Excess adiposity is a serious global health issue, with overweight/obesity impacting over 35 percent of adult men and women worldwide(Reference Ng, Fleming and Robinson1). These individuals are at increased risk of multiple negative physical, metabolic and psychological health outcomes, such as type II diabetes, CVD, certain types of cancers and depression(Reference Martin-Rodriguez, Guillen-Grima and Martí2–Reference Wardle and Cooke4). The high prevalence and probable health consequences of excessive adiposity emphasise the need for multifaceted interventions that reduce the impact of obesity through weight loss. Self-monitoring of dietary intake is a cornerstone of behavioural obesity treatment; however, the extent of monitoring needed to produce significant intervention effects has not been well explored.
Excessive adiposity is typically the result of positive energy imbalances, and first-line treatment includes decreasing energy intake and increasing energy expenditure(Reference Hill, Wyatt and Peters5). Lifestyle interventions targeting diet and physical activity are more effective in promoting weight loss when they encourage individuals to create and sustain behavioural modifications by employing strategies such as realistic goal setting and individual self-regulation skills(Reference Kirk, Penney and McHugh6,Reference Greaves, Sheppard and Abraham7) . One component of many behavioural weight loss interventions is dietary self-monitoring(Reference Greaves, Sheppard and Abraham7–Reference Yu, Sealey-Potts and Rodriguez9), in which individuals are responsible for logging or recording their dietary intake. The practice of dietary self-monitoring is grounded in self-regulation theory, which posits that self-evaluation and self-reinforcement necessitate behaviour change(Reference Vohs and Baumeister10). Self-monitoring requires an individual to have some level of understanding and awareness of their actions, thus supporting the development of self-regulation skills(Reference Burke, Wang and Sevick8). Although the theoretical basis for encouraging dietary self-monitoring is well-established, best practices for implementation are not clearly defined.
Dietary self-monitoring as a behaviour change technique evolves as weight loss intervention models modernise. In addition to conventional paper and pen methods, monitoring may now be performed on a variety of platforms including mobile apps and websites (such as CalorieKing or MyFitnessPal). Studies may also modify reporting guidelines (total intake, specific behaviours/foods) and reporting frequency (real time, once daily, five times a month) based on variations in study designs, targets or outcomes. Traditionally, dietary self-monitoring strategies involve recording of all daily food and beverage intake onto paper logs. Often, participants were required to look up the nutrient content of foods and calculate total intake by tallying points or energy content(Reference Burke, Wang and Sevick8). Participant’s adherence to these strategies decreases over time as the practice is labor-intensive and requires substantial internal motivation(Reference Burke, Wang and Sevick8). A 2016 meta-analysis showed that weight loss intervention participants who had greater adherence to dietary self-monitoring lost more weight(Reference Lemstra, Bird and Nwankwo11), thus improvement in monitoring may drive better weight loss outcomes. However, this finding may be confounded by increased individual motivation to practice self-monitoring and a coinciding motivation to utilise other self-regulatory behaviours(Reference Fitzpatrick, Appel and Bray12,Reference Burgess, Hassmén and Pumpa13) . That is, high adherence to dietary self-monitoring, may be an indication of a motivated participant.
Approaches to adapting traditional self-monitoring models to potentially reduce burden include digital recording options, reduction of monitoring scope or simplification of recording through smartphone photo features. Dietary monitoring smartphone applications have been created to make recording intake theoretically easier for participants to achieve and to provide richer feedback data for users(Reference Teasdale, Elhussein and Butcher14). Evidence has shown smartphone applications for self-monitoring dietary intake and physical activity are effective at supporting weight loss goals and promoting adherence to tracking protocols(Reference Semper, Povey and Clark-Carter15,Reference Cavero-Redondo, Martinez-Vizcaino and Fernandez-Rodriguez16) . However, the review looking at dietary tracking only concluded that there was no significant difference in the amount of weight lost between groups who recorded their diet on paper or electronically(Reference Semper, Povey and Clark-Carter15).
Another way to reduce the burden of recording a full day’s intake may be to decrease monitoring intensity (i.e. focusing on specific components of the diet or dietary behaviours as opposed to all food and beverage intake). For example, participants may be encouraged to monitor or track only those dietary behaviours theorised to impact weight loss success, such as drinking sugar-sweetened beverages or eating fruits and vegetables(Reference Bennett, Herring and Puleo17,Reference Lally, Chipperfield and Wardle18) . By decreasing the intensity of self-monitoring to only specific types of food intake, the labor associated with the task and the demand for intrinsic motivation may be reduced. However, it is unclear if this strategy is as effective in supporting weight loss as self-monitoring of the diet in its entirety. Because dietary self-monitoring remains a cornerstone of behavioural weight loss interventions, and new self-monitoring tools continue to emerge, a review of current approaches to dietary self-monitoring and their impact on weight loss is needed.
The goal of this systematic review is to examine the use of different dietary self-monitoring approaches in behavioural weight loss interventions in order to support the optimisation of these tools in future work. This review will be guided by the following research questions:
-
1. How is dietary self-monitoring implemented in weight loss interventions (current platforms (web, app, paper, etc.), intensity levels (all dietary intake v. dietary components), adherence metrics and feedback integration)?
-
2. How effective are interventions that use dietary self-monitoring to support weight loss among adults with overweight and obesity?
-
3. What are the weight loss outcomes in interventions that use higher intensity dietary self-monitoring v. lower intensity self-monitoring?
Methods
This systematic review was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.
Search methods
We performed a systematic search in Ovid MEDLINE, Ovid EMBASE, Ovid PsycINFO, Cochrane Library, PubMed, Web of Science and EBSCOhost CINAHL, from inception to September 18, 2019 (search strategies are available as supplementary material). An update was performed using identical searches from September 18, 2019, to December 15, 2020. Results of the two searches were combined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Flow Diagram for reporting. Search structures, subject headings and keywords were tailored to each database by a medical research librarian specialising in systematic reviews. Searches were not restricted by language but were restricted to human subjects. We searched Embase for grey literature resources such as conferences, dissertations, reports and other unpublished studies in order to identify additional relevant citations. References in the included articles were also searched. Our findings are reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines(Reference Moher, Liberati and Tetzlaff19).
Study selection
Behavioural weight loss intervention studies targeting adults with overweight or obesity that implemented dietary self-monitoring were included in this review. The inclusion criteria for the review are shown in Table 1. Interventions targeting people with severe mental illness or with an existing condition that would impact subsequent weight loss (such as pregnancy, post-partum, bariatric surgery) were excluded. Weight maintenance and weight gain prevention trials were also excluded. Studies using 24-h dietary recalls, food frequency questionnaires or other tools to assess diet as a study outcome, as opposed to as a behaviour change technique with a clearly defined monitoring protocol, were excluded. Trials were not limited by length of study, follow-up duration or country. Uncontrolled, pilot/feasibility, quasi-experimental or single-arm intervention studies were excluded, as were studies in which both the control and experimental groups were instructed to follow identical dietary self-monitoring procedures. The study selection process was conducted by a single reviewer (MR); a second reviewer (YL) analysed 10 % of total articles from the initial search and independently categorised them for inclusion and exclusion using an identical screening process. Agreement between the two reviewers was 98·5 %. Discrepancies were discussed among MR and YL and resolved by consensus and mediation with the senior author (KB).
Table 1 Inclusion criteria

Data extraction and quality assessment
The general study characteristics were extracted and are shown in Table 2. Two reviewers (MR and AR) extracted details from studies including: author, year, country, design, sample size, sampling frame, participant ages, intervention setting, study durationand main outcome measures (weight change). Dietary self-monitoring information that was extracted included: (1) platform of self-monitoring (web, app, paper, etc.); (2) dietary self-monitoring recording and submission processes (e.g. record on paper and mail in); (3) feedback messaging, if any (4) adherence; and (5) the intensity of the reported dietary intake (total diet, specific dietary components, etc.).
Table 2 Characteristics of included studies
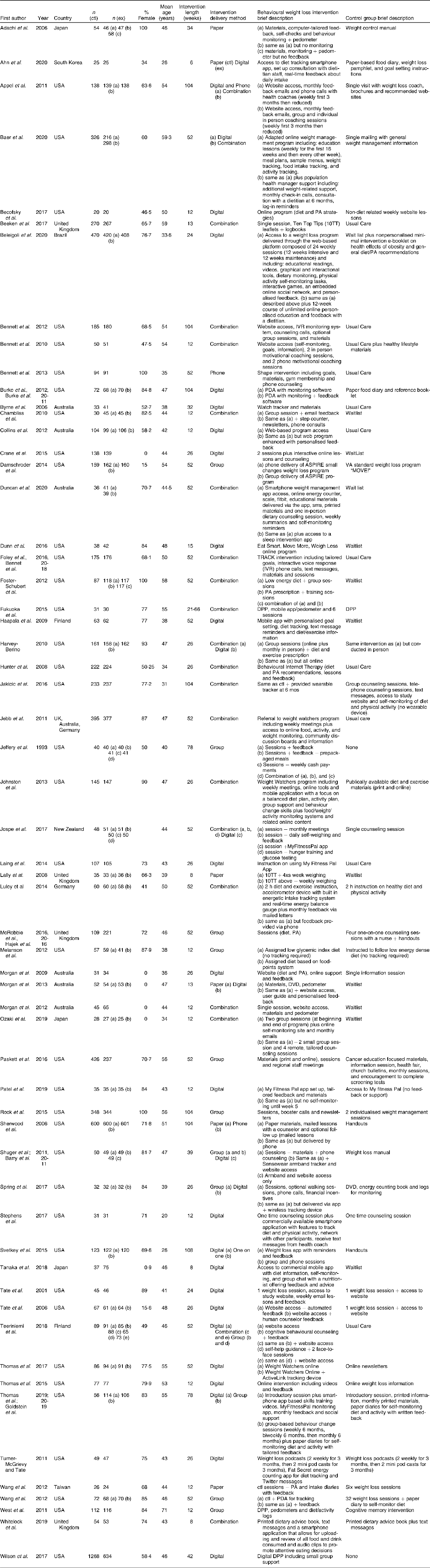
DPP, diabetes prevention program.
Data quality was assessed using a Consolidated Standards of Reporting Trials (CONSORT) statement risk of bias tool, previously adapted for weight loss intervention studies(Reference Young, Morgan and Plotnikoff20) (Table 3). Items were scored as present (✓), absent (✗) or ‘unclear or inadequately described’ (?). Some items were not applicable depending on the design of individual studies, and these were scored as N/A. Risk of bias categorization were based on total scores calculated using a previously developed system (✓ = 1 |✗ = 0 | ? = 0 | n/a = 0); risk of bias categories included: high risk (0–3), medium risk (4–7) or low risk (8–10)(Reference Young, Morgan and Plotnikoff20).
Table 3 Quality assessment of included studies

Data synthesis and analysis
Studies were collectively examined with regard to study characteristics and outcomes. Weight loss was the outcome of interest in this review. Each of the included articles used weight loss as the primary study outcome. Weight change from baseline to the end of treatment was examined in each study. Mid-point and later follow-up periods were not included, as this review was focused on initial weight loss rather than weight loss maintenance. P-values were extracted from studies and reported whenever available. Included studies were divided into two groups based on the intensity level of monitoring that include: (i) interventions that required self-monitoring of all dietary intake and (ii) interventions that required self-monitoring of less than all dietary intake hereto referred to as ‘abbreviated intake’ (e.g. vegetable intake only, snack intake). A meta-analysis of weight loss data was attempted, but high clinical and methodological study heterogeneity (I 2 > 95 %) limited interpretability.
Results
Search/screening results
Search results and screening flow chart are shown in Fig. 1. A total of 10 441 unique study records were identified by the search. Of these, a total of fifty-nine individual interventions met the criteria for inclusion and were included in this review(Reference Bennett, Herring and Puleo17,Reference Lally, Chipperfield and Wardle18,Reference Adachi, Sato and Yamatsu21–Reference Teeriniemi, Salonurmi and Jokelainen78) . Results from studies that represented duplicate or secondary reporting of the same intervention were combined with the principal outcomes paper.
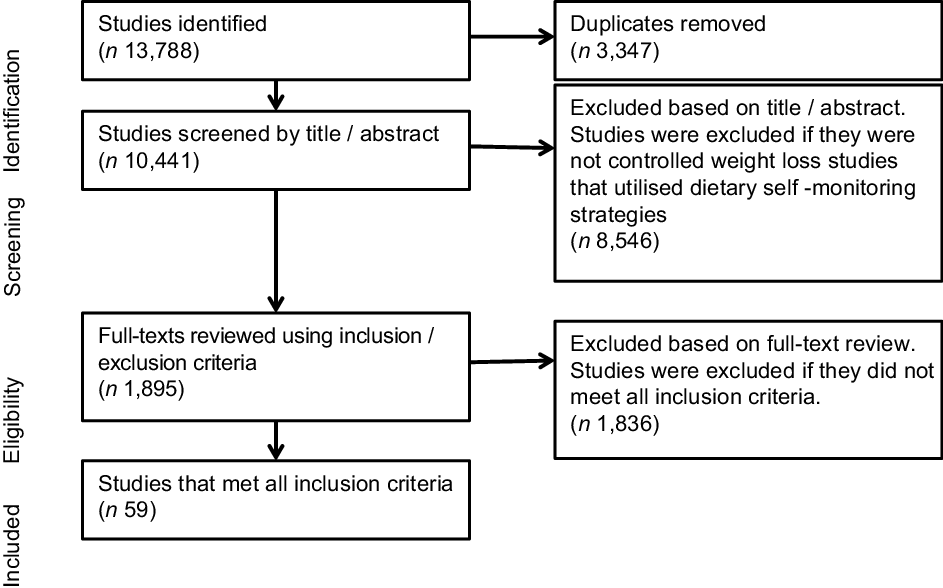
Fig. 1 PRISMA flow chart
Study characteristics
General study characteristics
Study characteristics are summarized in Table 2. Of the fifty-nine studies included, thirty-eight were conducted in the USA, seven in Australia/New Zealand, four in the United Kingdom, three in Japan, two in Finland and one each in Taiwan, Germany, Brazil and South Korea. One study had multiple international locations. The mean age of participants ranged from 20 to 71 years (IRQ = 10·0) with a majority of studies including participants older than 40 years (73 %, n 43). Five studies recruited only men and four studies recruited only women. Intervention durations varied from eight to 108 weeks (IRQ = 40·0). Based on the study selection criteria, all of the included studies had a comparison group: n 16 used a waitlist or a no or unrelated intervention control group; n 24 used a minimal intervention comparison group that typically consisted of one or two weight loss counseling sessions, handouts on healthy lifestyles, basic weight loss website access or some combination of these; and n 9 provided the comparison group with an alternative intervention. Alternative intervention studies were those in which two groups received substantial weight loss interventions but with variability in content, delivery or duration. Different intervention delivery methods were used to communicate weight loss content including group sessions, websites and other digital methods, one-on-one sessions, phone calls and paper materials such as books and leaflets. Studies recruiting in specialised obesity clinics or through primary care providers typically used usual care comparison groups (n 10).
Quality assessment
Risk of bias for each study is shown in Table 3. Quality scores indicated medium or low risk of bias for all studies. This was likely due to our inclusion criteria, which was limited to studies with comparison groups and excluded pilot studies. Forty-six out of the n 59 included studies (78 %) conducted some form of intent to treat analysis although different imputation methods were used. Eighteen (31 %) included assessor blinding, n 40 (68 %) described accounting for confounders in analysis and n 37 (63 %) met retention rate criteria with <20 % of the total sample dropping out before the end of the intervention.
Dietary self-monitoring methods
The methods for implementing dietary self-monitoring in the included studies are described in Table 4. This includes the scope of self-monitoring requested (all intake or abbreviated intake), platforms used, reporting and submission details, adherence metrics, adherence results and any reported relationships between self-monitoring adherence and weight loss outcomes. Several dietary self-monitoring platforms were used in the weight loss interventions including mobile phone apps (n 19), paper food diaries (n 22), wearables (n 2), websites (n 27) and personal digital assistants (PDAs) (n 2). Platforms were not always exclusive; some studies used different platforms for different intervention groups, or offered participants a choice of platform.
Table 4 Description of dietary self-monitoring, adherence and relationship to weight loss

NR, not reported; Ctl, control group; Ex, experimental group.
* Top ten tips (10TT) dietary goals: eat at roughly the same time each day, choose reduced fat foods, eat healthy snacks, check fat and sugar content on labels, avoid sugar sweetened beverages and alcohol, focus on your food while eating, eat at least 5 portions of fruit and vegetables/d.
† Interactive obesity treatment approach (iOTA) dietary goals: avoid sugary drinks, avoid eating fast food, eat breakfast every day, eat at least 5 fruits and vegetables/d, avoid high-fat meat, avoid high-calorie snacks, have low-fat dairy 3 times/d, avoid foods made with white flour, like white bread, regular pasta and white rice.
There was variability in the intensity level of dietary monitoring to be recorded. Forty-four studies (75 %) required dietary self-monitoring of all intake and n 15 studies required self-monitoring of abbreviated intake. Abbreviated dietary-self monitoring protocols varied among included studies. Two studies utilised the recording of meal patterns (e.g. how often one eats certain types of foods or meals), and nine focused on dietary behaviours such as eating fruit or vegetables or avoiding fast food. One study required participants to self-monitor dietary intake using a traffic light method. The traffic light method categorises foods based on nutrient and energy density into green, yellow and red. Using this method, participants were asked to report the overall number of foods consumed from each color category. Lastly, one study asked participants to estimate the portion sizes of their daily meals using a predefined ranking system (i.e. ‘mini’, ‘normal’ or ‘maxi’)(Reference Luley, Blaik and Götz50). Food photography was used in two studies. The first study had participants upload photos of all the foods and beverages they consumed and provide a self-review of their diet quality using a brief survey(Reference Whitelock, Kersbergen and Higgs70). The second study had participants upload only photos of their three main meals to the study website each day. The images were later reviewed with the participant(s) during a group chat with a nutritionist on the study website. The nutritionist offered immediate (within 3 h) feedback on meal choices and responded to specific questions from participants(Reference Tanaka, Sasai and Wakaba61).
A majority of the studies (n 45) provided feedback based on self-monitoring data that varied in delivery platform, frequency and timeliness. In several studies (n 15), feedback was delivered immediately through automated messaging or graphs; this was particularly common in studies that utilised commercial dietary tracking apps. In other instances, study personnel would review dietary inputs and offer weekly (n 10) or monthly (n 4) feedback. Six studies used a combination of these approaches, offering immediate feedback followed by additional weekly or monthly follow-ups.
Methods for assessing adherence to dietary self-monitoring and the corresponding metrics are provided in Table 4. Adherence to dietary self-monitoring was examined in thirty-three of the fofty-nine studies, although the definition of adherence was inconsistent. Metrics included the actual number of days or weeks participants completed monitoring diaries (n 9), the proportion of diaries completed out of the number requested (n 9), the proportion of participants completing a certain number of diaries (n 8) and the proportion or number of participants self-reporting monitoring diary use (n 6).
Reported relationships between adherence and weight loss are described in Table 4. Eighteen studies (all intake = 14; abbreviated intake = 4) examined adherence to self-monitoring and weight loss and 12 (all intake = 9; abbreviated intake = 3) identified significant positive relationships between adherence and weight loss while six did not (all intake = 5, abbreviated intake = 1). Twelve studies had both weight loss and adherence data but did not examine or report relationships.
Dietary self-monitoring and weight loss
The weight loss outcomes of included studies are shown in Table 5. Interventions that utilised all intake dietary self-monitoring (n 44) showed significant weight loss in the study group v. the comparison group in twenty-sevenm studies (61 %). Fifteen studies (34 %) did not report significant intervention effects between the study and comparison groups and one study reported a reverse effect(Reference Jakicic, Davis and Rogers45), although that study included an active weight loss program comparison group. Among interventions that utilised all intake dietary self-monitoring and had a true (waitlist, no or unrelated intervention) control group (n 10), seven studies (70 %) demonstrated a significant between group intervention effect on weight loss. Of interventions that utilised abbreviated dietary self-monitoring (n 15), ten (67 %) reported significantly greater weight loss in intervention groups v. comparison groups. Five reported no significant effects. Of the interventions that used abbreviated self-monitoring methods and had true control groups (n 5), four studies (80 %) reported a significantly greater weight loss among the study groups compared to controls. There was no apparent pattern indicating one type of abbreviated monitoring (specific behaviours v. traffic light, etc.) facilitated more weight loss. Direct comparisons between paper and digital self-monitoring were examined in nine studies(Reference Ahn, Lee and Kim24,Reference Burke, Conroy and Sereika32,Reference Harvey-Berino, West and Krukowski43,Reference Shuger, Barry and Sui57,Reference Spring, Pellegrini and Pfammatter58,Reference Thomas, Bond and Raynor65,Reference Wang, Sereika and Chasens67,Reference Morgan, Collins and Plotnikoff73,Reference Teeriniemi, Salonurmi and Jokelainen78) . Among these, only one study demonstrated significantly more weight loss between in digital v. paper dietary self-monitoring platforms(Reference Harvey-Berino, West and Krukowski43).
Table 5 Weight loss outcomes of included studies

* sd calculated from CI.
Discussion
This review, including fifty-nine intervention studies, examined: (1) the implementation of different dietary self-monitoring protocols in behavioural weight loss interventions including characteristics, adherence metrics and feedback utilisation; (2) the effectiveness of self-monitoring interventions to promote weight loss among adults with overweight/obesity and (3) differences in weight loss outcomes between interventions that use higher v. lower intensity dietary self-monitoring. A wide range of self-monitoring platforms and implementation protocols were identified across included studies. The majority of interventions demonstrated a significant reduction of weight compared with control groups. A similar proportion of studies that included self-monitoring of all dietary intake (61 %) and abbreviated intake (67 %) demonstrated significant intervention effects on weight loss; however, a formal meta-analysis was not conducted due to study heterogeneity.
Dietary self-monitoring was implemented in different ways across studies; digital and/or paper diaries were used to collect all intake or abbreviated intake with or without integrated feedback. Studies utilised all-dietary intake self-monitoring strategies more often than abbreviated-intake strategies. Study participants’ self-monitoring behaviour wanes over time, highlighting the issue of participant burden(Reference Burke, Wang and Sevick8). Several included studies (n 15) used abbreviated self-monitoring approaches, and it is reasonable to assume that these may be less burdensome and encourage more monitoring adherence, although the adherence data are not reported with sufficient consistency to allow formal tests of the monitoring adherence by types of self-monitoring. High variability in adherence metrics obfuscates the potential relationship between dietary monitoring intensity and weight loss outcomes.
The majority of included interventions found significant weight loss in experimental groups compared with control groups (all intake monitoring (61 %) and abbreviated intake monitoring (67 %)). This finding is in line with previous research highlighting the importance of dietary self-monitoring as a component of behavioural weight loss programmes. One meta-regression of 122 evaluations found self-monitoring in lifestyle interventions to be responsible for the greatest heterogeneity among studies and, when self-monitoring and one or more other behaviour change techniques were combined, weight loss success increased(Reference Michie, Abraham and Whittington79). This is further supported by literature suggesting interventions that include self-monitoring are particularly effective in promoting weight loss among certain populations including post-partum women(Reference Lim, O’Reilly and Behrens80) and cancer survivors(Reference Hoedjes, van Stralen and Joe81). Similar proportions of studies using higher and lower intensity monitoring demonstrated significant impact on weight loss, suggesting abbreviated self-monitoring may be an effective approach when higher intensity self-monitoring is not possible.
It is impossible to effectively disentangle the impact of dietary self-monitoring on weight loss from the other intervention components in included studies. Although self-monitoring may be a uniquely important aspect of behavioural weight loss interventions, deeper exploration of this concept is limited by a lack of consensus on self-monitoring adherence measures. Only thirty-three of the fifty-nine included studies (all intake = 26; abbreviated intake = 7) examined self-monitoring adherence, and definitions of adherence were inconsistent across included studies. Importantly, the cut-offs used to differentiate the ‘adherent’ v. the ‘non-adherent’ appeared to be arbitrarily set by researchers. A priori measures of self-monitoring adherence need to be established in order to understand the relative benefits of different platforms and intensity levels of monitoring. Comparable measures would also allow for the synthesis of data across studies, thus enabling a deeper understanding of how self-monitoring impacts weight loss and participant characteristics that may moderate this relationship. This topic is under active investigation; Turner-McGrievy et al. suggest the reporting of two or more eating occasions per day is an optimal definition of adherence to self-monitoring in the context of weight loss interventions(Reference Turner-McGrievy, Dunn and Wilcox82). A narrative review of the subject concluded that until a widely agreed-upon definition of adherence was established, multiple indicators of dietary self-report adherence may be appropriate to better understand the relationship between monitoring and weight loss success(Reference Payne, Turk and Kalarchian83).
Strengths of this review include utilising: eight databases including the gray literature for the search, a medical librarian to design the search strategy and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. This review is limited by the use of one reviewer in screening all articles and not conducting a meta-analysis. The extracted data were limited to information explicitly stated in the included papers, variability in article reporting made it challenging to determine what duration of time participants were requested to self-monitor (daily, weekly and monthly), and therefore this information was not included.
Behavioural weight loss interventions among adults with overweight/obesity are an essential element in the fight against excessive adiposity and associated chronic disease. Such interventions can be effective in achieving weight loss, but intervention components must be carefully structured in order to optimise implementation. This review adds to the literature by offering an overview of existing methods for collecting different levels of dietary-intake data and weight loss success among interventions utilising diverse dietary-monitoring strategies. This is the first review to examine weight loss interventions by intensity of self-monitoring. Abbreviated dietary self-monitoring may hold promise as a way to reduce participant burden, but carefully designed studies comparing all intake and abbreviated monitoring protocols are needed.
Acknowledgements
Acknowledgements: The authors would like to thank the librarians at the MD Anderson Research Library for their support. Financial support: This research was supported by the MD Anderson Cancer Center Support Grant (P30 CA16672), the Center for Energy Balance in Cancer Prevention and Survivorship and the Duncan Family Institute for Cancer Prevention and Risk Assessment at the University of Texas MD Anderson Cancer Center. M.R. is supported in part by the US Department of Agriculture, Agricultural Research Service under Cooperative Agreement No. 58-3092-0-001. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or USDA. MD Anderson, the NIH and the USDA had no role in the design, analysis or writing of this article. Conflicts of interest: There are no conflicts of interest. Authorship: M.R., Y.L. and K.B. developed the research questions, designed the review approach, conducted data extraction and analysis and wrote the manuscript. A.R. provided assistance in data extraction and manuscript development. S.S., L.S. and C.D. supported manuscript development, research question formulation and editing. K.K. provided librarian guidance in developing the search strategy and subsequent updates. Ethics of human subject participation: Not Applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S136898002100358X








