, vegetables, pulses, cereals, fish and seafood, monounsaturated fat:saturated fat ratio) and three negative components (dairy, meat and alcohol).
MDS_A_7P_2N and MDS_A_NA_7P_1N are directly based upon the original MDS for adults, considering alcohol as a negative component for adolescents in MDS_A_7P_2N and ignoring the alcohol component for this young population in MDS_A_NA_7P_1N. To ease the readability throughout the paper, two standard MDS_A scores that are closest to the original MDS have been abbreviated as MDS_A and MDS_A_NA, respectively.
Table 1 Overview of the components of the adapted MDS and the adapted KIDMED for adolescents*
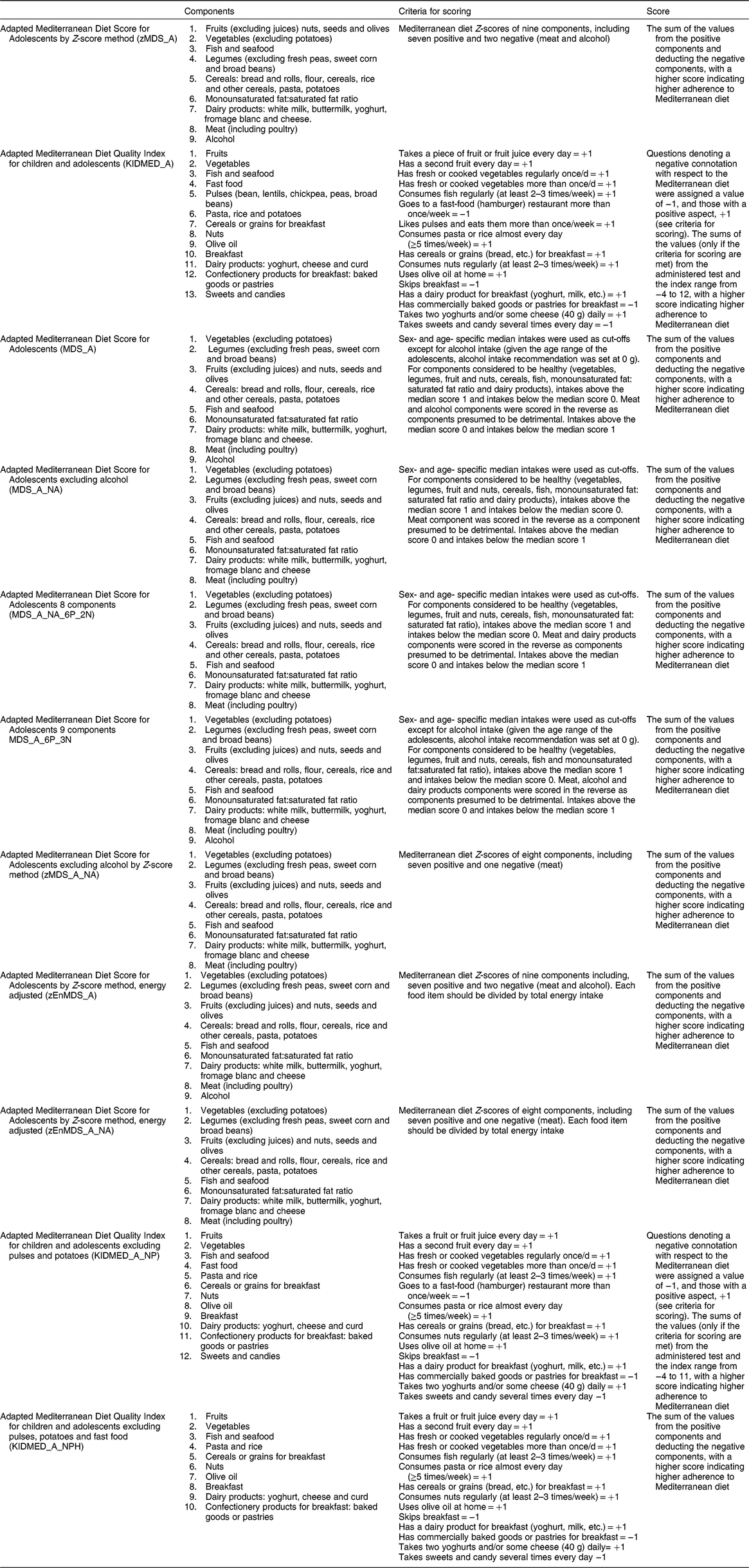
MDS, Mediterranean Diet Score; KIDMED, Mediterranean Diet Quality Index for children and adolescents.
* Construct details of the abbreviated scores: _A, adolescents; _NA, no alcohol category included in score; _xP, number of positive categories included in score (x = number); _xN, number of negative categories included in score (x = number); _NP, no pulses category included in score; _NPH, no pulses and fast food category included in score.
MDS_A and MDS_A_NA were also calculated as continuous variables using standardized Z-scores (in which each component was included as a Z-score rather than a binary variable): without energy adjustment, zMDS_A and zMDS_A_NA; and with energy adjustment, zEnMDS_A and ZEnMDS_A_NA. All indices were also analysed excluding under-reporters. Higher scores indicate higher adherence to the Mediterranean diet. More details of the components and cut-offs used to calculate the adapted zMDS_A are shown in Table 1.
Adapted KIDMED for adolescents
A second index to assess the adherence to the Mediterranean diet in our European adolescents was developed based on the KIDMED score, an index developed for children and adolescents proposed by Serra-Majem et al.(Reference Serra-Majem, Ribas and Ngo6).
The original KIDMED(Reference Serra-Majem, Ribas and Ngo6) is calculated based upon a test that includes sixteen questions which could be self-administered or conducted by interview (paediatrician, dietitian, etc.). The index ranged from −4 to 12, where questions denoting a negative connotation were assigned a value of −1 and those with a positive connotation, a value of +1.
In our adapted version, we used data from repeated 24 h dietary recalls, the FFQ and the FCP questionnaire. Information on the consumption of pulses, cereals or grains for breakfast, yoghurts and cheese, baked goods or pastries, dairy products, olive oil and nuts were taken from the 24 h recalls. Potatoes, pasta and rice were also obtained from the 24 h dietary recalls. Questions about the usual consumption of fruits, vegetables and sweets were obtained by means of the FFQ. Furthermore, questions related to fast food and breakfast skipping were taken from the FCP questionnaire. The KIDMED_A as a categorical variable ranged from −4 to 12, with a higher score indicating higher adherence to the Mediterranean diet. Furthermore, two variants of KIDMED_A were calculated as categorical variables (see Table 1 for more details):
1. KIDMED_A_NP, not including pulses and potatoes; and
2. KIDMED_A_NPH, not including pulses, potatoes and fast food (e.g. hamburger).
The components and the criteria for scoring the KIDMED_A are presented in Table 1.
Results pertaining to zMDS_A and KIDMED_A are provided in Tables 2–7 in the present paper. However, the detailed results for all other indices (MDS_A_7P_2N, MDS_A_7P_1N, MDS_A_NA_6P_3N, zMDS_A_NA, zEnMDS_A, zEnMDS_A_NA, KIDMED_A_NPH and KIDMED_A_NP) are presented in the online supplementary material, Supplemental Annexes 1, 2 and 3.
Table 2 Association between the adapted Mediterranean Diet Score for Adolescents by Z-score method (zMDS_A) and food intakes among adolescents aged 12·5–17·5 years from nine European countries, Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study
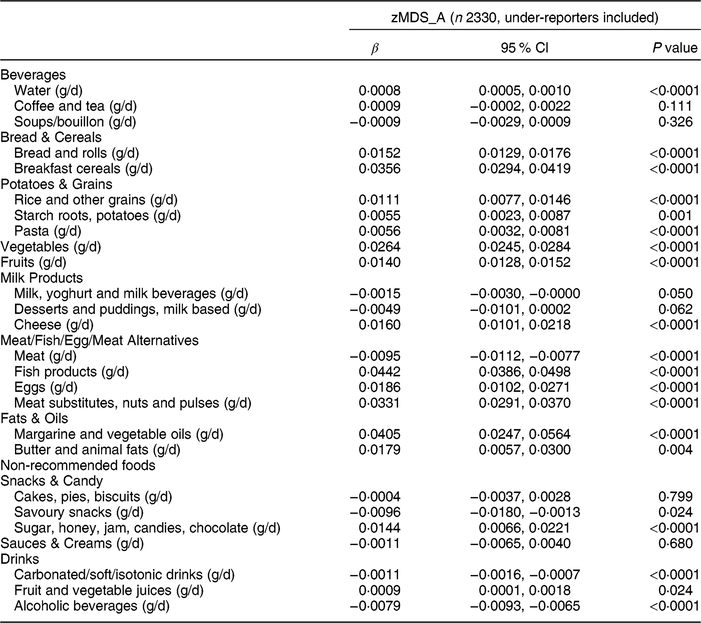
β, standardized regression coefficient.
Multilevel regression analyses with inclusion of a random intercept for centre and corrected for age and sex as independent variables. Bonferroni correction resulted in level of significance <0·0019.
Table 3 Association between the adapted KIDMED for adolescents (KIDMED_A) and food intakes among adolescents aged 12·5–17·5 years from nine European countries, Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study
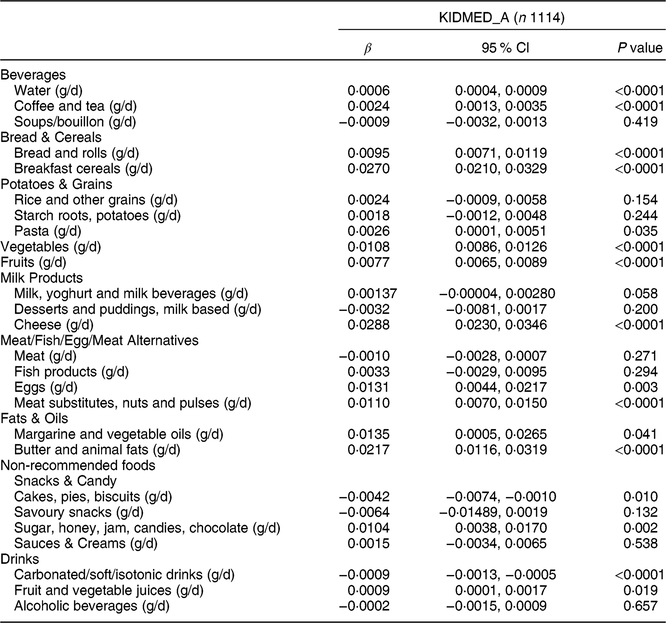
KIDMED, Mediterranean Diet Quality Index for children and adolescents; β, standardized regression coefficient.
Multilevel regression analyses with inclusion of a random intercept for centre and corrected for age and sex as independent variables. Bonferroni correction resulted in level of significance <0·0019.
Table 4 Association between the adapted Mediterranean Diet Score for Adolescents by Z-score method (zMDS_A) and usual intake of macro- and micronutrients among adolescents aged 12·5–17·5 years from nine European countries, Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study
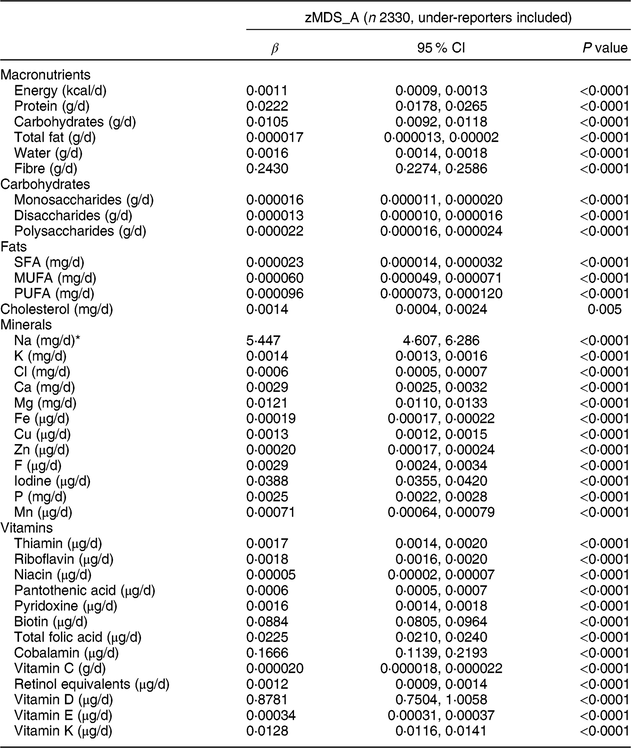
β, standardized regression coefficient.
Multilevel regression analyses with inclusion of a random intercept for centre and corrected for age and sex as independent variables. Bonferroni correction resulted in level of significance <0·0013.
* Variable was log-transformed to obtain a normal distribution.
Table 5 Association between the adapted KIDMED for adolescents (KIDMED_A) and usual intake of macro- and micronutrients among adolescents aged 12·5–17·5 years from nine European countries, Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study
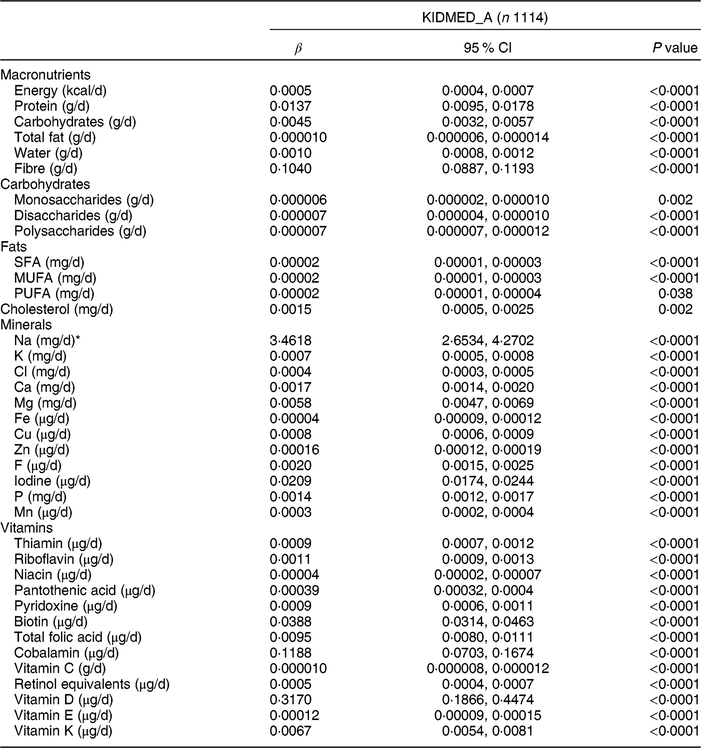
KIDMED, Mediterranean Diet Quality Index for children and adolescents; β, standardized regression coefficient.
Multilevel regression analyses with inclusion of a random intercept for centre and corrected for age and sex as independent variables. Bonferroni correction resulted in level of significance <0·0013.
* Variable was log-transformed to obtain a normal distribution.
Table 6 Association between the adapted Mediterranean Diet Score for Adolescents by Z-score method (zMDS_A) and nutritional biomarkers among adolescents aged 12·5–17·5 years from nine European countries, Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study
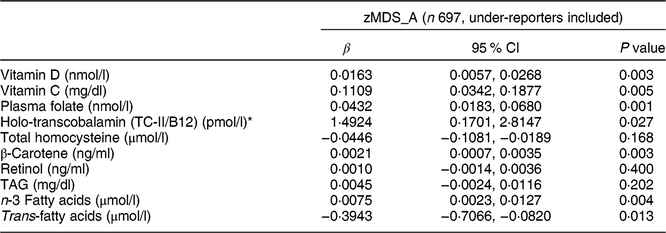
β, standardized regression coefficient.
Multilevel regression analyses with inclusion of a random intercept for centre and corrected for age and sex as independent variables. Bonferroni correction resulted in level of significance <0·005.
* Variable was log-transformed to obtain a normal distribution.
Table 7 Association between the adapted KIDMED for adolescents (KIDMED_A) and nutritional biomarkers among adolescents aged 12·5–17·5 years from nine European countries, Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study
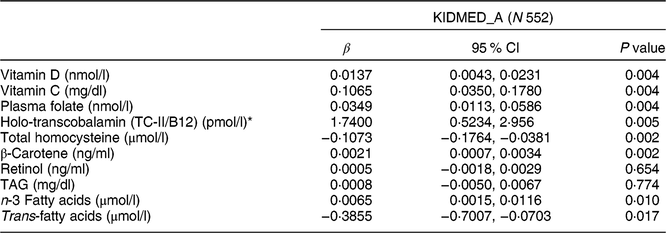
KIDMED, Mediterranean Diet Quality Index for children and adolescents; β, standardized regression coefficients.
Multilevel regression analyses with inclusion of a random intercept for centre and corrected for age and sex as independent variables. Bonferroni correction resulted in level of significance <0·005.
* Variable was log-transformed to obtain a normal distribution.
Biochemical analyses
Fasted blood samples were collected by venepuncture at school between 08.00 and 10.00 hours according to a standardized blood collection protocol. Details about the sampling, processing and transportation have been published elsewhere(Reference Gonzalez-Gross, Breidenassel and Gomez-Martinez31). For the current analyses, nutritional biomarkers and biomarkers with an important role in nutritional metabolism have been selected as objective indicators.
Plasma 25-hydroxyvitamin D was analysed by ELISA using a kit (OCTEIA 25-Hydroxy Vitamin D) from Immunodiagnostic Systems (Frankfurt, Germany) and measured with a SunriseTM Photometer by TECAN (Hamburg, Germany). The sensitivity of this method is 5 nmol/l for 25-hydroxyvitamin D and the variation is below 6 %. Although plasma vitamin D concentrations are influenced by numerous factors such as sunlight exposure and adiposity, evidence indicated moderate correlations with dietary intake(Reference Jacques, Sulsky and Sadowski32). Vitamin C was analysed in plasma by reversed-phase HPLC (Sykam, Gilching, Germany) using UV detection (UV–VIS 205; Merck, Darmstadt, Germany). The CV of vitamin C was 4·4 %. Strong correlations of dietary intake of vitamin C with serum ascorbic acid concentrations have been shown mainly when habitual dietary intakes of vitamin C are relatively modest(Reference Bates, Rutishauser and Black33,Reference Jenab, Slimani and Bictash34) .
Plasma folate (CV = 5·4 %) and total homocysteine (CV = 10·7 %) were determined by means of an immunoassay using the Immunolite 2000 analyser (DPC Biermann GmbH, Bad Nauheim, Germany) and holo-transcobalamin (CV = 5·1 %) was measured by an automated microparticle enzyme immunoassay with the AxSYM analyser (Abbott Laboratories, Abbott Park, IL, USA). Folate intakes correlate weakly with serum/plasma concentrations since many factors can influence the bioavailability of dietary folate and, therefore, serum/plasma folate concentrations(Reference Jacques, Sulsky and Sadowski32).
Retinol, α-tocopherol and β-carotene were analysed in plasma using reversed-phase HPLC (Sykam) using UV detection (UV–VIS 205; Merck). The variation of the method is below 3 % for all the vitamins. Plasma retinol concentrations are responsive to vitamin A intake only in individuals with inadequate vitamin A status(Reference Willett, Stampfer and Underwood35). Also, there are moderate correlations between blood concentrations of carotenoids and fruit and vegetable intakes(Reference Jenab, Slimani and Bictash34–Reference Rock, Swendseid and Jacob36).
Serum phospholipid fatty acid composition (CV = <4·4 %) was measured by capillary GC (model 3900; Varian GmbH, Darmstadt, Germany) after extraction performed by thin-layer chromatography. Serum TAG were determined enzymatically on the Dimension RxL clinical chemistry system (Dade Behring, Schwalbach, Germany) using the manufacturer’s reagents and instructions. There is moderate correlation between n-3 fatty acid intake and plasma phospholipid levels although reflecting intake in the short to medium term(Reference Andersen, Solvoll and Drevon37,Reference Hodge, Simpson and Gibson38) . Good correlations have been shown between the intake of specific types of trans-fatty acid and their levels in blood; however, the correlation between the total sum of trans-fatty acid intake and the sum of serum trans-fatty acid levels is only weak(Reference Hodson, Skeaff and Fielding39,Reference Baylin, Kim and Donovan-Palmer40) . Likewise, plasma TAG has been found to be positively correlated with total fat intake and negatively with fibre intake. Levels may to some extent be indicative of the level of dietary fibre intake, but the findings to date are contradictory(Reference Jenab, Slimani and Bictash34,Reference Sonnenberg, Quatromoni and Gagnon41) .
Statistical analysis
Statistical analyses were performed using the statistical software package IBM SPSS Statistics for Windows version 21.
Pearson χ 2 and t tests were used to test for differences between sexes in categorical and continuous variables, respectively. Normality was evaluated visually and based on the skewness of the data distributions. Skewness of variables on intake ranged from 0·869 (meat intake) to 9·809 (alcohol intake; due to a high number of non-consumers). Since these variables were studied in a large sample, it was considered that parametric tests were allowed without transformations. However, variables with a skewness > 3 were transformed (logarithmic transformation and square-root transformation were tested) and the multilevel analyses were repeated with the transformed data. A log-transformation of holo-transcobalamin was also performed in the present study.
Multilevel linear regression analysis with inclusion of a random intercept for study centre was used to examine the relationship of the different Mediterranean diet indices – MDS_A (see online supplementary material, Supplemental Annexes 1, 2 and 3), MDS_A_NA (Supplemental Annexes 1, 2 and 3), zMDS_A (Tables 2, 4 and 6), zMDS_A_NA (Supplemental Annexes 1, 2 and 3), zEnMDS_A (Supplemental Annexes 1, 2 and 3), zEnMDS_A_NA (Supplemental Annexes 1, 2 and 3) and KIDMED_A (Tables 3, 5 and 7), which were calculated with and without exclusion of the under-reporters except for KIDMED_A that was calculated only for the full population – with foods, nutrient intakes and blood biomarkers.
Multilevel linear regression analysis was also performed between MDS_A_NA_6P_2N, MDS_A_6P_3N, KIDMED_A_NPH and KIDMED_A_NP (Supplemental Annex 3) and nutritional biomarker status. Confounders (age and sex) were entered as covariates. The level of statistical significance was controlled for multiple testing and a Bonferroni correction was applied to lower the significance level (α) account of the number of tests (foods = 0·05/number of tests; nutrients = 0·05/number of tests; biomarkers = 0·05/number of tests) to control family-wise error rate. Therefore, statistical significance was considered with P values of 0·0019, 0·0013 and 0·005 for the association between all indices and foods, nutrients and biomarkers, respectively.
Results
For all adapted MDS, the total population consisted of 2330 participants (53·8 % females, mean age 14·7 (sd 1·2) years) or 1804 participants (52·6 % females, mean age 14·7 (sd 1·2) years) when excluding under-reporters. For all adapted KIDMED scores, the total population involved 1114 participants (56·5 % females, mean age 14·8 (sd 1·3) years).
Comparison of the different indices with food intakes
Multilevel regression analysis of the zMDS_A and KIDMED_A with the usual consumption of different foods is shown in Tables 2 and 3, respectively. A significant positive association was found between water (g/d) and both indices (β = 0·0008 and β = 0·0006, respectively; both P < 0·0001). In contrast, soft drinks (g/d) showed a significant negative relationship with zMDS_A (Table 2), KIDMED_A (Table 3) and MDS_A (Supplemental Table S1.4; β = −0·0011, β = −0·0009 and β = −0·0004, respectively; all P < 0·0001). Alcoholic beverages (g/d) and meat (g/d) presented a significant negative association (β = −0·0079 and β = −0·0095; both P < 0·0001, respectively) with zMDS_A. All non-recommended foods (all g/d) also presented a significant negative association with zEnMDS_A (Supplemental Table S1.8). Sugar/honey/jam/candies/chocolate (all g/d) showed a negative tendency association (β = −0·0076, P = 0·057). A significant positive link (P < 0·0001) was observed for rice, bread and cereals, pasta, fat and oil, vegetables, fruits and fish with zMDS_A and KIDMED_A (except fish; all g/d). Potatoes (g/d) showed a significant positive association with zMDS_A. zEnMDS_A presented positive and significant relationship with healthy foods such as fish, fruit, vegetables, meat, nuts and pulses (all g/d), among others (see Supplemental Table S1.8).
Comparison of the different indices with nutrient intakes
Tables 4 and 5 show the results of macronutrients for zMDS_A and KIDMED_A, respectively. For zMDS_A, apositive association (all P < 0·0001, except for cholesterol (mg/d), P = 0·005) was observed with all macro- and micronutrients (Table 4). The usual intake of macronutrients, minerals and vitamins was positively related to the KIDMED_A (all P < 0·0001, except for monosaccharides (mg/d), P = 0·002; PUFA (mg/d), P = 0·038; and cholesterol (mg/d), P = 0·002; Table 5). However, zEnMDS_A presented negative associations with the majority of macro- and micronutrients, vitamins and minerals (Supplemental Table S2.8).
Comparison of the different indices with nutritional biomarker levels
Tables 6 and 7 describe the results between the zMDS_A and KIDMED_A, respectively, and nutritional biomarkers in subgroups of 697 participants (under-reporters included) and 552 adolescents excluding under-reporters. A significant positive association (all P < 0·01) was observed between zMDS_A and KIDMED_A and vitamin D (nmol/l), vitamin C (mg/dl), plasma folate (nmol/l), holo-transcobalamin (pmol/l), β-carotene (ng/ml) and n-3 fatty acids (ng/ml). Trans-fatty acids (µmol/l) showed a significant negative association with zMDS_A, and KIDMED_A (β = −0·3943 and β = −0·3855, respectively; both P < 0·05). In addition, total homocysteine (µmol/l) showed a significant negative association with KIDMED_A (β = −0·1073, P = 0·002).
Discussion
Adherence to the Mediterranean diet has been quantified in diet quality indices that attempt to obtain a global evaluation of the quality of the diet based on a traditional reference pattern. However, this traditional reference pattern originally based upon adult populations has been adapted for use in children and adolescents, a population group with specific nutritional requirements when considering their growth needs. Therefore, different variants of the original MDS have been developed for use in child and adolescent populations, all considering different arguments for the inclusion of components such as alcohol and dairy products in the calculation of the MDS.
The results from the present cross-sectional study have shown that zMDS_A and KIDMED_A followed by MDS_A were significantly associated with most nutritional biomarkers in the theorized directions. Moreover, our data seem to indicate that similar results were obtained independently of the inclusion or exclusion of under-reporters (results considering under-reporters are shown in the online supplementary material, Supplemental Annexes 1, 2 and 3).
Nutrient-dense food items, such as fish, fruits and vegetables, were positively associated with zMDS_A and KIDMED_A, whereas most of the energy-dense and non-recommended foods (e.g. cakes, biscuits, soft drinks) showed negative associations.
At the level of food intake, Vyncke et al.(Reference Vyncke, Cruz Fernandez and Fajo-Pascual5) investigated similar associations in relation to the Diet Quality Index for Adolescents (DQI-A); nevertheless, they did not find a significant relationship between the DQI-A and meat and fish intake. In our study, a negative and positive significant association was obtained between meat and fish intake, respectively, for KIDMED_A and zMDS_A, which was also found by Grosso et al.(Reference Grosso, Marventano and Buscemi42). Moreover, a significant positive association was shown between MDS_A, zMDS_A and KIDMED_A and the consumption of fats and oils. A moderate intake of fat and oil is recommended because they are also important sources of essential fatty acids and vitamins. Furthermore, adolescents have higher fat intake needs, which is essential for growth(Reference Vyncke, Libuda and De Vriendt43). However, high-fat diets may decrease insulin sensitivity and are positively associated with increased cardiovascular risk(Reference Appel, Sacks and Carey44,Reference Ruiz, Avila and Valero45) , although a precise dose–response relationship has not been defined.
The preservation of an adequate hydration status in adolescents has been recognized as important, being related to the ability to regulate body temperature and cognitive performance(Reference Edmonds and Burford46). Water showed a positive association with all indices calculated (P < 0·0001). Carbonated drinks presented a negative relationship with zMDS_A and KIDMED_A, whereas there was a positive association between both indices and fruit and vegetables juices. No significant relationship between the MDS_A and zMDS_A and the usual intake of coffee was found. One reason for this could be a lower consumption of coffee and alcoholic beverages by the adolescent population included in the HELENA study(Reference Duffey, Huybrechts and Mouratidou47).
Another interesting finding was the positive association of MDS_A, zMDS_A and KIDMED_A with energy intake, in contrast with the negative association found by Vyncke et al.(Reference Vyncke, Libuda and De Vriendt43) when investigating the association between energy intake and the DQI_A. Complex carbohydrates were positively associated with all indices except the zEnMDS_A. An important advantage of this energy-adjusted zEnMDS_A index is that it was negatively associated with energy intake. Vyncke et al.(Reference Vyncke, Cruz Fernandez and Fajo-Pascual5) found a negative relationship between energy intake and the DQI-A. Our findings also indicate that there are positive associations between these indices and the usual intake of macro- and micronutrients. Furthermore, a positive and significant association was found for the zMDS_A, KIDMED_A and MDS_A with all minerals and vitamins. Our results are in concordance with Serra-Majem et al.(Reference Serra-Majem, Ribas and Garcia48) except for vitamin E. Vyncke et al.(Reference Vyncke, Cruz Fernandez and Fajo-Pascual5) did not find an association between vitamin C or carotene and the DQI-A. This would be considered an important point because the adequate intakes of vitamins and minerals are essential during childhood and adolescence(Reference Gonzalez-Gross, Valtuena and Breidenassel49,Reference Amit50).
Nutritional biomarkers may offer advantages and be able to improve the estimates of dietary intake assessment due to the independence of their random errors in relation to the errors inherent to intake questionnaires(Reference Yokota, Miyazaki and Ito51). Biomarkers have been recognized as potential tools for describing dietary patterns, since they may provide dietary information that is complementary to self-reported dietary intake(Reference Bach-Faig, Geleva and Carrasco52,Reference Prentice, Sugar and Wang53) . Not all nutrients have well-defined biological markers, and many are influenced by other factors than intake(Reference Vyncke, Cruz Fernandez and Fajo-Pascual5). In the present study, positive and strong associations were found between KIDMED_A and zMDS_A and levels of plasma folate and holo-transcobalamin in the expected direction. In addition, a significant negative association was shown between KIDMED_A and total homocysteine. Adequate folate and vitamin B12 levels are necessary for healthy growth and development, and deficiency at young ages is related to developmental delay, feeding problems, failure to thrive, anaemia and even irreversible neurological damage(Reference Selhub and Paul54). A deficiency of these vitamins usually leads to an increase in total homocysteine concentrations, which is considered an additional a marker for the status of folate and vitamin B12 (Reference Obeid, Munz and Jäger55). However, there are few studies analysing the association between these nutritional biomarkers and diet quality indices in adolescents; and therefore KIDMED_A has shown a good validity by confirming the expected association.
Among others, a significant and positive relationship was found between vitamin D and KIDMED_A and zMDS_A. This finding was important since vitamin D is involved in the immune system, bone mineralization and muscle tissue. This vitamin has been related with different pathologies such as cancer, diabetes, hyperactivity, hypertension and multiple sclerosis(Reference Gonzalez-Gross, Valtuena and Breidenassel49,Reference Bischoff-Ferrari56–Reference Elmadfa, Godina-Zarfl and Dichtl59) . KIDMED_A and zMDS_A also showed a significant positive relationship with vitamin C. Likewise, other authors observed these relationships between different diet quality indices and nutritional biomarkers in people aged over 18 years. For example, Weinstein et al.(Reference Weinstein, Vogt and Gerrior60) found a correlation between the Healthy Eating Index and serum vitamin C, serum folate and serum vitamin B12 in a large population. Bach-Faig et al.(Reference Bach-Faig, Geleva and Carrasco52) observed associations between high adherence to Mediterranean diet and high levels of β-carotene, vitamin C and folate. Okuda et al.(Reference Okuda, Sasaki and Bando61) found correlations between energy intake and β-carotene (P < 0·030). In our study, there was no association between zMDS_A and KIDMED_A and retinol. The absence of an association with retinol level might be attributed to the fact that this vitamin is prevalent in meat, and a significant negative relationship was found with zMDS_A and KIDMED_A for meat intake.
Regarding fatty acids, serum levels of trans-fatty acids showed an inverse relationship with the KIDMED_A and zMDS_A. This finding was also discovered by Vyncke et al.(Reference Vyncke, Cruz Fernandez and Fajo-Pascual5) with the DQI-A. This is an interesting result because higher levels of trans-fatty acids are related to an increase of cardiovascular risk(Reference de Souza, Mente and Maroleanu62).
The aim of the present study was to obtain a holistic measure for the overall diet quality of an individual, using the Mediterranean diet as a reference. All different indices have advantages and disadvantages and the main disadvantage of the MDS, in comparison with the DQI-A from Vyncke et al.(Reference Vyncke, Cruz Fernandez and Fajo-Pascual5), is that energy intake was positively associated with zMDS_A and KIDMED_A. This is mainly a disadvantage when thinking about obesity prevention among youth. However, zEnMDS_A could be considered when energy intake will be analysed due to the negative association with usual energy intake. In our study, statistically significant associations were found with biomarkers representing long-term and short- to medium-term dietary intakes except for TAG and retinol.
Strengths and limitations
There are few studies investigating the use and validity of these indices in a European adolescent population. The MDS_A and KIDMED_A in the present study were not based on nutrient intakes, avoiding the limitations that coincide with the use of food composition data (such as the use of various tables in different countries with different methods of analysis used, unavailability of food items, loss of dietary information from mixed dishes, etc.).
All indices were calculated based on two self-administered, computer-assisted, non-consecutive 24 h recalls, among others. Following recommendations of the European Food Consumption Survey method (EFCOSUM), 24 h dietary recalls were preferred as these are open-ended questionnaires from which detailed information can be obtained. Moreover, the European Food Safety Authority indicates in all publications on dietary misreporting among children and adolescents, that more research on validity of dietary methods is needed in this young population group, considering also the issue of misreporting(63). For that reason, under-reporters have been taken accounted for in the present study.
One limitation of the used method is, however, that information of only two days was obtained. Although this allows inclusion of exceptional intakes at the individual level, this effect is neutralized by the large number of observations. The 24 h dietary recall method does not allow quantifying proportions of non-consumers for particular food items, especially for infrequently consumed foods. In order to decrease this influence, nutrient intakes were corrected for within-person variability by applying the Multiple Source Method. In this respect, the 24 h dietary recalls were performed through the computer-assisted HELENA-DIAT software to standardize the recall procedures as much as possible.
The same food composition table for conversion of food intake data to estimated nutrient intakes was used for all survey centres(Reference Julian-Almarcegui, Bel-Serrat and Kersting25). This identified differences in definitions, analytical methods, units and modes of expression, which were overcome. The fact that one single database of food composition was used could be a limitation due to the composition of some foods that may vary between countries and this might have reduced the correlation with some biomarkers. Another limitation may be that the FFQ has been validated only in a Belgian youth population and not in the HELENA study(Reference Vereecken and Maes29).
The present study used data from the largest European study investigating the association of lifestyle factors (such as diet) with health outcomes (like obesity and health biomarkers) among European adolescents. Even though the sample size is reduced for some of the sub-analyses, as described in the ‘Methods and materials’ section, all the variables included in the present study were well framed for this sample size.
Conclusion
The zMDS_A and KIDMED_A showed associations with nutrient and food intakes, especially with nutritional biomarkers, in the hypothesized directions. Although other variants also showed good associations with the indicators under study, they were less significant compared with the zMDS_A and KIDMED_A. Altogether, based on these results, we recommend the use of the continuous MDS_A score using the Z-score method or the adapted KIDMED score for use in European adolescents when investigating adherence to the Mediterranean diet among adolescents.
Acknowledgements
Acknowledgements: Many thanks to Christel Bierschbach, Adelheid Schuch, Anke Berchtold, Petra Pickert, Andre Spinneker, Ulrike Albers and Anke Carstensen for their contribution to laboratory work. Financial support: The HELENA study was support by the European Community Sixth RTD Framework Programme (Contract FOOD-CT-2005-007034). There was additional support from the Spanish Ministry of Education (AGL2007-29784-E/ALI), Axis-Shield Diagnostics Ltd (Oslo, Norway), Abbot Científica S.A. (Spain) and Cognis GmbH. R.A.-U. obtained a travel grant from the Consejo Social de la Universidad Politécnica de Madrid and the work reported in this paper was undertaken while she was hosted by the International Agency for Research on Cancer. R.A.-U. is also supported by Universidad Politécnica de Madrid with a predoctoral scholarship. The funders had no role in the design, analysis or writing of this article. The content of this article reflects only the authors’ views and the European Community is not liable for any use that may be made of the information contained herein. Conflict of interest: None. Authorship: L.A.M. coordinated the project. L.A.M., M.G.-G. and M.K. designed and carried out the HELENA project. R.A.-U. and M.G.-C. carried out data analyses and drafted the manuscript. S.B.-S., M.G.-G. and I.H. provided critical input on the data analyses and on earlier versions of the manuscript. All authors contributed to interpretation of the data and reviewed the submitted manuscript. Ethics of human subject participation: The HELENA study was conducted following the ethical guidelines of the Declaration of Helsinki, the Good Clinical Practice rules, and the legislation about clinical research in human subjects in each of the participating countries. Written informed consent was obtained from all adolescents, their parents or guardians, and the protocol was approved by the Human Research Review Committees of the centres involved. IARC disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980019001022
Author ORCID
Silvia Bel-Serrat, 0000-0003-3698-2619.









