INTRODUCTION
Recently, radiocarbon (14C) dating has become a valuable dating method in cultural heritage for technological and authentication studies (Van Strydonck et al. Reference Van Strydonck, De Moor and Benazeth2004; Richardin and Gandolfo Reference Richardin and Gandolfo2013; Caforio et al. Reference Caforio, Fedi, Mando, Minarelli, Peccenini, Pellicori, Petrucci, Schwartzbaum and Taccetti2014; Jull and Burr Reference Jull and Burr2014). In combination with material identification, 14C analysis enables a temporal classification of an artwork. Measured 14C ages can establish whether the dated material in the artwork is consistent with the activity period of the attributed artist. Hence, 14C may be a key method to resolve puzzles in artwork authentication such as items suspected to be forgeries (Caforio et al. Reference Caforio, Fedi, Mando, Minarelli, Peccenini, Pellicori, Petrucci, Schwartzbaum and Taccetti2014) or when reattributing artwork to its painter (Van Strydonck et al. Reference Van Strydonck, Masschelein-Kleiner, Alderliesten and de Jong1998). Continuous research in the field regarding the removal of restoration products on painting samples prior to 14C measurement (Fedi et al. Reference Fedi, Caforio, Liccioli, Mandò, Salvini and Taccetti2014; Liccioli et al. Reference Liccioli, Fedi, Carraresi and Mandò2016) or exploiting the particular feature of the so-called bomb peak to date contemporary art demonstrates the potential of the method (Fedi et al. Reference Fedi, Caforio, Mando, Petrucci and Taccetti2013; Petrucci et al. Reference Petrucci, Caforio, Fedi, Mando, Peccenini, Pellicori, Rylands, Schwartzbaum and Taccetti2016). One of the most critical issues in 14C dating is the sampling process, not only the sample size but also the chosen material. For instance, paintings may be varnished and this practice was commonly executed with natural resins, which tend to yellow upon aging. During restoration, the yellow varnish layer is replaced and thus no longer isochronous to the paint layer. Additionally the growing use of synthetic material in modern painting must also be considered, as the use of such compounds will significantly impact 14C dating. These hydrocarbons were formed millions of years ago and their 14C content has decayed long ago, hereby resulting in misleading older 14C ages. Hence sampling art objects requires a broad understanding of technological practices and their materials in order to treat the sample accordingly and to retrieve reliable and explicit 14C ages.
Commonly, 14C analyses are restricted to the artwork’s support material, such as the wooden frame, wooden panel, the canvas or paper, where sufficient material can be sampled. The results, however, may be subjected to discussion, as a possible later reuse of an older artwork’s support, either by the artist himself or a forger, cannot be excluded. Old and damaged canvases are often relined, hence presenting a mixture of old and younger material, rendering sampling tedious or even unfeasible. The frame may have been replaced through time and thus is no longer original source material. Sample selection is therefore crucial for reliable interpretation of the results. As mentioned by Fedi and co-workers, the solution remains in dating carbon from a short-lived organism, which cannot be reused (Fedi et al. Reference Fedi, Caforio, Mando, Petrucci and Taccetti2013). When considering artists’ materials, not only the support material may be carbon based but the binding media, pigments or additives may also bear carbon atoms and hence are potentially datable by 14C. If of natural origin the binding medium (oil, natural resin, plant gums, animal glues, etc.) is an ideal material for 14C dating, as it cannot be reused. When creating a forgery, it is conceivable that forgers could acquire older canvases and purposely avoid pigment anachronisms. By exposing the object to artificial aging, the appearance of centuries-old, dried oil paint could be mimicked, yet only the physical appearance of oil would be altered. The 14C content of the oil used, however, remains representative of the time of oil production regardless of the artificial aging.
The idea of dating linseed oil or other drying oils used as painting media was first proposed by Keisch and Miller in 1972 to detect post-1950 forgeries (Keisch and Miller Reference Keisch and Miller1972). Indeed, the testing of atmospheric nuclear weapons in early 1950 lead to an increase in production of 14C in the atmosphere, which almost doubled the natural atmospheric 14C concentration (Nydal and Lovseth Reference Nydal and Lovseth1965). Hence using different specimens of linseed oil, Keisch and Miller showed that the post-1950 14C signal made it possible to discriminate between oil paintings of the early 1950s and those produced in the 1960s based on the higher concentration of 14C. Their approach, however, has not been popularized as the application suffered from practical limitations such as sample size. Specifically, the study was conducted using the liquid scintillation counting method of the time to measure the radioactive decay of the sample. As a consequence as much as 100 mg of material was required.
The advent of accelerator mass spectrometery 40 years ago (Nelson et al. Reference Nelson, Korteling and Stott1977; Purser et al. Reference Purser, Liebert, Litherland, Beukens, Gove, Bennett, Clover and Sondheim1977) had a significant impact on sample size requirement as the amount of carbon needed for standard analysis was successfully reduced to as low as 1 mg. Such advances in 14C dating have made it possible to determine the age of the object of interest itself rather than the organic remains found in the same context. Rock art dating using 14C AMS has hence become a well-established method where the material of choice is charcoal. In the case where inorganic pigmented paints is found, the vehicle used to bind the pigments is dated (Li et al. Reference Li, Baker, DeRoo and Armitage2012; Baker and Armitage Reference Baker and Armitage2013). However, due to relatively large amounts of sample material required (>20 mg material; Bonneau et al. Reference Bonneau, Staff, Higham, Brock, Pearce and Mitchell2016), such methods have not been applied routinely for the analysis of modern and contemporary art.
The first milestone in dating the organic binder using 14C AMS was established by dating a paint sample taken from the artist’s palette (Hendriks et al. Reference Hendriks, Hajdas, McIntyre, Kuffner, Scherrer and Ferreira2016), as the recurrent presence of carbonates in the investigated paintings themselves was deemed problematic.
The present work aims to render the approach of dating the natural organic binder more broadly applicable. In principle, most of the common inorganic pigments do not influence the measurement. However, exceptions are carbonates, where the anions contain a carbon atom. Lead white, a basic lead carbonate, has been the most widely used white pigment for centuries. Furthermore, it has been admixed to many paint products with the objective of lightening the paint color. It has the added role of shortening the drying process of the oil paint. Carbonates such as calcite and dolomite have been and still are common filler materials. Due to these reasons, carbonates can be encountered in many paints. We thus investigated, in particular, the options of selectively removing carbonate additives prior to the dating of the binding medium. To validate the method, paint and canvas samples, were collected from a signed and dated painting from Franz Rederer (1899 Zurich–1965 Zurich). Prior to 14C analysis, a thorough and detailed characterization of all paint components was carried out, which required a multi-technique approach involving XRF, FT-IR and Raman analysis to identify the pigments present in the sample and the binder type, respectively. The paint identification leading to the selection of suitable pigments was carried out at the SIK-ISEA and at HKB on micro-samples for sample suitability assessment. All 14C measurements were conducted on the MICADAS instrument at ETH Zurich, where the possibility to measure minimal amounts of sample material was addressed by the possibility to graphitize small samples containing down to 100 µg C or directly measuring CO2 from the samples (Ruff et al. Reference Ruff, Wacker, Gaggeler, Suter, Synal and Szidat2007, Reference Ruff, Szidat, Gaggeler, Suter, Synal and Wacker2010).
CASE STUDY
An oil painting entitled Bildnis Margrit mit roter Jacke und Konzertkleid from Franz Rederer, a Swiss artist of the 20th century was chosen as subject for our study. The painting is a property of the SIK-ISEA and was inventoried in 2010. The painting delivered ideal source material for a case study due to its legitimate authorship and consequently a reliable signed date, unvarnished surface and no history of restoration since the time of its creation. Moreover the painting was signed by the artist with 1963 i.e., right at the beginning of the 14C bomb peak allowing conversion to precise calendar ages (outside the extensive 19/20th century “wiggle” on the 14C calibration curve). A canvas sample was collected in the excess material around the frame on the backside of the painting, while two paint samples were collected from the front and back of the painting, as displayed in Figure 1. A more detailed overview of the sampling is given in Figures 2 and 3.

Figure 1 Bildnis Margrit mit roter Jacke und Konzertkleid, Franz Rederer, 1962, oil on canvas, 100×140 cm, SIK-ISEA, Zurich. Photograph: SIK-ISEA (Philipp Hitz). The three sampling locations are marked (in red in the online version).

Figure 2 Sampling of the green paint P05 on the rear of the painting (→48.6 cm, ↑25.2 cm). On the left, after sample collection the respective damage can be observed. The used scale represents 5 mm. On the right, the collected sample weighed 8.5 mg. (See online version for color.)
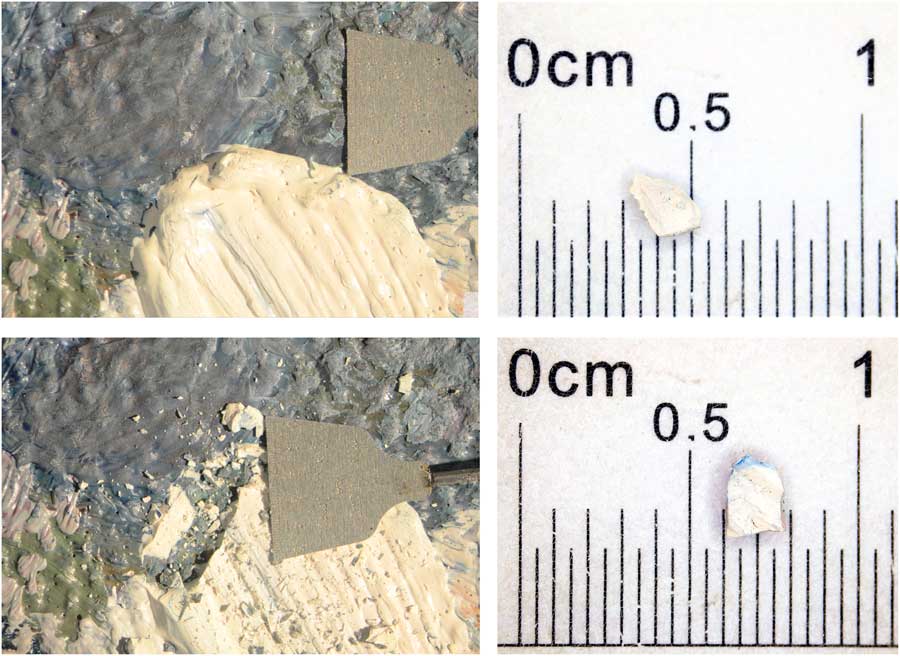
Figure 3 Sampling of the white paint P04. The sample was taken at 33.5 cm from the left and 111.0 cm from the bottom of the painting. The pictures on the left depict the sampling process, before (top) and after (bottom) sample collection. On the right the actual sample size of both sub-samples ETH-69023 1.1 and 2.1, which weighed 2.6 and 4.9 mg respectively before carbonate removal and 14C analysis. The used microscale in both pictures on the left is 5 mm wide.
EXPERIMENTAL
Spectroscopic Analysis
As already established in the pilot study (Hendriks et al. Reference Hendriks, Hajdas, McIntyre, Kuffner, Scherrer and Ferreira2016), the paint sample locations were selected after identification of the binder type and pigments, based on a multi-technique approach, combining optical light microscopy, X-ray fluorescence (XRF), Fourier transform infrared (FT-IR) and Raman spectroscopy. Ideal sampling locations were defined as paint areas containing no other carbon sources than the natural organic binder, therefore inorganic pigments are paint samples of choice. When organic pigments are present, a mixed signal combining the oil and pigments will be measured, which prevents straightforward data interpretation. Depending on the source, different cases may be observed: when extracted from a plant the pigment might carry the 14C signature of the atmosphere, its age may be synchronous to the age of the natural organic binder or somewhat older hereby potentially causing a small offset. On the other hand, synthetically manufactured pigments will afford misleading older ages. In the late 19th century, the so-called aniline-dyes and coal-tar-based synthetic products have come into use. Since these are derivatives of a geologic source material, they strongly distort the age determination. Their separation from the sample material on a macroscopic level is difficult to achieve and thus their presence must be ruled out by spectroscopic analysis. Furthermore, petroleum-derived waxes like paraffin used as additives in modern oil paint must also be excluded.
Microscopic observations were conducted on an AXIO light microscope mounted on a mechanical arm (Zeiss, Thornwood, NY, USA) and equipped with a digital camera (Nikon, Tokyo, Japan).
The elemental composition was determined at the SIK-ISEA directly on the painting by XRF using a Bruker AXS ARTAX 800 system equipped with an Rh target and polycapillary lens (excitation spot\100 µm). Used settings were: generator voltage 50 kV, current 600 µA, Helium atmosphere, and acquisition time 100 s (Bruker, Karlsruhe, Germany).
Raman and FT-IR spectroscopy analyses were undertaken at the art technological laboratory, University of Applied Sciences, Bern. The FT-IR spectra were acquired on a Perkin Elmer system 2000 (Perkin Elmer, MA, USA) in transmission mode within a spectral range of 4000–580 cm–1 and accumulation of 32 scans per spectrum at a resolution of 4 cm–1. Samples were prepared on a CVD diamond window.
Raman spectroscopy was performed on a Renishaw InVia dispersive Raman system. The instrument was equipped with a Leica DM microscope and a 785 nm (diode-type), Renishaw HP NIR785 (300 mW) laser source (Renishaw, Gloucestershire, UK). The spectra were recorded using a laser power of 0.01–1 mW on sample, microscope objectives of 50×(NA 0.55) and 100×(NA 0.90) magnification, and a measurement time between 30 and 200 s.
The evaluation and interpretation of the spectra was based on HKB spectral reference databases as well as published data (Bell et al. Reference Bell, Clark and Gibbs1997; Burgio and Clark Reference Burgio and Clark2001; Scherrer et al. Reference Scherrer, Zumbuehl, Delavy, Fritsch and Kuehnen2009).
Sample Preparation
The sampled canvas was cleaned by Soxhlet. (Bruhn et al. Reference Bruhn, Duhr, Grootes, Mintrop and Nadeau2001), which included successive extractions in solvents under reflux, namely hexane, acetone, and ethanol for 1 hr each, in order to remove potential contaminants such as oils, fats, and waxes. After drying the sample was cleaned following conventional acid-base-acid (ABA) procedures (Hajdas et al. Reference Hajdas, Bonani, Thut, Leone, Pfenninger and Maden2004; Hajdas Reference Hajdas2008).
Before treating the samples collected from the paintings, preliminary studies were conducted on some prepared trial paint of known composition (Umbra 40710, linseed oil 73054, Kremer Pigmente GmbH & Co, Aichstetten/Allgäu, Germany, purchased in 2005). An inorganic pigment, umber, which is a mixture of iron and manganese oxide, being carbon free was chosen as pigment for the dating analysis of the oil. The standard ABA procedure (Hajdas et al. Reference Hajdas, Bonani, Thut, Leone, Pfenninger and Maden2004) could not be applied since the alkali solution may hydrolyze and solubilize the oil, hereby leading to undesired saponification reactions. The umber trial paint was hence treated with 0.5 M HCl for 10 min at 65°C, then rinsed with Milli-Q® water. Due to the systematic presence of carbonates within the painting of Rederer, a batch of umber trial paint mixed with calcium carbonate (champagne chalk, Kremer, Aichstetten/Allgäu, Germany) was also prepared in order to investigate the possibility of removing carbonates mixed within the paint samples. Two procedures were compared: simple treatment with 0.5 M HCl at 65°C, in contrast to the same acid treatment at 80°C in the shaker for different time durations from 15 min to 5 hr, followed by measurement as graphite targets. The paint samples from Rederer’s painting were cleaned by a single warm acid wash with HCl 0.5 M at 80°C in the shaker for 1–4 hr, depending on sample size, then rinsed with Milli-Q® water.
Cleaned and dried samples were transferred into tin capsules (4×4×11mm, Elementar, Germany) and graphitized for AMS 14C measurement using the fully automated graphitization unit AGE (Wacker et al. Reference Wacker, Nemec and Bourquin2010b).
Radiocarbon Measurement by AMS
All 14C measurements discussed in this study were carried out on the Mini Carbon Dating System MICADAS at the Physics Department of ETH (Synal et al. Reference Synal, Stocker and Suter2007). To correct for background and isotopic fractionation, NBS oxalic acid II standards (Standard Reference Materials, Gaithersburg, USA) and phthalic anhydride blanks (Sigma-Aldrich, Buchs, Switzerland) were also measured. Data evaluation was performed using the AMS data reduction program BATS (Wacker et al. Reference Wacker, Christl and Synal2010a). Calendar ages were determined using the OxCal v.4.2.4 software (Bronk Ramsey Reference Bronk Ramsey2008, Reference Bronk Ramsey2009) and the post-bomb atmospheric NH1 calibration curve (Bomb13NH1) (Hua et al. Reference Hua, Barbetti and Rakowski2013; Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatte, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013). The resulting calibrated time intervals correspond to 95.4% probability and are expressed in years cal AD.
RESULTS AND DISCUSSION
Spectroscopic Analysis Results
The dating of the binding element required careful pigment examination, as only paint prepared with carbon-free pigments provide reliable 14C analysis of the oil. The results from the multi-technique approach, combining XRF, FT-IR and Raman spectroscopy are displayed in Table 1. Both chosen paint samples P04 white and P05 green contain a mixture of different white pigments, zinc, lead, and titanium white. Information regarding the binder was gained by FTIR spectroscopy, where an oil component was identified. The green pigment owes its color to ultramarine, a synthetic inorganic blue and trace amounts of a synthetic organic green PG7. This green pigment is a so-called phthalocyanine green a copper (II) complex with chlorinated phthalocyanine, which contains 35–37 wt% C depending on the extent of chlorine substitution. As mentioned above, the presence of organic pigments may interfere with the dating of the binder, especially if it is of synthetic origin. In this particular case, the dating of the binder was nevertheless pursued as only trace amounts of the organic pigment were identified. The expected 14C post-1950 signal of the oil production would either obscure the PG7 signal, or if not allow to detect the potential contamination caused by PG7 14C-free carbon.
Table 1 Combined results from the multi-technique approach to assess the paint composition of samples P04 white and P05 green from the investigated painting Bildnis Margrit mit roter Jacke und Konzertkleid by Franz Rederer.

Carbonate-Removal Efficiency
As previously reported (Hendriks et al. Reference Hendriks, Hajdas, McIntyre, Kuffner, Scherrer and Ferreira2016), the pervasive presence of carbonates within the painting of Rederer prevented the dating of the organic binder. To circumvent this limitation a method to remove the carbonates in the paint samples and hence overcome the problem of misleading older 14C ages was developed. Preliminary studies were conducted on prepared trial paint with calcium carbonates to evaluate the feasibility of removing carbonates prior to 14C measurements. The paint used was a mixture of umber and champagne chalk (CaCO3), which even in trace amounts will afford erroneous older 14C ages. The mockup samples were thus treated with hydrochloric acid in excess, hereby forming a calcium chloride salt, water and carbon dioxide as displayed in Equation 1.
The measured 14C concentration in the samples, if carbonate free, is expected to match the value obtained by dating the umber trial paint (F14C=1.070 ± 0.003) as the same raw materials were used (Hendriks et al. Reference Hendriks, Hajdas, McIntyre, Kuffner, Scherrer and Ferreira2016). As expected, samples still containing chalk (F14C=0) yield lower F14C values than the reference measurement (see Figure 4). The remaining carbonate contamination is strongly dependant on the treatment duration. The temperature seems to play a minor role, although the treatment at 80°C seems to speed up the process a little. As evident from Figure 4 the treatment for 15 min to 1 hr, regardless of the treatment temperature, is insufficient to remove all carbonates. In strong contrast, samples treated for more than 90 min with HCl provide carbonate free samples as the measured 14C ages match the expected value within the 1σ error. Hence by exposing carbonate containing paint pigments to acid for several hours, the problematic carbonates are successfully removed and the natural organic binder can be dated. This study was conducted on samples weighing several milligrams, hence when dealing with samples in the microgram range adjusting the reaction time to 1 hr is worth considering. The samples from Rederer’s painting were thus treated with 0.5 M hydrochloric acid at 80°C in the shaker, for 1–4 hr, depending on sample size.
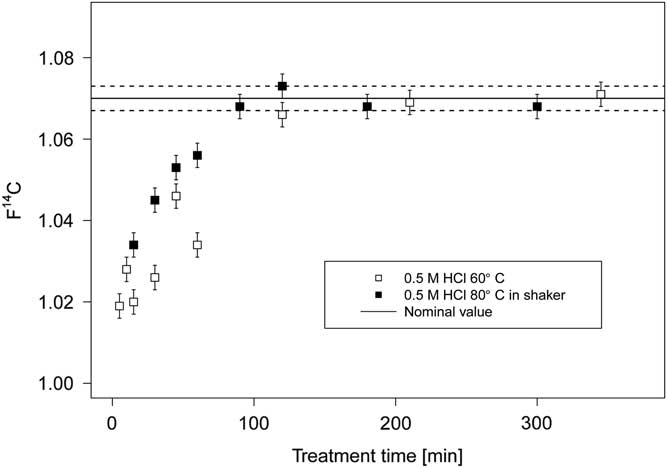
Figure 4 Carbonate removal efficiency in paint samples with respect to the treatment time. The nominal value represents the measured F14C in the umber trial paint, which is carbonate free and serves as reference material in order to assess the efficiency of the carbonate removal study.
Carbonates may, however, be found in a variety of forms in paint samples: calcium carbonates are often used as an extender, while lead carbonate is a white pigment and copper carbonate, also known as malachite is a green pigment. The proposed carbonate removal procedure should be applicable to all carbonate types regardless of the cation. Indeed when treated with acid, carbonates will react to form the corresponding metal salt under formation of water and carbon dioxide as displayed in Equation 2. In our particular case, the metal is lead and is converted to lead chloride and no extraneous C other than the organic binder remains (see Equation 3). The water is removed by drying the sample in the oven and the produced CO2 is formed as gas, hence none of the reaction products interfere with the 14C dating of the binder. The neutral form of lead carbonate, as described in Equation 3, is seldom found in paint samples. However, its basic form is much more common, which also reacts with acid to form a lead chloride salt as displayed in Equation 4.
In the case of Rederer’s painting, the basic form of lead carbonate was identified and was expected to react following Equation 4 upon acid treatment. The production of lead chloride in the paint samples was barely visible due to the small quantities of material, although in some cases the formation of white needles was witnessed. Using a spectro Arcos ICP-OES instrument (SPECTRO, Kleeve, Germany) the salt composition was measured as a mixture of Pb and Cl. In order to confirm the reaction displayed in Equation 4, a test sample of basic lead carbonate (2PbCO3·Pb(OH)2), provided by the SIK-ISEA, was treated with HCl in excess. From this test sample the relative ratio of Cl to Pb was determined using selected spectral lines Cl 134.724 and Pb 220.353. For calibration, mixed standards solutions were prepared from 1000 ppm stock solution of Cl (CPI International Peak Performance, Amsterdam, Netherlands) and Pb (Inorganic Ventures Christiansburg, Virginia, USA) by dilution with a solution of 1% nitric acid. A calibration curve from blank to 100 mg/kg using 3 standards was established. The resulting white crystals from the test sample were diluted by a factor of 2000 to reach a final concentration of 400 ppm. The measured concentration of Cl and Pb, respectively 19.5 ppm and 56.8 ppm, resulted in a Cl to Pb ratio of 2:1. This result indicates that the basic lead carbonate 2PbCO3·Pb(OH)2 does indeed react to form PbCl2 after treatment with hydrochloric acid and hence does no longer affect the dating of the binder.
14C Measurements on Canvas and Binder from Rederer’s Painting
Radiocarbon data of the canvas and both paint samples, carbonate-free after the acid treatment, are summarized in Table 2 and illustrated in Figure 5, where the calibrated ages are compared to the painter’s time of activity. All F14C values are larger than 1, thus indicating that the dated samples are modern, namely post-1950.
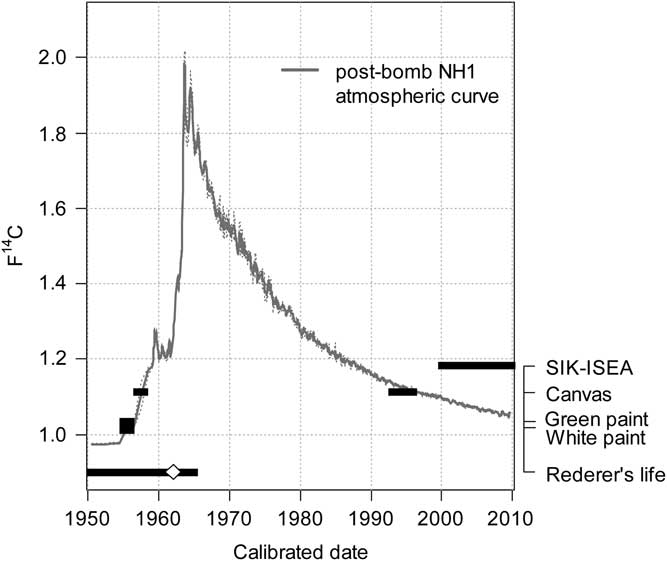
Figure 5 Comparison of calibrated age interval of the canvas and two paint samples against the time period of the painter’s activity and when the painting became property of the SIK-ISEA. The white marker indicates the signed date of the painting. Also displayed is the post-bomb atmospheric NH1 curve, which represents the yearly fraction modern F14C concentration.
Table 2 Sample label and code, respective starting sample size before sample treatment, resulting carbon mass available for measurement, measured fraction modern concentration and uncertainty, respective calibrated age range.

When comparing the 14C data gained from the canvas and the two paint samples, the fraction modern F14C of all samples is in agreement within the 1σ interval. Due to the particular feature of the 14C bomb peak, after calibration, narrow time ranges are derived. In the case of the canvas alone, two time intervals were dated 1957–1958 and 1993–1996. The first time period is in line with the signed date, while the later period occurs after the artist’s death. In the present case no issue of authenticity is to be raised as the painting has a solid provenance history, hence the later time period is not applicable.
The natural organic binder, in this case linseed oil, was dated using two paint samples. From the white paint sample two sub-samples were prepared and measured, both affording the same results (see Table 2). The conversion to calendar ages yields a single time interval 1955–1956, thus leaving no doubt to the painting’s authenticity. One can conclude that the proposed method for the removal of carbonates from paint sample offers an objective mean of dating the natural organic binder by 14C AMS. As in the spectroscopic analysis results section, the green paint sample taken from the rear of the canvas mainly composed of lead white, i.e. carbonates, also contained traces of PG7, a synthetic organic pigment. The presence of this organic pigment was debated as being problematic as this second source of carbon other than the binder will interfere with the 14C analysis. Gratifyingly, the measured 14C concentration in the green paint is consistent with the measured white paint. In addition the calendar dates cover the exact same time period. This result can be explained by several factors. The first being the particular feature of the bomb peak signal, where the strong increase of atmospheric 14C caused by the nuclear weapon testing was transferred to all living organisms of the 1960s. The high levels of atmospheric 14C of the bomb peak are hereby reflected in the linseed oil. The ratio of the PG7 to the oil content is also to be considered, that is the ratio of contaminant mass to the total mass of carbon. Since only trace amounts of PG7 were detected, the carbon contribution of the linseed oil, namely 400 µg C, was sufficient to supress the PG7 signal. However when dealing with much smaller sample size, as for instance 20 µg C, and due to the heterogeneous spatial distribution of the pigment particles within the paint, the PG7 could possibly have an effect on the 14C content (the ratio of contaminant mass to the total carbon amount might be higher).
The signed date is 1962 and hence both the canvas and binder predate the painting by a few years. This offset can be explained by taking into account the time necessary for either the canvas manufacturing or oil production as well as due to trade, storage in trade and storage in the atelier. As displayed in Figure 5, one can conclude that all materials used in the production of the painting originate from the time of the painter’s activity. Thus, the possibility of dating new material other than the artwork’s support has been demonstrated.
It is imperative to highlight that when dealing with cultural heritage objects, sampling is often very limited. In the present study, the white paint that was taken from the front of the painting amounts to 7.5 mg initial material, while the green paint collected on the backside of the painting weighed about 8 mg. After carbonate removal, the paint samples contained less than 20% of carbon. Assessing a lower limit for sampling is not an easy task, as each paint composition is different. The sampling amount depends on the selected paint location, which elements make the paint composition, the contribution of heavy metals and in which proportions are the pigments mixed with the binder. Therefore, the initial assumption of less than 20% of C content for any paint sample appears an adequate starting point when considering sampling. The possibility to reduce sample sizes to the microgram range will be subjected to our future work, especially as the presented data herein showcases the feasibility of the method and emphasizes its potential to become a standard method for oil painting dating. Nevertheless, each case study would have to be carefully considered as the precision presented here requires at least 200 µg of carbon (see Table 2).
Note that the work presented in the context of this manuscript is a feasibility study, carried out on a next-to-ideal sample. The painting is exceptional in regards to being neither varnished nor having been restored. A later intervention (restoration of painting) by addition of a more modern varnish or a more modern consolidant may cause problems in the dating process. For such cases, compound specific dating may be more appropriate and will be investigated in the future.
CONCLUSION
The possibility of dating other materials than those in support of a work of art has been exemplified and is applicable, as long as a suitable sample zone can be identified. This relies on the efficiency of the multi-technique approach combining XRF, FTIR and Raman spectroscopy. Inorganic pigments are paint samples of choice, as they are not primarily carbon based. White paint, for instance, is ideal as it is often based on lead, titanium or zinc white and hence is an excellent candidate for further 14C analysis. The presence of extraneous carbon content within the lead white may compete with the dating of the binding medium, but can be removed by chemical treatment. In an adjusted acid step with hydrochloric acid, the lead carbonate is converted to lead chloride and hence rendered suitable for further 14C measurements. Dating the binder allows to make sense of the canvas ages subjected to discussion regarding the possible reuse of old canvases. The age obtained from the oil has a high probability of being representative of the time of creation and hence is less questionable than the support material.
The proposed method will encounter major difficulties in producing reliable age of the binder when considering varnished and or retouched paintings. Where there is later addition of new varnish layers or consolidation lining materials, additional carbon sources other than the binder are present and each part will contribute to the 14C content of the sample. Thus in such cases the proposed method would require extensive pre-cleaning of the varnish layer and removal of the consolidation material. Additionally, one must bear in mind that the painting techniques have evolved over time and hence old and modern paintings present different challenges in dating. Old paintings typically do not originally include any synthetic material but may show multiple layers of varnish. These generate condensates or oxidized products through the aging process, thus rendering the separation of these layers tedious. Modern paintings must also be treated with caution as the advent of industrialization metamorphosed the artist’s material supply. Products, which used to be extracted from natural sources, become synthetically manufactured using 14C-free, petroleum-derived sources.
It is, however, worth keeping in mind that 14C analysis dates the time of death of the organic material, i.e., the plant used to make the canvas or pressed to oil for the binder, not the time of painting, which is indisputably the event of interest. 14C dating cannot authenticate the authorship of a work of art, yet it can allow temporal classification, which is a crucial piece of information in authentication processes.
ACKNOWLEDGMENTS
The authors thank the SIK-ISEA for granting access to their in-house collection of paintings, providing an ideal case study and allowing for sampling. The support of the HKB for the pigment analysis is gratefully acknowledged. Both Maria Belen Röttig and Mantana Maurer are thanked for their support in the lab. We also thank Beat Aeschlimann for the ICP-OES measurements. Funding was supported by an ETH Research Grant ETH-21 15-1.









