Dietary Na is an important micronutrient required for a variety of essential physiological functions within the human body. However, there is convincing evidence that high Na excretion (as a consequence of high Na intake) plays a role in endothelial dysfunction, ventricular hypertrophy, and has deleterious effects on blood pressure (BP), resulting in an increased hypertension risk( Reference Rust and Ekmekcioglu 1 – Reference Mancia, Oparil and Whelton 4 ).
Hypertension, a multifactorial disease associated with modifiable and non-modifiable risk factors( Reference Mamudu, Paul and Wang 5 , Reference Koch, Romero and Romero 6 ), is the most important risk factor for premature mortality worldwide and a major risk factor for CVD and kidney disease( Reference Rust and Ekmekcioglu 1 , Reference Ohta, Kimura and Kitaoka 3 , Reference Zhang, Mahapatra and Huang 7 – 10 ). More than one in five adults worldwide have high BP, and it has been estimated that each year 9·4 million deaths worldwide are caused by complications related to hypertension( 11 ). Lifestyle changes such as reducing dietary Na intake are key to preventing and reducing the prevalence of hypertension( 10 ). The WHO currently recommends an intake of less than 5 g salt/d (equivalent to <2 g Na/d) in the normotensive population and an intake of less than 1·5 g Na/d in individuals with hypertension( Reference Chobanian, Bakris and Black 8 , 11 ). However, the average consumption is 4·0 g Na/d (equivalent to 10 g salt/d) worldwide( 10 ). Na intake, therefore, is a key behaviour that can be modified to reduce the risk of high BP( Reference He, Li and Macgregor 12 ).
Over the last three decades, Latin American countries, including Chile, have experienced a rapid lifestyle transition owing to their populations adopting a more Westernised lifestyle, including an increasingly unhealthy diet and reductions in physical activity (PA)( Reference Celis-Morales, Salas and Alduhishy 13 – Reference Labraña, Durán and Martínez 16 ). In fact, Chile has the highest prevalence of obesity in Latin America. Seven out of ten adults are overweight or obese( 17 ) and consequently at high risk of hypertension( Reference Labraña, Durán and Martínez 16 ). It is perhaps not surprising, therefore, that in the last Chilean National Health Survey 2016–2017, 27·6 % of the population were found to have hypertension (2·6 % more than in 2010)( 18 ), with an average Na excretion of 3·7 g/d (equivalent to 9·4 g salt/d)( 18 ). As a result, Chile has the third highest prevalence of hypertension in Latin America( Reference Miranda, Herrera and Chirinos 19 ).
Given the rapid lifestyle transition and current levels of hypertension in Chile, identifying the main factors associated with higher Na excretion could help to develop and tailor more effective public health policies. The aim of the present study, therefore, was to determine the main factors (sociodemographic, anthropometric, lifestyle and health status) associated with high Na excretion in a representative population of Chile.
Methods
Study design
The present study was an observational, cross-sectional analysis assessing the association of Na excretion with hypertension in Chilean adults. The present paper is reported in adherence to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines( Reference von Elm, Altman and Egger 20 ).
Study population
The present study was based on participants aged ≥15 years from the 2009–2010 Chilean National Health Survey (CNHS 2009–2010). The CNHS 2009–2010 is a large, nationally representative population-based study of biological and lifestyle risk factors, dietary status and health, conducted every six years in Chile in both urban and rural zones. One participant was randomly selected per household, with pregnant individuals and individuals with violent behaviour excluded, as described elsewhere( 17 ). Data were collected by trained staff in two visits where individuals were administered questionnaires and anthropometric and physiological measures, as well as biological samples, were obtained. The response rate from the eligible population to the CNHS 2009–2010 was 54 % (n 5412). Of these, only 2913 participants had Na excretion measured and were therefore included in the present study.
Anthropometric and metabolic measurements
Weight was measured by a digital scale (Tanita HD-313®) and height with a height rod in their home, with participants not wearing shoes and in light clothing, through standardized methods and by trained nurses or midwives, as described elsewhere( 17 ). BMI was calculated as weight/height2 and classified using the WHO criteria (normal weight: 18·5–24·9 kg/m2; overweight: 25·0–29·9 kg/m2; obese: ≥30·0 kg/m2)( 21 ). Underweight individuals were not included as only forty-six participants were classified with a BMI < 18·5 kg/m2. Central obesity was defined as waist circumference >88 cm for women and >102 cm for men( 17 ) and was measured at the mid-axillary line at the midpoint between the costal margin and the iliac crest by an ergonomic circumference-measuring tape.
BP was measured using the Omron HEM 742® BP monitor and cuff by trained staff and derived from the mean of three readings recorded after 15 min of rest. Mean arterial pressure was derived as: [(diastolic BP) × 2 + systolic BP]/3. Hypertension was defined as systolic BP ≥ 140 mmHg (SBP) and diastolic BP ≥ 90 mmHg (DBP) or currently receiving treatment for hypertension( Reference Chobanian, Bakris and Black 22 ). These categories were established according to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High BP( 23 ). Medication for lowering BP was collected using self-reported questionnaires (694 participants were on medication). Type 2 diabetes was defined as fasting glucose ≥ 7·0 mmol/l or current treatment for diabetes( 24 ). The presence of metabolic syndrome was defined using the National Cholesterol Education Program Adult Treatment Panel III criteria( Reference Alberti, Eckel and Grundy 25 ): waist circumference >102 for men and >88 cm for women; serum TAG > 1·7 mmol/l; HDL-cholesterol < 1·03 mmol/l; SBP ≥ 130 mmHg or DBP ≥ 85 mmHg; and fasting glucose > 5·6 mmol/l or current treatment for diabetes. Each metabolic syndrome component was classified as either present or absent as per the above criteria. The number of metabolic syndrome components present for each participant was calculated to provide an ordinal measure of cardiometabolic health. The presence of three or more components was used to indicate the presence of metabolic syndrome.
Sociodemographic characteristics
Sociodemographic data were collected for all participants, including age, sex, education level (primary, <8 years; secondary, <12 years; beyond secondary, >12 years), monthly household income (low, ≤$US 480; middle, $US 481–865; high, ≥$US 865) and smoking status (non-smoker, ex-smoker, smoker), using nationally validated questionnaires. Dietary intake of fruit and vegetables was reported using an FFQ which was administered once. Participants were asked, ‘In a typical/ordinary week, how many days do you eat fruit?’ and ‘In a typical/ordinary week, how many days do you eat vegetables?’, which was then converted into grams( 17 ). Alcohol consumption was self-reported and collected using the Alcohol Use Disorders Identification Test (AUDIT) questionnaire developed by WHO( Reference Saunders, Aasland and Babor 26 ) and adapted for use in Chile( Reference Alvarado, Garmendia and Acuña 27 ).
Sodium excretion
Daily Na excretion was determined using a urine sample collected by trained nurses using a standardised protocol. The indirect ion-selective method was utilised to measure Na electrolyte in the urine (indirect potentiometry). Although measurement of urinary Na excretion over 24 h is the gold standard for assessing Na excretion in population surveys, it is time-consuming and has a high burden for participants and is therefore not always possible. In this context, to calculate 24 h urinary Na excretion (24HUNa), conversion formulas by Tanaka et al. were used. Tanaka’s formulas require the measurement of Na and creatinine in a spot urine sample as well as the age in years, body weight in kilograms and height in centimetres for each individual. This method is simple and has been previously validated( Reference Tanaka, Okamura and Miura 28 ). The formulas are as follows:
 $$\openup-7pt \eqalignno{& {\rm \hskip -22.5pt PRCr }\,\left( {{\rm mg/d}} \right){\equals}\cr \,& \,{\minus}{\rm 2\!\cdot\!04}{\times}{\rm age }\;\left( {{\rm years}} ) \hskip 0pt\right \,{\plus}{\rm 14\!\cdot\!89}{\times}{\rm body \;weight \;}\left( {{\rm kg}} \right) \cr \,&\,{\plus}{\rm 16\!\cdot\!14}{\times}{\rm height \;}\left( {{\rm cm}} \right){\minus}{\rm 2244\!\cdot\!45,\hskip 0pt} $$
$$\openup-7pt \eqalignno{& {\rm \hskip -22.5pt PRCr }\,\left( {{\rm mg/d}} \right){\equals}\cr \,& \,{\minus}{\rm 2\!\cdot\!04}{\times}{\rm age }\;\left( {{\rm years}} ) \hskip 0pt\right \,{\plus}{\rm 14\!\cdot\!89}{\times}{\rm body \;weight \;}\left( {{\rm kg}} \right) \cr \,&\,{\plus}{\rm 16\!\cdot\!14}{\times}{\rm height \;}\left( {{\rm cm}} \right){\minus}{\rm 2244\!\cdot\!45,\hskip 0pt} $$
where PRCr is predicted value of 24 h urinary creatinine, SUNa is Na concentration in the spot voiding urine and SUCr is creatinine concentration in the spot voiding urine.
Physical activity and sedentary behaviour
PA levels, including moderate and vigorous intensities and transport-related PA, were determined using the Global Physical Activity Questionnaire version 2 (QPAQ v2)( 29 ). PA was categorised into: inactive individuals (<600 MET-min/week) and active individuals (≥600 MET-min/week)( 30 ), where MET is metabolic equivalent of task. Sedentary behaviour was derived using the following question: ‘How much time do you usually spend sitting or reclining on a typical day?’( 30 ).
Statistical analyses
Statistical analyses were performed using survey-weighted values and the statistical software package Stata version 14. Descriptive characteristics are presented as adjusted means with 95 % CI for quantitative variables or as a proportion for categorical variables. High Na excretion was defined as an excretion ≥3·6 g/d, which corresponds to the population median. To determine the main sociodemographic, health and lifestyle correlates of Na excretion, we used logistic regression analysis where Na excretion was the outcome coded as a binary variable (0=low, 1=high). The exposures were fitted into the model as categorical variables. For continuous variables, we derived tertiles to investigate the odds of being in the high Na excretion group by low, middle or high level of the exposure variable of interest (low=0, middle=1, high =2).
Associations between Na excretion and BP (mean arterial pressure, SBP and DBP) were investigated using linear regression analysis. Individuals taking BP medication (n 694) were excluded from this analysis. The association between Na excretion and hypertension (no=0, yes=1) was investigated using multivariate logistic regression analyses for the linear association and splines logistic regression with four equally distributed knots (at the 20th, 40th, 60th and 80th percentiles) for non-linear associations.
Results are presented as means and 95 % CI, and as OR with their respective 95 % CI, for BP and hypertension outcomes, respectively. Analyses were performed using incremental models. Model 0 was unadjusted; Model 1 was minimally adjusted (age, sex, area of residence (rural, urban), city of residence, education level and BP treatment); and Model 2 was fully adjusted (age, sex, area of residence, city of residence, education level, BP treatment, smoking, sedentary behaviour, total PA and BMI category). A P value of <0·05 was considered significant in all analyses.
Results
The main characteristics of participants by Na excretion category (<3·6 g/d and ≥3·6 g/d) are summarised in Table 1. Individuals who had Na excretion ≥3·6 g/d (equivalent to >9 g salt/d) were older (47·4 years), more likely to be male, and had lower levels of education and household income. Furthermore, those with high Na excretion were also more likely to be obese and have more metabolic complications such as diabetes and metabolic syndrome (34·0, 11·9 and 39·3 %, respectively; Table 1). The characteristics of participants without data available for Na excretion are presented in the online supplementary material, Table S1. However, no major differences were observed between those with and without data available for Na excretion.
Table 1 Characteristics, by urinary sodium excretion, of Chileans aged ≥15 years (n 2913) from the Chilean National Health Survey 2009–2010

WC, waist circumference; PA, physical activity; MET, metabolic equivalent of task; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Data presented as mean and sd for continuous variables, or as n and % for categorical variables. Na excretion was determined through a urine sample and then transformed into a 24 h Na excretion value using formulas by Tanaka et al.( Reference Tanaka, Okamura and Miura 28 ). Na excretion <3·6 g/d was used as a reference group.
There was a significant association between Na excretion and systolic, diastolic and mean arterial BP as is shown in the online supplementary material, Table S2 and Fig. 1. Per 0·4 g increase in Na excretion, SBP increased by 2·06 mmHg (95 % CI 1·7, 2·4 mmHg, P<0·0001; Fig. 1(a)), DBP increased by 0·85 mmHg (95 % CI 0·7, 1·0 mmHg, P<0·0001; Fig. 1(b)) and mean arterial pressure increased by 3·76 mmHg (95 % CI 3·1, 4·4 mmHg, P<0·0001; Fig. 1(c)). These associations were independent of major confounding factors as demonstrated in Table S2.
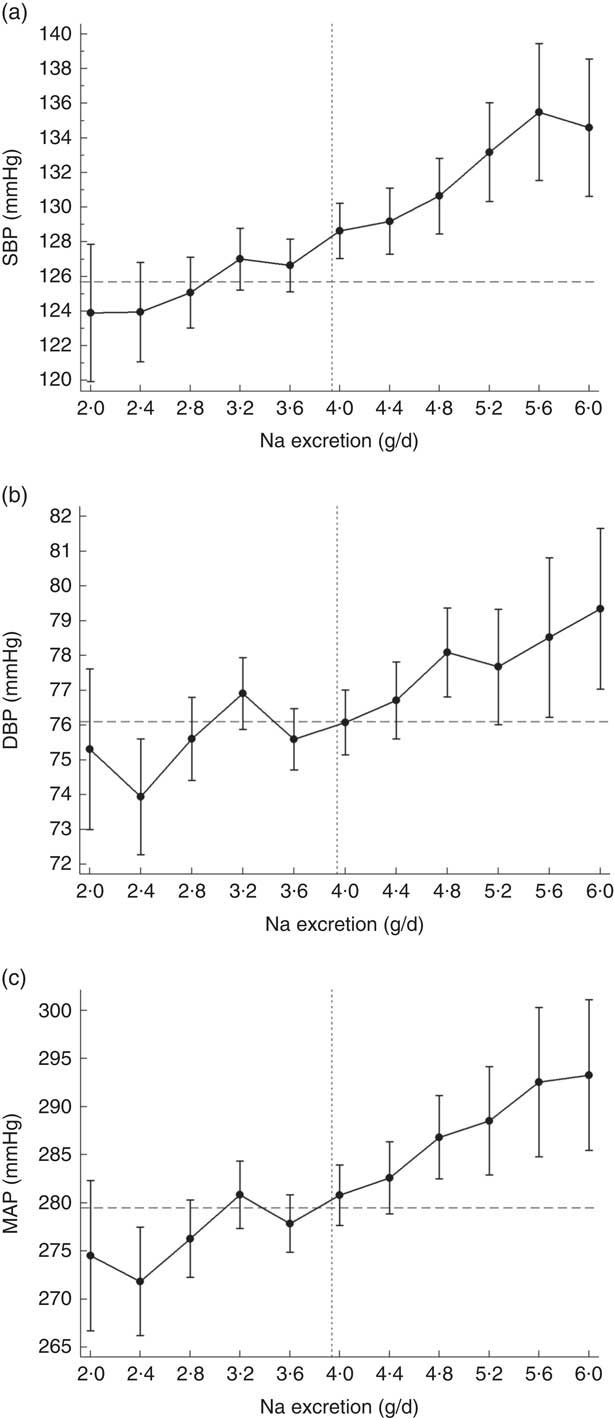
Fig. 1 Asociation of sodium excretion with (a) systolic blood pressure (SBP), (b) diastolic blood pressure (DBP) and (c) mean arterial blood pressure (MAP) in Chileans aged ≥15 years (n 2913) from the Chilean National Health Survey 2009–2010. Data are presented as means with their 95 % CI indicated by vertical bars; ![]() indicates the population average blood pressure,
indicates the population average blood pressure, ![]() indicates the population average Na excretion. Analyses were adjusted age, sex, area of residence (rural, urban), city of residence, education level, smoking, sedentary behaviour, total physical activity and BMI category. Participants who were on blood pressure-lowering medication were removed from this analysis (n 694)
indicates the population average Na excretion. Analyses were adjusted age, sex, area of residence (rural, urban), city of residence, education level, smoking, sedentary behaviour, total physical activity and BMI category. Participants who were on blood pressure-lowering medication were removed from this analysis (n 694)
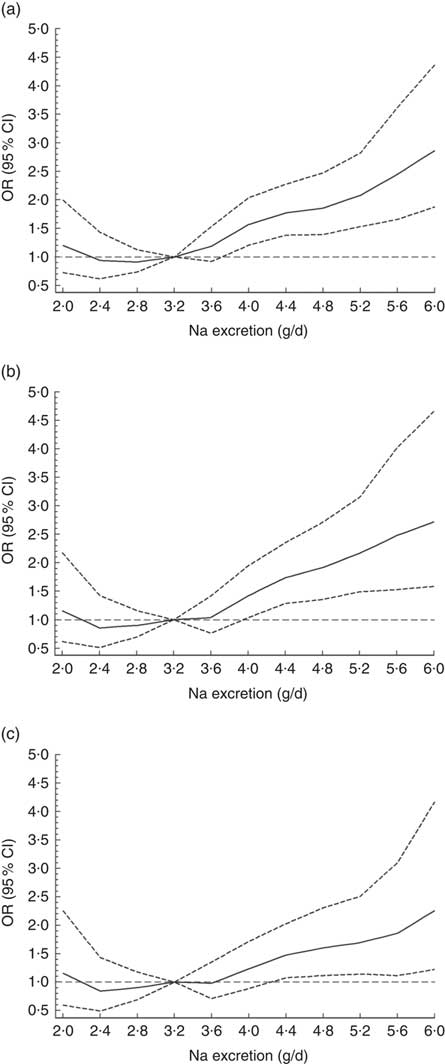
The odds for hypertension increased by 10·2 % per each 0·4 g increment of Na excretion (OR=1·10; 95 % CI 1·06, 1·14; P<0·0001). When a non-linear association was investigated, using splines regression with four knots equally distributed (20th, 40th, 60th and 80th percentiles), the results showed that the biggest increase in the odds for hypertension was observed when Na excretion was above 8 g/d (OR=1·21; 95 % CI 1·01, 1·45; P<0·0001; Fig. 2 and online supplementary material, Table S3). These findings were independent of age, sex, education level, BP-lowering medication, smoking, sedentary behaviour, total PA and BMI category (Fig. 2 and Table S3).
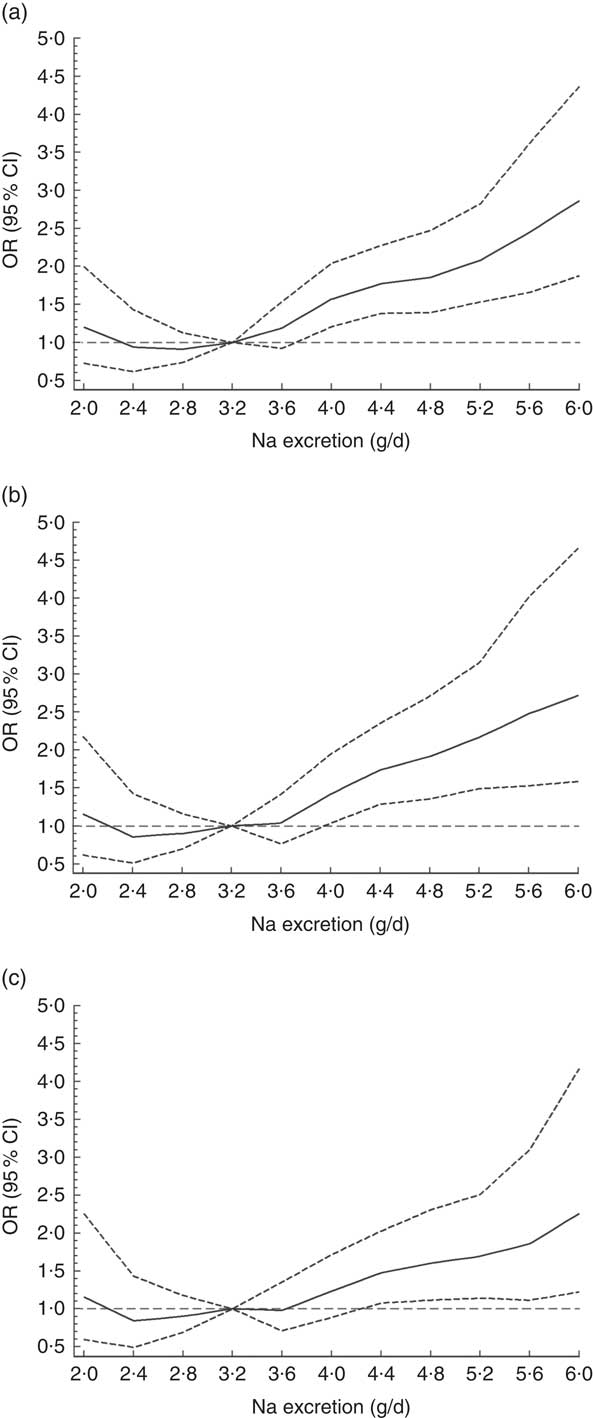
Fig. 2 Association between sodium excretion and hypertension in Chileans aged ≥15 years (n 2913) from the Chilean National Health Survey 2009–2010. Data are presented as OR (———) and their 95 % CI (![]() );
); ![]() denotes OR = 1. (a) Model 0, unadjusted; (b) Model 1, adjusted for age, sex, area of residence (rural, urban), city of residence, education level and blood pressure treatment; (c) Model 2, adjusted for Model 1 plus smoking, sedentary behaviour, total physical activity and BMI category
denotes OR = 1. (a) Model 0, unadjusted; (b) Model 1, adjusted for age, sex, area of residence (rural, urban), city of residence, education level and blood pressure treatment; (c) Model 2, adjusted for Model 1 plus smoking, sedentary behaviour, total physical activity and BMI category
On the other hand, correlate analysis of high Na excretion (≥3·6 g/d) showed that individuals aged over 25 years, those who were overweight, obese or centrally obese, individuals who reported to be taking BP-lowering medication and individuals with hypertension, diabetes and metabolic syndrome were more likely to have a high Na excretion (Fig. 3). Obesity and central obesity were the strongest factors associated with high Na excretion (OR=2·50; 95 % CI 2·01, 3·11; P<0·0001 and OR=2·26; 95 % CI 1·88, 2·72; P<0·0001, respectively). By contrast, individuals living in an urban setting, females, those with >12 years of education, those in middle- or high-income groups and those who spent more time sitting were less likely to be in the higher Na excretion category (Fig. 3).
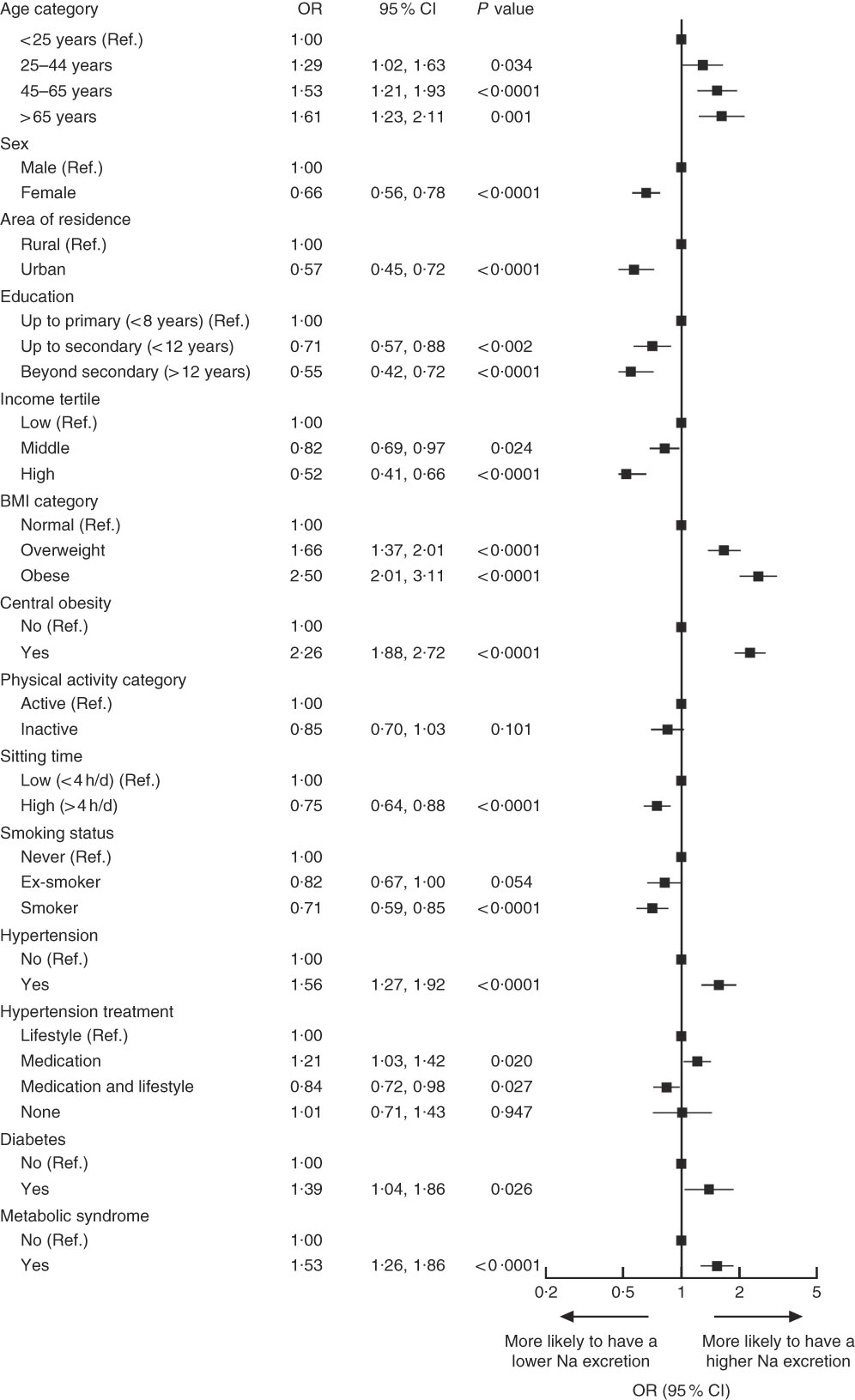
Fig. 3 Correlates of high sodium excretion in Chileans aged ≥15 years (n 2913) from the Chilean National Health Survey 2009–2010. Data are presented as OR (■) with their 95 % CI represented by horizontal bars. Low sodium excretion (<3·6 g/d) was used as a reference category (Ref.), therefore OR above 1 indicates that individuals were more likely to have higher sodium intake. Analyses were adjusted for age, sex, area of residence (rural, urban), city of residence, education level, smoking, sedentary behaviour, total physical activity and BMI category, except when these variables were used as main exposures
Discussion
The main findings of the present study are that males, individuals who are older, individuals who are overweight or obese, and those who have been diagnosed with hypertension, metabolic syndrome or diabetes are more likely to have higher Na excretion (≥3·6 g/d) within the Chilean population. Conversely, females, individuals with high levels of education or income, those living in urban cities, those who are more physically active and also people who reported spending more time sitting were less likely to be in the high excretion group. A possible explanation for these findings may be related to this population being more aware of, and having increased access to, health information( Reference Zhao, Zhang and Zhang 31 ). Additionally, the odds of having hypertension increased by 10·2 % per each 0·4 g increment in Na intake. These findings could be relevant for informing national public health policy makers of the need for strategies to decrease the current levels of Na consumption in the population, which are currently reported as 3·7 g/d( 18 ). WHO is currently providing support to governments in a bid to implement policies aimed at reducing the global Na intake, with a target of a 30 % reduction by 2025( 10 ). However, if Na intake continues to increase in Chile, the country could be at an increased risk of poor health and face a greater economic burden from the many chronic health conditions associated with high Na intake. In fact, Na intake was shown to be the primary factor accounting for the rising hypertension prevalence in Chile, which has increased from 26·9 % in 2010 to 27·6 % in 2017( 17 , 18 ).
Despite the present study being the first to investigate correlates of higher Na excretion in the Chilean population, research conducted in different middle- and high-income countries (China, Denmark, France, Slovenia, Japan and the USA) has shown similar associations( Reference Drewnowski, Henderson and Driscoll 32 , Reference Liu 33 ). Indeed, previous studies have shown that those with higher Na intakes were more likely to be older individuals( Reference Drewnowski, Henderson and Driscoll 32 , Reference Liu 33 ), male( Reference Meneton, Lafay and Tard 34 ), have a diagnosis of metabolic syndrome( Reference Soleimani 35 ) and hypertension( Reference Soleimani 35 , Reference Ribic, Zakotnik and Vertnik 36 ), and a high BMI( Reference Ribic, Zakotnik and Vertnik 36 – Reference Takahashi, Tanabe and Adachi 38 ).
In the last two decades, the effects of dietary Na on human BP, predominantly through Na intake, have been investigated in several epidemiological and interventional studies( Reference Takase, Sugiura and Kimura 39 , Reference Johnson, Mohan and Rogers 40 ). In Japan, a 2-year follow-up study of 6103 individuals showed that the risk of developing hypertension was higher in those with higher Na intake (hazard ratio=1·25; 95 % CI 1·04, 1·50)( Reference Takase, Sugiura and Kimura 39 ). Mozaffarian et al., as part of the Global Burden of Diseases Nutrition and Chronic Diseases Expert Group (NUTRICODE), conducted a meta-analysis on Na consumption worldwide and calculated the dose–response relationship between Na and BP( Reference Mozaffarian, Fahimi and Singh 41 ). The data showed a linear dose–response relationship between reduced Na intake and SBP, where a decrease of 2·3 g Na/d was associated with a reduction in BP of 3·82 mmHg( Reference Mozaffarian, Fahimi and Singh 41 ). Similarly, a meta-analysis of thirty-six studies showed that a reduction of Na intake significantly reduced SBP by 3·39 mmHg and DBP by 1·54 mmHg( Reference Aburto, Ziolkovska and Hooper 42 ). Mente et al. showed that the exclusion of individuals on medication did not substantially alter the magnitude of the association of 2·24 mmHg increment in SBP per 0·4 g of Na( Reference Mente, O’Donnell and Rangarajan 43 ). Our findings are consistent with these results where a 0·4 g increment in Na excretion was associated with 2·06 and 0·85 mmHg higher SBP and DBP, respectively.
Although Chile has the third highest prevalence of hypertension within Latin American countries, the present study is the first to investigate the correlates of Na excretion (a proxy of salt intake) and its association with hypertension. This is particularly important because, even if similar associations have been shown in other middle- and high-income countries, such local data are needed to inform the policies and public health interventions implemented in Chile. Our non-linear association analysis shows that hypertension risk increases significantly when Na excretion reaches 3·2 g/d. This is clinically relevant as more than 67 % of the Chilean population consumes ≥9 g salt/d. If intake at this level continues, the prevalence of hypertension, and consequently the burden of CVD, is likely to increase significantly in the short to middle term. In this context, new and effective strategies towards Na intake and therefore BP reductions are needed. A clear example of the effect of Na intake reduction on BP is the findings identified by He et al. in a meta-analysis of thirty-four trials (3230 participants) which suggested that reducing Na intake from 3·6–4·8 g/d to 2·0–2·4 g/d would have a major effect on BP, but a further reduction to 1·2 g/d would have an even greater effect and should become the long-term target for population( Reference He, Li and Macgregor 12 ).
Factors such as age, sex, BMI and metabolic syndrome have been shown to be correlated with Na intake in previous studies( Reference Cogswell, Zhang and Carriquiry 44 ). To our knowledge, the present study is the first to investigate the relationship of the lifestyle factors, PA and sitting time, with Na excretion. Both physically active and sedentary individuals were less likely to have a high-Na diet. This observation exposes potential discrepancies in health behaviours. Further investigation into the motivations for the low Na intake in these groups is required because, if there is a health-conscious motivation for this, exploring why individuals remain sedentary when making other healthy lifestyle decisions could further inform public health policies and interventions.
Limitations of the study
The CNHS 2009–2010 is a nationally representative sample of the adult Chilean population. Although these data were collected several years ago, which may reduce their influence in a country with a rapidly changing lifestyle/obesity landscape, they are the most up-to-date information available from Chile. Although the inclusion of a wide range of health, demographic and behavioural variables in the data set allowed for comprehensive adjustment for the effect of confounding factors, the CNHS has limited data available regarding other dietary behaviours such as fruit and vegetable, processed meat and other food intakes, which has limited our ability to take into account their potential confounding effect in the current study. Another limitation was the lack of data on energy expenditure or energy intake to account for their potential modifying effect on the associations described herein; however, we have used the total PA as a proxy of energy expenditure to account for some of these effects. Moreover, Na excretion was derived from validated equations using a fasted urine sample( Reference Tanaka, Okamura and Miura 28 ), which has been shown to be a better estimator of Na excretion than self-reported questionnaires. Nevertheless, 24HUNa is still considered the gold standard method for assessing Na excretion and consequently the use of a spot urine sample may have impacted on the validity of these results. Only seventy-two (2 %) participants met the WHO’s Na intake recommendation (<2·0 g/d) in the current study, therefore the population median was used to classified people as low and high Na intake, which could influence some of the estimated OR. On the other hand, as with all observational studies, our findings cannot prove causality, although previous randomised controlled trials have shown the causal effect of Na excretion on BP and hypertension risk( Reference He, Li and Macgregor 12 ). In addition, lifestyle exposures such as dietary intake and PA were self-reported, which has been shown to be prone to bias previously. This could obscure the true association between lifestyle factors and Na intake, and therefore future studies using objective measures for PA and better methods for dietary intake assessment are required. Another limitation that could cause self-selection bias is differences between the response rate in the total population and those who agreed to urine sampling. However, the characteristics of those without urinary Na excretion data were very similar to those with data available.
Conclusion
The present data have identified the main factors associated with higher Na excretion in a nationally representative population of Chile. The study has also corroborated the association of Na excretion with BP and hypertension, independent of major confounding factors. Given that Chile has the third highest prevalence of hypertension in Latin America, these findings can help create urgent policies and public health interventions for this at-risk population.
Acknowledgements
Acknowledgements: The authors thank all participants for their cooperation and the Chilean Health Ministry and Department of Public Health, The Pontificia Universidad Católica de Chile for commissioning, designing and conducting the second National Health Survey 2009–2010. Financial support: The Chilean National Health Survey (CNHS) 2009–2010 was funded by the Chilean Ministry of Health and led by the Department of Public Health, The Pontificia Universidad Católica de Chile. The present study was funded by the Chilean Health Ministry as part of the second health surveillance in Chile. The funders had no role in study design, data collection, data analysis, data interpretation or any decision related to this manuscript. Conflict of interest: None. Authorship: F.P.-R., A.S. and R.B. are joint first authors. S.R.G. and C.C.-M. are joint senior authors. F.P.-R. and C.C.-M. generated the research question. F.P.-R. and C.C.-M. planned the analysis. F.P.-R. performed the literature search. F.P.-R. performed the analysis with support from S.R.G. and C.C.-M. F.P.-R. tabulated the data. F.P.-R., A.S., R.B., S.R.G. and C.C.-M. interpreted the findings. F.P.-R., A.S. and R.B. wrote the first draft of the manuscript. All authors critically reviewed this and previous drafts. All authors approved the final draft for submission. C.C.-M. is the guarantor. Ethics of human subject participation: The CNHS 2009–2010 was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Research Committee of the Faculty of Medicine at the Pontificia Universidad Católica of Chile. Written informed consent was obtained from all subjects.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980018003889