Over the course of many years, numerous studies have reported that intakes of SFA and trans-fatty acids (TFA) are related to lipidic risk factors for CVD. In the case of SFA intake, the strength of this association is estimated to be as follows: every 1 % increase of energy coming from SFA causes an increase in LDL cholesterol (LDL-C) concentration by 12·7–17·4 mg/l and in HDL cholesterol (HDL-C) concentration by 4·3–5 mg/l( Reference Mensink, Zock and Kester 1 ). This was confirmed in a systematic review and regression analysis prepared for the WHO in 2016, which covered seventy-four randomised studies. At the same time, it has been demonstrated that replacement of SFA with cis-MUFA or cis-PUFA normalises the lipid profile more effectively than replacing them with a mixture of carbohydrates. The decrease in total cholesterol (TC), LDL-C and TAG concentrations was greatest when cis-PUFA were used( Reference Mensink 2 ). Regarding TFA, their adverse influence on lipid parameters is also well documented. Beginning in the 1990s, a number of studies have been published indicating that, compared with the same amount of energy from cis-unsaturated fatty acids or SFA, intake of TFA increases LDL-C level, decreases HDL-C level and increases TC:HDL-C( Reference Laine, Snodgrass and Dawson 3 – Reference Judd, Clevidence and Muesing 6 ). Compared with other fatty acids, the concentration of TAG and lipoproteins also increases( Reference Mensink, Zock and Kester 1 , Reference Ascherio, Katan and Zock 7 ). In a meta-analysis of numerous studies, Mozaffarian and Clarke concluded that intake of 1 % of energy from TFA in place of other fats increases TC:HDL-C by 0·022 if SFA are replaced; by 0·051 if MUFA are replaced; and by 0·057 if PUFA are replaced( Reference Mozaffarian and Clarke 8 ).
Many studies have demonstrated the effectiveness of replacing SFA and TFA with other macronutrients, especially unsaturated fatty acids, in improving the lipid profile( Reference Mensink, Zock and Kester 1 ). This formed a basis for developing population-based recommendations in which reduction of SFA and TFA intakes is one of the basic dietary targets aimed at decreasing CVD risk. The current recommendations for SFA intake, by the WHO as well as European and American scientific societies, suggest that in order to decrease the risk of myocardial infarction and stroke, SFA intake should be reduced to below 10 % of total dietary energy (5–6 % in persons who would benefit from decreasing LDL-C concentration), and in the case of TFA, their intake should be decreased to below 1 % of total dietary energy( Reference Lichtenstein 9 – Reference Eckel, Jakicic and Ard 12 ). Still, SFA remain a significant source of energy in developed countries, fluctuating around 12 % of total dietary energy( Reference Wright, Wnag and Kennedy-Stephenson 13 , Reference Bates, Lennox and Prentice 14 ). As for TFA, however, some countries have managed to achieve a marked reduction in their consumption.
Recently, doubts have also arisen about whether the current recommendations to reduce SFA and TFA intakes, in addition to benefits resulting from their impact on risk factors, translate into a notable effect on health in the form of CVD risk reduction. The results of studies published in 2017 pertaining to the effects of consumption of different types of fatty acids on the risk of CVD are being widely discussed and raise the question of whether a revision of the current recommendations is necessary. The present paper is a review of the most recent studies, reviews and meta-analyses on the effects of SFA and TFA intakes, as well as various models for replacing them in the diet, on CVD risk.
Literature searches (prospective cohort studies, systematic reviews and meta-analyses) were conducted in two databases, MEDLINE® (PubMed) and Scopus®. Searches spanned the period from January 2014 to August 2017. Results from these two searches were combined and filtered for human studies published in the English language.
SFA and models of their replacement in the diet
In recent years, studies have emerged on SFA and models of their replacement with regard to cardiovascular risk. Major meta-analyses, systematic reviews and results of large prospective cohort studies are shown in Table 1. They include studies that cast doubt on the effectiveness of the recommendations made to date focused on decreasing SFA intake.
Table 1 SFA and models of their replacement in regard to cardiovascular risk: meta-analyses, systematic reviews and prospective cohort studies published in the years 2014–2017
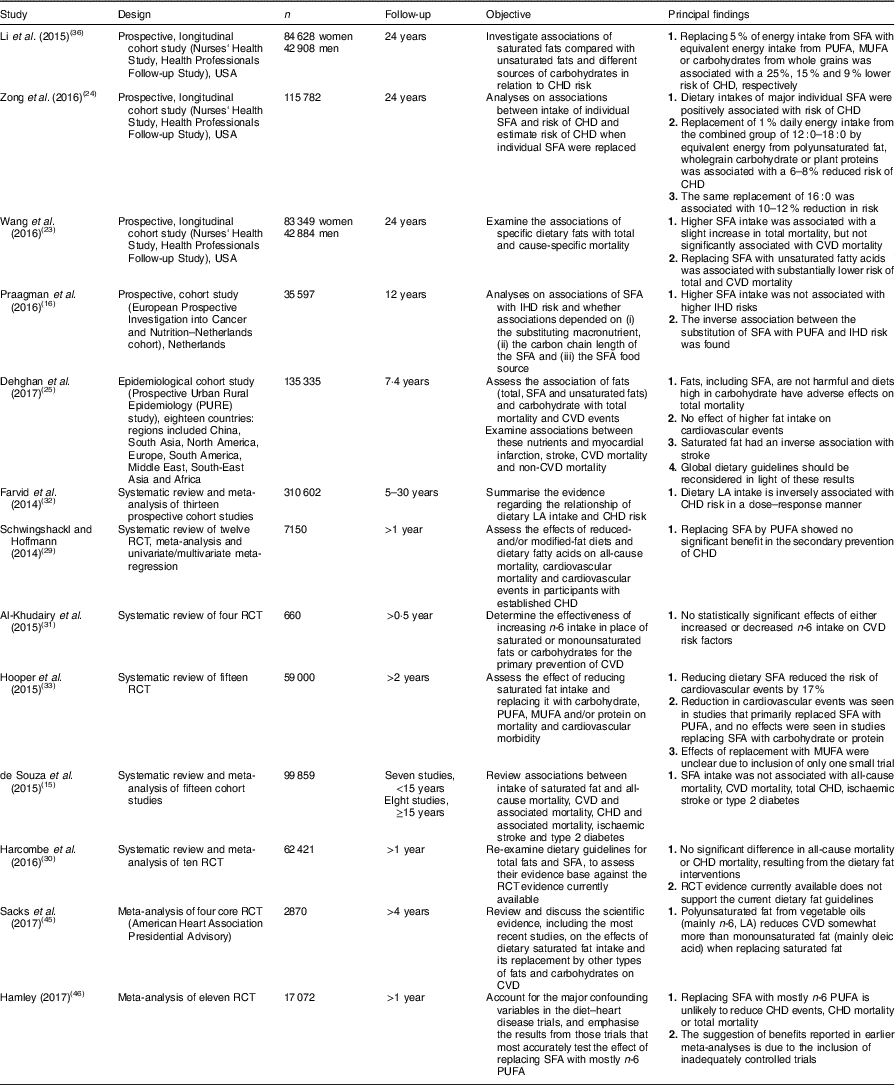
RCT, randomised controlled trial; LA, linoleic acid.
In 2015, de Souza et al. published a systematic analysis of studies which described the relationship between SFA, unsaturated fatty acids with the trans configuration and all-cause mortality, CHD mortality, ischaemic stroke and type 2 diabetes( Reference de Souza, Mentem and Maroleanu 15 ). The authors failed to find any clear relationship between high SFA intake and all-cause mortality, CHD mortality, IHD, ischaemic stroke or type 2 diabetes. At the same time, they demonstrated that intake of trans-unsaturated fatty acids was associated with a 34 % increase in all-cause mortality and a 28 % increase in CHD mortality as well as a 21 % increase in CHD risk( Reference de Souza, Mentem and Maroleanu 15 ). However, no significant relationship was observed between high trans-fat intake and ischaemic stroke or type 2 diabetes.
The EPIC-NL (European Prospective Investigation into Cancer and Nutrition–Netherlands) cohort study also revealed surprising results. A low risk of CHD was observed in persons with high SFA intake (the mean follow-up time was 12 years). It had been demonstrated that replacement of SFA with animal protein, cis-MUFA or even cis-PUFA or carbohydrates was associated with a significantly higher risk of IHD (hazard ratio per 5 % of energy=1·27–1·37)( Reference Praagman, Beulens and Alssema 16 ). However, the authors of the study noted the specific sources of SFA among the participants (mostly dairy products) as well as the distribution of individual fatty acids in the SFA pool, a large proportion of which comprised short- and medium-chain acids( Reference Praagman, Beulens and Alssema 16 ). This observation seems to confirm the results of the MESA (Multi-Ethnic Study of Atherosclerosis), in which high intake of SFA coming from dairy products was associated with a lower risk of IHD, while high intake of SFA coming from meat was associated with a higher CVD risk. The follow-up was carried out for 10 years( Reference de Oliveira Otto, Mozaffarian and Kromhout 17 ). The authors of both papers, however, pointed out that further studies are required in populations with a greater variety of SFA sources, as well as the need to assess the negative role of TFA in cases in which SFA are replaced with plant sources of fatty acids. The results of these studies seem to confirm meta-analyses on the effect of dairy product consumption on the risk of CVD. A meta-analysis by Alexander et al. covering thirty-one prospective cohort studies revealed a possible link between dairy product consumption and decreased risk of CVD( Reference Alexander, Bylsma and Vargas 18 ). Similar observations were demonstrated in an earlier meta-analysis by Qin et al.( Reference Qin, Xu and Han 19 ), which covered twenty-two prospective cohort studies. The authors observed that dairy product consumption has a negative correlation with the risk of CVD and stroke. They also concluded that consumption of dairy products with decreased fat content leads to decreased incidence of stroke, while consumption of cheese may reduce the incidence of both stroke and CHD( Reference Qin, Xu and Han 19 ). It was shown in a large multicentre study by Brassard et al. that SFA from cheese or butter do not have a significant effect on non-lipid cardiometabolic risk factors, such as inflammation markers, arterial blood pressure and homeostatic model assessment of insulin resistance, which can partly explain why observational studies have not shown a link between consumption of cheese and an increased risk of coronary artery disease( Reference Brassard, Tessier-Grenier and Allaire 20 ). Other meta-analyses indicated beneficial effects of dairy product consumption in terms of the risk of both type 2 diabetes( Reference Gijsbers, Ding and Malik 21 ) and obesity( Reference Abargouei, Janghorbani and Salehi-Marzijarani 22 ). It seems therefore that elimination of dairy products as the source of SFA may be detrimental to health.
Results of some large prospective studies have been published recently. In 2016, Wang et al. and Zong et al. published results from American prospective studies, the Nurses’ Health Study and the Health Professionals Follow-up Study, which indicated that larger intake of SFA contributes to a slight increase in total mortality (no link to CVD mortality) and risk of CHD( Reference Wang, Li, Chiuve and Stampfer 23 , Reference Zong, Li and Wanders 24 ). Completely different data were provided by the PURE (Prospective Urban Rural Epidemiology) study, whose findings were published in 2017. This was a large, prospective study which included a population of over 135 000 adults aged 35–70 years from eighteen countries of Asia, Europe, North and South America and the Middle East, lasting an average of 7·4 years. Consumption of total fat and other types of fatty acids, including SFA, was not linked to the risk of CVD, myocardial infarction or mortality caused by CVD. It was the first so-broad study to describe the relationship between low intake of SFA (e.g. <7 % of energy) and total mortality and CVD. A reverse correlation was observed between intake of SFA and the risk of stroke. The authors claimed that the available data do not justify the recommendation for reducing consumption of SFA to less than 10 % of total energy and that their very low intake (i.e. below about 7 % of energy) can even be harmful( Reference Dehghan, Mente and Zhang 25 ). However, it should be noted that the PURE study has numerous limitations such as use of the FFQ only at baseline, huge economic variation within the cohorts and limited data collection.
Studies on models of replacement of SFA in the diet with other macronutrients have also given inconsistent results. The paradigm of benefits stemming from the replacement of SFA with PUFA (i.e. linoleic acid (LA)) was called into question by the authors of a repeated analysis of the MCE (Minnesota Coronary Experiment) results( Reference Ramsden, Zamora and Majchrzak-Hong 26 ). Ramsden et al. confirmed the effectiveness of replacing SFA with LA in decreasing cholesterol concentrations: in the intervention group, a significant decrease in serum cholesterol concentrations was achieved compared with the control group (mean change from baseline=−13·8 % v. −1·0 %, P<0·001). However, the expected benefits to health associated with this fact in the intervention group were not achieved in terms of decreased incidence of coronary artery atherosclerosis or myocardial infarction. Importantly, the mean dietary intervention period was 1063 d, i.e. just under 3 years( Reference Ramsden, Zamora and Majchrzak-Hong 26 ). In 2013, the same authors published a similar article involving a repeated analysis of medical data of SDHS (Sydney Diet Heart Study) participants( Reference Ramsden, Zamora and Leelarthaepin 27 ). This revealed that patients receiving a diet of decreased SFA content and increased LA content (n 221) had a higher mortality rate than patients in the control group (n 237; all causes of death, 17·6 % v. 11·8 %; CVD, 17·2 % v. 11·0 %; CHD, 16·3 % v. 10·1 %). The follow-up was carried out for 12 months. The dietary intervention in the SDHS led to an increase in PUFA intake to as much as about 15 % of total dietary energy (mostly LA) as well as a decrease in SFA intake to less than 10 % of total dietary energy and a decrease of food cholesterol intake to below 300 mg daily( Reference Ramsden, Zamora and Leelarthaepin 27 ). On the other hand, the MCE study revealed an increase in LA to 13·2 % of total dietary energy and a decrease in SFA intake to 9·2 %( Reference Ramsden, Zamora and Majchrzak-Hong 26 ). Both studies involved decreasing the amount of SFA through reduction of their rich sources, such as butter, and replacing them with fats rich in LA in the form of corn oil or safflower oil, or margarines with a high content thereof( Reference Ramsden, Zamora and Majchrzak-Hong 26 , Reference Ramsden, Zamora and Leelarthaepin 27 ). Thus, the intervention focused on fat substitution while disregarding other important aspects of diet, for instance dietary fibre or antioxidants. There was also no information regarding the patients’ diet after completion of follow-up, so we do not know whether the changes introduced in the study were continued. Critics of this intervention also point to the fact that the provided plant fats had the form of hydrogenated oil or margarines rich in TFA( Reference Michas, Micha and Zampelas 28 ). Inclusion of new data from the SDHS in a meta-analysis of dietary interventions performed by the same research team, in which SFA were replaced with LA, did not reveal any benefits associated with all-cause mortality and mortality due to CVD resulting from such a dietary change( Reference Ramsden, Zamora and Leelarthaepin 27 ). These findings are consistent with those of the meta-analysis of thirteen randomised controlled trials conducted by Schwingshackl and Hoffmann, which showed that an increase in consumption of PUFA in place of SFA does not benefit secondary prevention of CHD( Reference Schwingshackl and Hoffmann 29 ). In their meta-analysis concerning the effectiveness of dietary interventions in prophylaxis, Harcomb et al. also did not find reduced intake of SFA to have a beneficial effect on total mortality or mortality due to CHD( Reference Harcombe, Baker and DiNicolantonio 30 ). The conclusions from a Cochrane systematic review performed by Al-Khudairy et al. on the potential of using n-6 acids in primary prevention also undermined any benefits associated with their use. The analysis following application of the inclusion criteria included only four European controlled clinical trials (the oldest one from 1998) involving 660 participants. More than 140 clinical trials were excluded from the analysis because of a lack of randomisation, too short duration or too small groups. The Cochrane systematic review failed to demonstrate any relationship between PUFA intake and decreased or increased CVD risk. According to the authors, there is a need for randomised studies assessing cardiovascular events as well as the presence of cardiovascular risk factors with larger study groups( Reference Al-Khudairy, Hartley and Clar 31 ).
Different results came from a meta-analysis by Farvid et al., which included thirteen published and unpublished cohort studies involving a total of 310 602 persons and 12 479 cardiovascular events, including 5882 CHD deaths. It was demonstrated that a 5 % increase in energy coming from LA, substituted for energy from SFA, was associated with a 9 % lower risk of cardiovascular events (relative risk=0·91; 95 % CI 0·87, 0·96) and a 13 % lower risk of death due to CHD (relative risk=0·87; 95 % CI 0·82, 0·94)( Reference Farvid, Ding and Pan 32 ).
Hooper et al. performed an analysis of fifteen randomised controlled trials with 59 000 participants. The analysis included only those studies carried out for more than 24 months. The results revealed a 17 % decrease in the risk of cardiovascular events resulting from reduced intake of SFA, which did not, however, translate into a decrease in all-cause mortality or mortality due to CVD. The authors said that better effects are achieved when SFA are replaced with PUFA. Significantly worse effects are achieved through replacement of SFA with carbohydrates, while the effect of using MUFA remains unclear, probably due to the low number of studies meeting the inclusion criteria( Reference Hooper, Martin and Abdelhamid 33 ).
The results of that analysis are in line with those of a previous meta-analysis by Jacobsen et al., in which it was concluded that replacement of SFA with n-6 PUFA decreases the risk of cardiovascular events by 13 % and of cardiovascular death by 26 %( Reference Jakobsen, O’Reilly and Heitmann 34 ). Regarding the possibility of using MUFA to replace SFA in the diet, most authors of meta-analyses point to the decidedly lower number of cohort studies and clinical studies as well as meta-analyses and systematic reviews than for those concerning n-6 PUFA, which means that no definite conclusions can be drawn( Reference Schwingshackl and Hoffmann 35 ). Despite this fact, when considering the effectiveness of SFA replacement, one must not forget the updated Nurses’ Health Study, covering 30 years of follow-up of 84 628 American women, and the Health Professionals Follow-up Study, covering 34 years of follow-up of 42 908 American men. In both studies, replacement of 5 % of energy from SFA with an equivalent amount of cis-MUFA led to a 15 % decrease in CHD risk (95 % CI 3, 26 %)( Reference Li, Hruby and Bernstein 36 ). Another study excluded from the analysis by Hooper et al. (due to lack of a clearly defined target regarding the overall level of fat intake), which utilised nutrition models with a high MUFA content, is the PREDIMED trial involving 7447 patients who were put on a Mediterranean diet and additional intake of 1 litre of olive oil per week or one portion of 30 g of nuts per day. Both study groups showed a good level of patient compliance and a significant reduction in the number cardiovascular events compared with the control group that used a standard low-fat diet. However, the proportion of individual fatty acid groups in the diet of the study groups was not specified( Reference Estruch, Ros and Salas-Salvadó 37 ).
In their analysis of studies on the possibility of using MUFA in primary and secondary CVD prevention which were published in the years 2013–2015, Joris and Mensink concluded that the results of the most recent studies confirm the previously suggested beneficial effects of these acids, comparable with those of LA and α-linoleic acid. At the same time, they indicated the necessity of further properly prepared clinical trials with cardiovascular events as end points( Reference Joris and Mensink 38 ).
What is remarkable about the aforementioned literature on the replacement of SFA with other macronutrients is the ambiguity of results pertaining to replacement of SFA with PUFA. There are several possible causes of this. In some studies, n-6 fatty acid intake exceeded 10 %, which – as shown in experimental studies – may be associated with pro-inflammatory activity. The authors of the SDHS pointed out a potential mechanism of increasing cardiovascular risk by LA, which is thought to contribute to increased production of bioactive oxidised LA metabolites (e.g. 9- and 13-hydroperoxyoctadecadienoic acid as well as 9- and 13-hydroxyoctadecadienoic acid), which play a role in atherosclerotic plaque formation( Reference Ramsden, Zamora and Leelarthaepin 27 ). The fact that participants in certain studies smoked tobacco and drank alcohol (a common occurrence in the case of interventions taking place in the 1960s and 1970s) may also be of importance, since this may enhance oxidative stress and oxidation processes, thereby increasing the cardiovascular risk( Reference Waddington, Croft and Sienuarine 39 , Reference Yokode, Ueyama and Arai 40 ). A factor of high importance may be the type of substitution used, especially whether TFA and SFA have been replaced with cis n-6 PUFA alone, a mixture of cis and trans n-6 PUFA (which may have been the case in studies conducted in the 1960s) or with a mixture of cis n-6 and n-3 PUFA.
A meta-analysis performed by Ramsden et al. demonstrated that mixtures of n-3/n-6 PUFA and a mixture of n-6 PUFA alone affect the risk of non-fatal myocardial infarction and death due to CHD in different ways. The authors claimed that substitution of a n-3/n-6 PUFA mixture for TFA and SFA causes a decreased CHD risk, while n-6 PUFA substitution demonstrates a tendency towards increased risk coronary artery disease( Reference Ramsden, Hibbeln and Majchrzak 41 ). However, due to a small number of studies included in this analysis, especially those concerning the use of a n-3/n-6 mixture, and inclusion of results from the SDHS and the MCE in evaluation of the efficacy of a mixture of n-6, these findings should be treated with caution. Some doubts have been raised concerning the clinical usefulness of the proportion between n-3 and n-6 fatty acids consumed. Some authors claim that this ratio serves no purpose and is confusing, and since it is believed that both PUFA types have beneficial effects, it should be omitted in modern recommendations( Reference Willett 42 ). However, Hammad et al. indicated that the results of studies in which SFA and TFA are replaced with increased intake of n-6 and n-3 PUFA should not be interpreted as demonstrating the effect of n-6 PUFA, since their outcome was affected both by the presence of n-3 fatty acids and the decreased proportion of TFA. They believe that preventive measures should be aimed at the elimination of TFA from the diet, reducing SFA and n-6 PUFA to less than 7 % and 10 % of energy, respectively, and achievement of a ratio of n-6 to n-3 fatty acids which is as close as possible to 1:1 with a sufficient amount of essential unsaturated fatty acids( Reference Hammad, Pu and Jones 43 ). This seems important in the light of a study by Ninomiya et al., which demonstrated that a lower ratio of EPA to arachidonic acid in serum is linked to a higher risk of CVD, especially CHD, in people with a higher level of high-sensitivity C-reactive protein in the general population of Japan( Reference Ninomiya, Nagata and Hata 44 ).
Controversies concerning the findings and methodology of the meta-analyses of studies on an effect of substitution of SFA with PUFA have been widely discussed in papers published in 2017: the American Heart Association Presidential Advisory and Hamley’s meta-analysis( Reference Sacks, Lichtenstein and Wu 45 , Reference Hamley 46 ). The authors of the American Heart Association position statement claimed that studies whose methodology is questioned because of their short duration, small study groups and use of margarines potentially containing TFA should be excluded from analyses of an effect on CHD risk (e.g. MCE, SDHS). After taking these factors into account, only four studies were included in the final meta-analysis; this was reflected in the conclusion, which confirmed that polyunsaturated fat from vegetable oils reduces risk of CVD a little more than monounsaturated fats in the substitution of saturated fats( Reference Sacks, Lichtenstein and Wu 45 ). On the other hand, Hamley adopted as the main exclusion criterion the absence of a simultaneously decreasing intake of SFA and increasing intake of PUFA by at least 20 % in the intervention group compared with the control group. Additionally, the author categorised the studies into properly controlled and improperly controlled, i.e. those with too many dietary and/or non-dietary differences between groups to regard a test of substituting SFA by n-6 PUFA as valid. In effect, following application of such inclusion and exclusion criteria, only one study included in the American Heart Association meta-analysis was among the five studies included in the group of properly controlled ones in Hamley’s meta-analysis. Due to debate over high TFA intake in the SDHS experimental group, this trial was also excluded in a sensitivity analysis of the adequately controlled trials. In consequence, completely different results were obtained, indicating that substituting SFA with PUFA does not reduce the risk of CHD, death due to CHD or total mortality( Reference Hamley 46 ).
Trans-fatty acids and their impact on CVD risk
Unsaturated TFA isomers in the diet originate from two sources: as natural ingredients of products coming from ruminants (beef, lamb and dairy products) and as industrial products of the process of vegetable oil hydrogenation, during which 30–50 % of double bonds change their configuration from cis to trans ( Reference Baum, Kris-Etherton and Willett 47 ). TFA from both sources contain the same isomers, but in different proportions. Elaidic acid isomers (C18:1D10t and D9t) are found in larger quantities in industrially produced fat, while vaccenic acid (C18:1D11t) is usually the main component of the TFA pool coming from ruminants( Reference Hulshof, van Erp-Baart and Anttolainen 48 ). In a systematic review for the WHO, Bouwer pointed out that replacement of the total amount of TFA (the sum of industrial TFA (iTFA) and ruminant TFA) with cis-MUFA, cis-PUFA and carbohydrates leads to increased HDL-C concentrations and decreased concentrations of TC and LDL-C, as well as to a decrease in the ratios TC:LDL-C and TC:HDL-C. At the same time, the strongest effects are observed when TFA are replaced with cis-PUFA. Only substitution of cis-MUFA and cis-PUFA demonstrated a significant decrease in TAG concentrations, which was not observed in the case of carbohydrates( Reference Brouwer 49 ).
In their meta-analysis of twenty-eight cohort studies, Skeaff and Miller demonstrated a strong link between TFA intake and CHD incidence and mortality( Reference Skeaff and Miller 50 ). This was confirmed in a study by Mozzafarian et al., which made it possible to determine the effect of TFA intake level on CVD: a 2 % increase in TFA intake is associated with a 23 % increase in the number of cardiovascular events( Reference Mozaffarian, Katan and Ascherio 51 ). According to an assessment by the US Centers for Disease Control and Prevention, elimination of total TFA intake in the USA would decrease the number of coronary events by 20 000 each year and the number of cardiac deaths by 7000( Reference Brownell and Pomeranz 52 ).
The beneficial effect of TFA intake reduction on the risk of CVD may also be explained by studies on LDL-P (LDL particle number), a new risk factor with a high potential for use in prevention. People with higher LDL-P levels may have a two- to threefold higher risk of CVD, irrespective of LDL fraction concentrations( Reference Ip, Lichtenstein and Chung 53 ). A study by Garshick et al. demonstrated that a decrease in TFA intake of nearly 1 % over the course of a year resulted in significant decrease in LDL-P, irrespective of other factors and covariates. This suggests that a decrease in LDL-P may be one of the mechanisms by which a decrease in dietary TFA content lowers cardiovascular risk( Reference Garshick, Mochari-Greenberger and Mosca 54 ).
Certain studies seem to prove that the assessment of the role of TFA in the development of CVD should take account of their source of origin as well as specific fatty acids from the TFA pool. As early as in 2008, the TRANSFACT (Trans Fatty Acids Collaboration) study revealed that TFA coming from natural products and industrially hardened oils have different effects on CVD risk factors, such as LDL-C and HDL-C concentrations as well as apoA and apoB1. The low number of subjects in this study and the visible difference in results depending on participant sex did not, however, make it possible to draw conclusions for the entire population( Reference Chardigny, Destaillats and Malpuech-Brugère 55 ). A systematic review and meta-analysis of observational studies performed by de Souza et al. demonstrated that, overall, the intake of TFA was associated with increased all-cause mortality, CHD mortality as well as with development of CHD. No relationship, however, was demonstrated with ischaemic stroke and type 2 diabetes. An analysis of the sources of TFA confirmed that intake of industrially produced isomers increases CHD mortality, but it failed to confirm such effects for TFA coming from ruminants. Interestingly, intake of trans-palmitoleic acid was inversely related to the incidence of type 2 diabetes (relative risk=0·58, 95% CI 0·46, 0·74)( Reference de Souza, Mentem and Maroleanu 15 ). In the Cardiovascular Health Study covering 2742 adult patients aged over 65 years, concentrations of t/t-18:2 were most adversely related to all-cause mortality, mainly due to increased CVD risk. Concentrations of t/c-18:2 were also positively related to all-cause mortality and CHD, but only after accounting for the effects of other TFA( Reference Wang, Imamura and Lemaitre 56 ).
The Ludwigshafen Risk and Cardiovascular Health Study demonstrated that overall TFA content in erythrocyte cell membranes in the studied population was associated with a lower risk of cardiovascular risk in this population( Reference Kleber, Delgado and Lorkowski 57 ). However, the study population was composed of people qualified for coronary angiography in Germany, a country in which the overall intake of TFA, especially iTFA, is relatively low, and the majority of TFA consumed comprise those from dairy products( Reference Hulshof, van Erp-Baart and Anttolainen 48 ). This study also led to a conclusion that high TFA concentrations in erythrocyte cell membranes are correlated with a favourable metabolic profile characterised by lower TAG concentrations, lower blood pressure and lower fasting glucose concentrations( Reference Kleber, Delgado and Lorkowski 57 ). In contrast to these findings, NHANES (National Health and Nutrition Examination Survey) data showed plasma elaidic acid levels to be associated with higher risks of all-cause and CVD-related mortality( Reference Li, Zhang and Song 58 ).
Liska et al. noted that drawing any conclusions regarding the effect of TFA intake on cholesterol concentrations is very difficult. The authors noted that many studies fail to describe the methodology of iTFA production and hence may be misleading in their description of food products used, for example margarines. Another problematic task is determination of TFA intake, which is described by some authors in grams per day and by some as a percentage of total dietary energy or the proportion of total fat. It is also difficult to assess the isolated effect of increasing iTFA intake on lipid parameters, since in most studies this is associated with reduced intake of cis-PUFA and/or cis-MUFA. There is also an insufficient number of studies assessing the impact of low intake of iTFA, especially those coming from partially hydrogenated oils, on CHD risk( Reference Liska, Cook and Wang 59 ). In the majority of available studies, the level of iTFA intake <1 % (which corresponds to its present average intake in the USA) is classified as the control group for study groups consuming 1–2 % of energy as iTFA or more than 2 % of energy as iTFA( Reference Liska, Cook and Wang 59 ). Therefore, further studies are necessary, especially as the literature provides greater proof of varied atherogenic effect of TFA exerted through various mechanisms, such as increasing the inflammatory condition or oxidative stress( Reference Monguchi, Hara and Hasokawa 60 ). Moreover, a study conducted by Nakamoto et al. indicated that increased intake of TFA, reflected in an elevated level of elaidic acid in plasma, can increase instability of atherosclerotic plaque in vivo ( Reference Nagasawa, Shinke and Toh 61 ).
Summary
Conclusions arising from studies published in 2014–2017 concerning replacement of SFA and TFA in the diet as an element of CVD prevention require a high level of caution in interpretation. The observed inconsistencies, especially in clinical trials of SFA substitution and their meta-analyses, may stem from different methodologies of dietary parameter changes (supplementation, replacement of selected food products with other products, change of the entire nutrition model), varying duration of studies, as well as the time at which they were carried out. One should take account of the fact that the risk of CVD is affected not only by fatty acids, but also by a number of other dietary and non-dietary elements of lifestyle. Therefore, these might have also influenced the results of individual studies, especially when the controlled replacement pertained only to fat. This also points to the differences in follow-up duration and timing of observation of the pre-specified end points. The results also seem to be affected by the population in which the dietary intervention was performed, as well as the baseline nutrition model, which – especially in the 1960s and 1970s – was different from now. It is extremely difficult to extrapolate such studies to contemporary recommendations. It seems that there is a need for properly randomised studies on large groups, with good control of dietary and non-dietary parameters, which account for not only the sum of SFA and TFA, but also their source: dairy products and meat for SFA, ruminant-derived and industrial products for TFA. Only such studies will allow for full evaluation of an effect of substituting SFA and TFA on cardiovascular risk.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: M.M.-W. contributed to conception and design, drafted the manuscript, critically revised the manuscript and gave final approval. A.D. contributed to conception and interpretation, critically revised the manuscript and gave final approval. M.K.W. contributed to design, critically revised the manuscript and gave final approval. Ethics of human subject participation: Not applicable.



