Low levels of vitamin D are reported in diverse populations around the world( Reference Mithal, Wahl and Bonjour 1 – Reference Lips 3 ), with one review suggesting that as many as 14 % of the world’s population have levels inadequate for general well-being( Reference Holick 4 ). Serum concentration of 25-hydroxyvitamin D (s-25(OH)D) is the standard assessment for vitamin D status in the body and s-25(OH)D concentration of 50 nmol/l or more is considered sufficient for optimal bone-related health outcomes by the Institute of Medicine( Reference Ross, Taylor and Yaktine 5 ).
Studies from Turkey, the Middle East and India have revealed that up to 50 % of children have vitamin D deficiency( Reference El-Hajj Fuleihan 6 ). A number of determinants that affect s-25(OH)D concentration in children have been identified and include skin pigmentation, prolonged breast-feeding without vitamin D supplementation and low consumption of foods rich in vitamin D( Reference Babu and Calvo 7 ). However, little is known about the vitamin D status of Nepalese children.
Nepal is a predominantly Hindu nation bordering Tibet and India, located between the latitudes of 26°N and 31°N. Nepalese cultural values do not restrict sun exposure and about 300 d per year are sunny( 8 ). A few studies that have investigated vitamin D status in special groups in Nepal, such as pregnant women and alcohol-abusing populations, report a wide range of vitamin D deficiency prevalence rates (4–64 %)( Reference Neupane, Lien and Hilberg 9 , Reference Jiang, Christian and Khatry 10 ). One recent study found 19 % of pre-adolescent Nepalese children to have vitamin D deficiency( Reference Cole, Ruczinski and Schulze 11 ). The samples were taken year-round and no assessment of clothing habits or dietary intake was conducted, which makes the findings difficult to interpret. Children under 5 years of age are particularly vulnerable to different infections and have greater mineral demands due to their growing bones; however, there are no data on vitamin D status in this age group in the South Asian region. Thus, we sought to determine the vitamin D status among children aged 12–60 months in rural Nepal and to identify its possible associations with sociodemographic indicators, breast-feeding practices and nutritional status.
Methods
Study population
The present community-based, cross-sectional study was conducted in rural Nepal, at latitude of 27·39°N and during the dry season, between September and November 2012. Children from 12 to 60 months of age were recruited from the nine wards of Ugrachandi Nala Village Development Committee in Kavrepalanchowk district of Central Nepal. We utilized the existing national vitamin A supplementation programme that maintains a national registry of children in this age group( 12 ). From the records available at the local health post, we assigned numbers to all 411 children aged 12–60 months living in the target village. By using an online randomizer, a set of 320 uniquely sorted numbers was generated to randomly select the participants. Female community health volunteers were requested to identify the selected children and invite their guardians for participation in the study. None of the children we screened had known metabolic bone disease or chronic disease associated with bone abnormalities, and none used medications likely to interfere with vitamin D metabolism. A total of 280 participants, all of whom provided complete questionnaires, were included in our analysis.
Collection of background information
The guardians of each child provided sociodemographic information, including information about family size, occupation and household income. Information on breast-feeding practices, consumption of milk products, use of vitamin supplements, medical history and sun exposure was also collected by a trained nurse from the same area, using a structured questionnaire. Household income and type of housing (made from natural or manufactured materials) provided proxy measures of socio-economic status. Pre-testing of the questionnaire among mothers from the same setting showed that foods naturally rich in vitamin D did not constitute the dietary supply in general. Therefore, dietary assessment of vitamin D-providing foods was not conducted.
Milk product intake was calculated by multiplying the amount per serving by the food frequency for each type of milk product. Ca intake was computed by multiplying the Ca content of the specific type of milk product by the daily consumed amount. Total Ca intake from milk products was calculated by summing the Ca intake from different milk products for each child. Ca values in milk products were obtained from the US Department of Agriculture’s National Nutrient Database for Standard Reference( 13 ). Weight was measured with a digital weight scale made by Microlife Company in Switzerland, certified for ISO 9001 with accuracy of 100 g. Height was measured upright, using a wall stadiometer (Bio-Plus 200 cm, model 265 M/1013522). Standing height for children younger than 24 months was equalized to the recumbent length by adding 0·7 cm. All measurements were taken without shoes and with minimal clothing.
Assessment of nutritional status
The nutritional status of the children was determined by comparing measurements of weight-for-height, weight-for-age and height-for-age with the WHO reference standards for wasting, underweight and stunting, respectively( 14 ). Weight-for-height (indicator of wasting, i.e. acute or recent malnutrition), weight-for-age (indicator of underweight, i.e. acute or chronic malnutrition) and height-for-age (indicator of stunting, i.e. chronic or long-standing malnutrition) were defined according to WHO standards( 15 ).
Dried blood spot sampling and vitamin D assay
Capillary blood was collected by a certified nurse after pricking the fingertip with a single-use safety lancet. The total amount collected was approximately 4–5 drops. Blood drops were applied directly on the sampling filter card within pre-marked circles. The cards were then air dried for 2 h; sample cards were stored in low gas-permeable zip-lock bags with desiccant packages. Samples were kept at room temperature during the first 7 d, and later refrigerated. At the end of sample collection, the dried blood spot (DBS) kits were shifted to the laboratory of Vitas AS in Norway by courier service for further analysis. Serum concentrations of 25-hydroxycholecalciferol (s-25(OH)D3) and 25-hydroxyergocalciferol (s-25(OH)D2) were quantified separately using liquid chromatography–tandem mass spectrometry (LC–MS/MS). S-25(OH)D was expressed as the sum of s-25(OH)D2 and s-25(OH)D3. Punches from the DBS were added to water, shaken and diluted with 2-propanol–methanol containing the internal standard [2H]6-25(OH)D3 and butylated hydroxytoluene as an antioxidant. After mixing and centrifugation the supernatant was transferred to an insert and centrifuged again, and an aliquot of 50 µl was injected into the HPLC system. HPLC was performed with an Agilent 1200 liquid chromatograph (Agilent Technologies, Palo Alta, CA, USA) interfaced by atmospheric pressure chemical ionization to an Agilent mass spectrometric detector operated in Multiple Reaction Monitoring mode. Vitamin D analogues were separated on a 4·6 mm×150 mm reversed-phase column with 2·7 µm particles. The column temperature was 40°C. A two-point calibration curve was made from analysis of DBS calibrators with known vitamin D concentrations. The LC-MS/MS DBS method was internally validated. The inter- and intra-assay CV were 7·6 % (35·8 nmol/l) and 10·2 % (78·3 nmol/l), respectively.
The LC-MS/MS DBS method was chosen as a minimally invasive and convenient to use technique for paediatric research participants. The laboratory performing the analysis is part of the Vitamin D Quality Assessment Scheme and is compliant.
A common universal normal range for s-25(OH)D does not exist; however, we chose to use the commonly used cut-off points, where vitamin D status is classified as severely deficient (0–12·5 nmol/l), moderately deficient (12·6–25·0 nmol/l), mildly deficient (25·1–49·9 nmol/l) and sufficient (≥50·0 nmol/l)( Reference Lips 16 ).
Ethical considerations
After explaining the study aims and data collection procedure, consenting guardians were asked to provide written or witnessed verbal consent. The guardians were also the informants. Verbal consent was acquired when guardians had a decision-making capacity but could not read or write due to illiteracy; in these cases, verbal consent was witnessed by an adult from the same community who was not a member of the study team and formally recorded. The study was conducted according to the guidelines provided in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Nepal Health Research Council and the Regional Committee for Medical and Health Research in Norway. Written or verbal, voluntary informed consent was obtained from all subjects/patients.
Statistical analysis
WHO Anthro software version 3·2·2 was used to evaluate sex-specific growth indicators according to WHO standards( 14 ). Descriptive statistics are presented as means and standard deviations. To compare the mean s-25(OH)D concentrations according to explanatory variables, we used an independent-sample t test (two-tailed) and one-way ANOVA. The relationships between s-25(OH)D and potentially associated variables were tested primarily using linear regression models, both for continuous and categorical explanatory variables. Assumptions of the regression analysis (linearity and equal variance for the dependent variable) were checked by inspecting plots of residuals against predicted values. Statistical analysis of the data was performed using the statistical software package IBM SPSS Statistics version 20. Significance level was set to 0·05.
Results
Sample characteristics
A total of 280 children aged 12–60 months, mean age 35·8 (sd 13·8) months, were included in the study. Most children (81·1 %) from our sample (157 boys and 123 girls) were from low to medium socio-economic groups. The majority of parents (98 %) reported trousers and long-sleeved shirts as regular clothing for their children; 16 % mentioned T-shirts as an option for warm days. In our sample, exclusive breast-feeding during the first 6 months was universal (99·3 %). The mean duration for breast-feeding was estimated to be 27 (sd 7) months. Moreover, 38 % of the mothers reported that their children were still breast-feeding. The total Ca intake for all the children was 318 (sd 235) mg/d. About 45 % of the children were classified as stunted, 6 % as wasted and 24 % as underweight (Table 1).
Table 1 Demographic and nutritional characteristics and s-25(OH)D concentrations (nmol/l) of rural Nepalese children (n 280) aged 12–60 months, September–November 2012
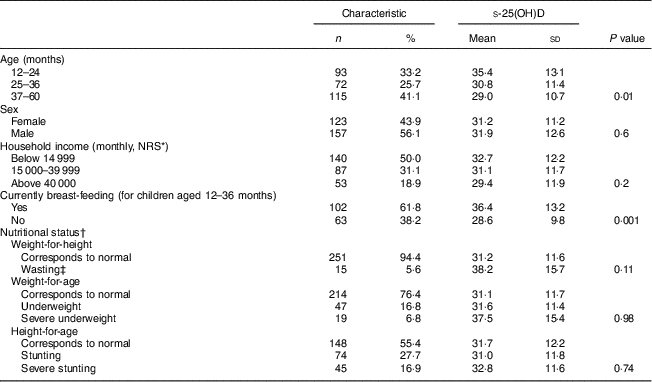
s-25(OH)D, serum 25-hydroxyvitamin D.
* NRS, Nepalese Rupees; $US 1=approx. NRS 100 as of 30 September 2012.
† Nutritional status defined according to WHO standards.
‡ Nutritional status determined with cut-offs −2 sd, includes three cases with score below −3 sd.
Vitamin D status
The mean concentration of total s-25(OH)D was 31·5 (sd 12·0) nmol/l for all infants, ranging between 6·9 and 74·5 nmol/l. S-25(OH)D2 concentration was detected only in nine children, with a mean of 8·4 (sd 5·6) nmol/l. As shown in Table 2, over 90 % of the children had s-25(OH)D concentrations below 50 nmol/l, whereas about a third of the children had s-25(OH)D concentrations in the moderate to severe deficiency range and only 9 % had sufficient s-25(OH)D concentrations.
Table 2 Vitamin D status on the basis of s-25(OH)D concentration among rural Nepalese children (n 280) aged 12–60 months, September–November 2012
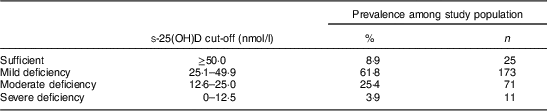
s-25(OH)D, serum 25-hydroxyvitamin D.
Table 1 also shows s-25(OH)D concentrations according to the different sociodemographic and nutritional categories. There was no difference in mean s-25(OH)D concentrations between boys and girls. Younger children (12–24 months) had higher s-25(OH)D concentrations than older children (P<0·01). The mean concentration of s-25(OH)D was 36·4 (sd 13·2) nmol/l and 28·6 (sd 9·8) nmol/l for children younger than 36 months who were currently breast-fed and not currently breast-fed, respectively. In linear regression models analysing the relationship between s-25(OH)D and potentially associated factors in Nepalese children, s-25(OH)D concentration was significantly higher among children who were currently breast-fed compared with those not currently breast-fed (P<0·001). Children living in mud/wooden houses had higher s-25(OH)D concentrations than those living in concrete houses (P 0·048). Other variables such as nutritional status, household income, time spent outdoors daily and Ca intake were not associated with s-25(OH)D concentrations (Table 3).
Table 3 Relationship between s-25(OH)D (nmol/l) and potentially associated factors in rural Nepalese children (n 280) aged 12–60 months, September–November 2012
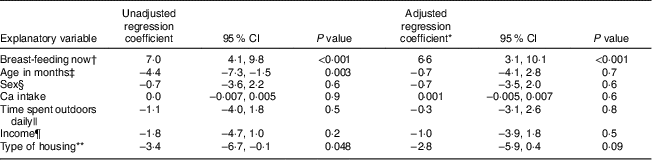
s-25(OH)D, serum 25-hydroxyvitamin D.
* Adjusted for variables that were significant in simple linear regression (breast-feeding now, type of housing and age).
† Breast-feeding now coded as: 0=no; 1=yes.
‡ Age coded as: 1=12–36 months; 2=37–60 months.
§ Sex coded as: 1=boy; 2=girl.
|| Time spent outdoors coded as: 1=≤2 h; 2=>2 h.
¶ Income coded as: 1=<NRS 15 000; 2=>NRS 15 000.
** Type of housing coded as: 1=mud and wood; 2=concrete.
Discussion
We found that vitamin D deficiency is common among children in rural Nepal, with over 90 % of children 1–5 years of age having s-25(OH)D concentrations under 50 nmol/l. Severe vitamin D deficiency (<12·5 nmol/l) was found in 4 % of the studied children. There are limited data on vitamin D status among children of pre-school age in the South Asian region; however, the findings of the current study are consistent with the few reports of vitamin D status among healthy pre-school children( Reference Wayse, Yousafzai and Mogale 17 , Reference Roth, Shah and Black 18 ). Using the cut-off concentration of 50 nmol/l, Wayse et al. reported vitamin D deficiency among 61 % of Indian children under 5 years of age( Reference Wayse, Yousafzai and Mogale 17 ). Roth et al. found a mean s-25(OH)D concentration of 39·2 nmol/l among Bangladeshi children aged 1–23 months( Reference Roth, Shah and Black 18 ). Similarly high prevalence of vitamin D deficiency was identified among pregnant women and their newborns, adolescents and adults in the region( Reference Sachan, Gupta and Das 19 – Reference Goswami, Gupta and Goswami 23 ).
Factors associated with serum 25-hydroxyvitamin D concentrations
In contrast to findings from studies conducted among Indian children, which showed that boys had significantly higher s-25(OH)D concentrations than girls( Reference Marwaha, Tandon and Reddy 20 , Reference Sahu, Bhatia and Aggarwal 24 ), we did not observe any significant sex difference in vitamin D status among our Nepalese children. This could be explained by the similar clothing practices and outdoor activities of both sexes in our sample, as noted by Sahu et al. with regard to teenagers in rural India( Reference Sahu, Bhatia and Aggarwal 24 ). As compared with samples of Pakistani infants( Reference Atiq, Suria and Nizami 25 ) and Indian schoolgirls( Reference Puri, Marwaha and Agarwal 21 ), we did not observe differences in s-25(OH)D concentration with regard to socio-economic factors such as income level of the family. Nevertheless, children living in mud/wooden houses had slightly higher s-25(OH)D concentrations than those living in concrete houses. This difference disappeared, however, after we adjusted for breast-feeding practices because more children who were currently breast-fed lived in a mud/wooden house. In the Nepalese context, concrete housing is a marker of affluence, but at the same time may indicate decreased physical activity and limited opportunity for sun exposure. Findings from Neupane et al.( Reference Neupane, Lien and Hilberg 9 ) suggested that rather than having a formal job, peasants who more often live in mud/wooden houses were more likely to have a better vitamin D status in Nepal. This factor as a potential predictor of vitamin D status remains to be tested specifically in different population groups.
High s-25(OH)D concentrations among low socio-economic strata have previously been explained with reference to prolonged sun exposure due to outdoor occupation, as opposed to better household conditions and higher levels of education that may associated with restricted outdoor activities( Reference Puri, Marwaha and Agarwal 21 , Reference Atiq, Suria and Nizami 25 ). It seems for children of pre-school age in the present study that vitamin D status is not determined by socio-economic status because of similarly poor dietary supply of vitamin D and comparable exposure to sunlight across social strata.
In the literature, prolonged exclusive breast-feeding was reported as a risk factor for the development of hypovitaminosis D( Reference Misra, Pacaud and Petryk 26 , Reference Wagner and Greer 27 ). In our study, we tested whether prolonged breast-feeding was associated with vitamin D status. Surprisingly, we found that children who received breast milk in addition to traditional foods had better vitamin D status compared with those who did not. This difference was still significant when adjusted for age, household income, type of housing, daily time spent outdoors and Ca intake. This may be attributed not only to the nutritional component of breast-feeding but also to the practice itself. Given the fact that, in Nepalese culture, women breast-feed minimally clothed children outdoors and under the sun, we can assume that prolonged breast-feeding provides children with more opportunities for sun exposure. Also, younger children had significantly higher concentrations of s-25(OH)D than those in the older groups. Most of the children had poor growth and were malnourished; however, we did not find a significant difference in s-25(OH)D concentration when comparing by nutritional status of the children.
Ca intake calculated from milk and milk products was low (318 mg/d). During the data collection, it also was observed that children are quite often given cow or buffalo milk and homemade yoghurt, but the servings were very small.
Even though this finding coincides with similar studies conducted in the region, the reason for this high magnitude of poor vitamin D status remains unclear. In the literature, several factors have been postulated, such as skin pigmentation, traditionally poor diets with few natural sources of vitamin D, prolonged breast-feeding and unavailability of a national vitamin D supplementation programme( Reference Mithal, Wahl and Bonjour 1 , Reference Prentice 28 ).
Limitations and strengths of the study
The present study has several limitations. When selecting the population sample, randomization was applied. Due to the absence of demographic data in the region, we had to create our own databases from the vitamin A supplementation programme’s demographic data available at the local health post. Although officially reported coverage for Nepal’s national vitamin A supplementation programme for children aged 6–59 months was 90·4 % as of 2011( 12 ), it could be that those children who for some reason did not receive vitamin A supplements during last 6 months were not included in our databases and consequently excluded from selection potential for the current study. This kind of selection bias might suggest that our sample was more representative for those who have better access to health-care programmes than for the general population. Apparently, Ca intake was not calculated accurately, as we used reported regular consumption of milk products and therefore cannot rule out recall bias; also, we did not account for other sources of dietary Ca.
Despite these limitations, the study suggests a general pattern of vitamin D status among pre-school children in rural Nepal. Full sample size was achieved with a response rate of 87·5 %. The use of the DBS method is another strength and is a minimally invasive means of obtaining s-25(OH)D measurements, particularly in a rural setting without sample storage and transport possibilities. However, we should take into consideration that Nepal extends from less than 100 to 8850 m above sea level, which has an effect on vitamin D production throughout the whole year( Reference Holick 29 ). Therefore this result needs to be interpreted with respect to the study population and geographical coordinates.
Conclusion
We report a high prevalence of vitamin D deficiency among pre-school children in a rural area of Nepal at latitude 27·39°N. In our sample, sociodemographic factors did not affect the vitamin D status of children, but prolonged breast-feeding was associated with better vitamin D status. Further research is required to investigate the health consequences of low vitamin D status for this population.
Acknowledgements
Acknowledgements: The authors are grateful to their research assistant, Pratibha Khatri, and to all community health volunteers from the Nala Ugrachandi Village Development Committee for their cooperation. They thank all the families and children who participated in the study. Financial support: This study was funded by the University of Oslo and supported in part by Vitas AS, Oslo, Norway for serum analysis. The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: D.A. and S.P.N. contributed equally to this work in terms of the analysis, interpretation and manuscript writing. D.A., S.P.N. and A.A.M. designed the study. D.A. carried out the fieldwork. D.A. and S.P.N. carried out the analysis and drafted the manuscript. T.E.G. performed laboratory analysis. A.A.M., T.E.G., D.A. and S.P.N. contributed to the interpretation of data, as well as critical revision of the manuscript. All authors approved the final version. A.A.M. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Ethics of human subject participation: The study was approved by the Nepal Health Research Council and the Regional Committee for Medical and Health Research in Norway. Written or verbal, voluntary informed consent was obtained from all subjects/patients.






