INTRODUCTION
Identifying and obtaining suitable natural materials for use as radiocarbon (14C) standards to provide quality assurance for radiocarbon measurements is of primary importance (Bryant et al. Reference Bryant, Carmi, Cook, Gulliksen, Harkness, Heinemeier, McGee, Naysmith, Possnert, Scott, van der Plicht and van Strydonck2000; Scott et al. Reference Scott, Boaretto, Bryant, Cook, Gulliksen, Harkness, Heinemeier, McGee, Naysmith, Possnert, van der Plicht and van Strydonck2004). Radiocarbon standards should be analyzed alongside every batch of samples and should follow the same pretreatment procedures to assess any contamination arising from the laboratory protocol. Creating radiocarbon standards involves finding materials of a similar nature to the samples, which are homogeneous and available in sufficient quantities to enable their long-term use by the radiocarbon community (Bryant et al. Reference Bryant, Carmi, Cook, Gulliksen, Harkness, Heinemeier, McGee, Naysmith, Possnert, Scott, van der Plicht and van Strydonck2000; Boaretto et al. Reference Boaretto, Bryant, Carmi, Cook, Gulliksen, Harkness, Heinemeier, McClure, McGee, Naysmith, Possnert, Scott, van der Plicht and van Strydonck2002; Scott et al. Reference Scott, Boaretto, Bryant, Cook, Gulliksen, Harkness, Heinemeier, McGee, Naysmith, Possnert, van der Plicht and van Strydonck2004). Robust consensus values of new radiocarbon standards are evaluated by their radiocarbon dating in inter-comparison exercises (Rozanski et al. Reference Rozanski, Stichler, Gonfiantini, Scott, Beukens, Kromer and van der Plicht1992; Bryant et al. Reference Bryant, Carmi, Cook, Gulliksen, Harkness, Heinemeier, McGee, Naysmith, Possnert, Scott, van der Plicht and van Strydonck2000; Scott et al. Reference Scott, Boaretto, Bryant, Cook, Gulliksen, Harkness, Heinemeier, McGee, Naysmith, Possnert, van der Plicht and van Strydonck2004). Inter-laboratory comparisons on standard materials are also important to aid radiocarbon laboratories in detecting possible biases (offsets) in their 14C measurements (Rozanski et al. Reference Rozanski, Stichler, Gonfiantini, Scott, Beukens, Kromer and van der Plicht1992; Bryant et al. Reference Bryant, Carmi, Cook, Gulliksen, Harkness, Heinemeier, McGee, Naysmith, Possnert, Scott, van der Plicht and van Strydonck2000; Scott et al. Reference Scott, Boaretto, Bryant, Cook, Gulliksen, Harkness, Heinemeier, McGee, Naysmith, Possnert, van der Plicht and van Strydonck2004).
At the University of Bristol, we recently developed a new method for radiocarbon dating potsherds from their absorbed lipid residues (Casanova et al. Reference Casanova, Knowles, Williams, Crump and Evershed2017, Reference Casanova, Knowles, Williams, Crump and Evershed2018, Reference Casanova, Knowles, Bayliss, Dunne, Barański, Denaire, Lefranc, di Lernia, Roffet-Salque, Smyth, Barclay, Gillard, Claßen, Coles, Illet, Jeunesse, Krueger, Marciniak, Minnitt, Rotunno, van de Velde, van Wijk, Cotton, Daykin and Evershed2020a). This method uses a compound-specific radiocarbon analysis (CSRA) approach based on commonly recovered food residues: C16:0 and C18:0 fatty acids (FAs), characteristic of degraded animal fats (Casanova et al. Reference Casanova, Knowles, Williams, Crump and Evershed2018). The pretreatment procedure initially involves the simultaneous extraction of FAs (and other lipids) from the clay matrix and their methylation into fatty acid methyl esters (FAMEs) using a methanolic/sulphuric acid solution. This is followed by the isolation of individual FAME compounds using preparative capillary gas chromatography (pcGC). These isolated FAMEs are then graphitised and 14C dates determined by accelerator mass spectrometry (AMS). These dates are subsequently corrected for the presence of the methyl C introduced during methylation using a mass balance approach (Casanova et al. Reference Casanova, Knowles, Williams, Crump and Evershed2018, Reference Casanova, Knowles, Bayliss, Dunne, Barański, Denaire, Lefranc, di Lernia, Roffet-Salque, Smyth, Barclay, Gillard, Claßen, Coles, Illet, Jeunesse, Krueger, Marciniak, Minnitt, Rotunno, van de Velde, van Wijk, Cotton, Daykin and Evershed2020a). To our knowledge, all commercially available fatty acid standards contain “modern” levels of 14C, and certainly none of the extensive collection we have measured has shown otherwise. Therefore, in the absence of commercially available FAME standards of background and intermediate ages, our work developing and refining our compound-specific lipid dating technique was performed using a solution of modern C16:0 and C18:0 FAMEs and was further validated using archaeological animal fats from archaeological bog butter finds spanning ca. 3000 years (Casanova et al. Reference Casanova, Knowles, Williams, Crump and Evershed2017, Reference Casanova, Knowles, Williams, Crump and Evershed2018). Our first radiocarbon dates of pottery vessels from well-dated sites/cultural phases proved to be entirely compatible with site chronologies and radiocarbon dates on conventional materials (Casanova et al. Reference Casanova, Knowles, Bayliss, Dunne, Barański, Denaire, Lefranc, di Lernia, Roffet-Salque, Smyth, Barclay, Gillard, Claßen, Coles, Illet, Jeunesse, Krueger, Marciniak, Minnitt, Rotunno, van de Velde, van Wijk, Cotton, Daykin and Evershed2020a). We then applied the new CSRA method to the dating of sites lacking conventionally dateable materials (Casanova et al. Reference Casanova, Knowles, Bayliss, Dunne, Barański, Denaire, Lefranc, di Lernia, Roffet-Salque, Smyth, Barclay, Gillard, Claßen, Coles, Illet, Jeunesse, Krueger, Marciniak, Minnitt, Rotunno, van de Velde, van Wijk, Cotton, Daykin and Evershed2020a; Stojanovski et al. Reference Stojanovski, Roffet-Salque, Casanova, Knowles, Oosterbeek, Evershed, Cruz, Thissen and Arzarello2020a), to verify the antiquity of lipids within pots (Dunne et al. Reference Dunne, Grillo, Casanova, Whelton and Evershed2019; Fewlass et al. Reference Fewlass, Mitchell, Casanova and Cramp2020), to directly date the use of specific food products (ruminant dairy and adipose fats) (Casanova et al. Reference Casanova, Knowles, Bayliss, Dunne, Barański, Denaire, Lefranc, di Lernia, Roffet-Salque, Smyth, Barclay, Gillard, Claßen, Coles, Illet, Jeunesse, Krueger, Marciniak, Minnitt, Rotunno, van de Velde, van Wijk, Cotton, Daykin and Evershed2020a, Reference Casanova, Arbogast, Denaire, Jeunesse, Lefranc and Evershed2020b; Stojanovski et al. Reference Stojanovski, Živaljević, Dimitrijević, Dunne, Evershed, Balasse, Dowle, Hendy, McGrath, Fischer, Speller, Jovanović, Casanova, Knowles, Balj, Naumov, Putica, Starović and Stefanović2020b) and to evaluate marine reservoir effects arising from marine product processing in pottery vessels (Casanova et al. Reference Casanova, Knowles, Ford, Cramp, Sharples and Evershed2020c).
No archaeological pottery vessel would, however, be suitable for use as a radiocarbon standard due to the amount of material needed for this purpose as the analyses are destructive in nature. In addition, the vessel would need to be extremely rich in lipids, with a homogeneous concentration and partitioning of lipid in the clay matrix. These, however, are known to be highly heterogenous and variable, depending on the function of the vessel and levels to which it was filled (Charters et al. Reference Charters, Evershed, Goad, Leyden, Blinkhorn and Denham1993). Up to now, we have been using pseudo-processing standards obtained by performing an injection of solvent solely (n-hexane) rather than FAME solutions, with trapping sequences mimicking the isolation of the two FAMEs in order to isolate all exogenous C that would be co-isolated with trapped compounds. The glass wool containing this exogenous C is transferred along with either IAEA-C7 (approaching the age of archaeological pottery vessels) or a radiocarbon “dead” blank (phthalic anhydride) into an Al capsule before combustion and graphitization (Casanova et al. Reference Casanova, Knowles, Williams, Crump and Evershed2017, Reference Casanova, Knowles, Williams, Crump and Evershed2018). Additionally, a procedural blank is performed alongside every batch of pot lipid extraction and analyzed by GC to ensure no FA contamination occurred in pot lipid extracts before the pcGC step. The pcGC isolation ensures that any contamination (with the exception of FA contamination) from pretreatment processes is completely removed. Therefore, the potential for the introduction of exogenous C only begins with the isolation of individual FAs in the pcGC. It is of no consequence, therefore, whether or not a processing standard for assessing the accuracy of radiocarbon dates on pot lipids consists of FAs contained within a potsherd.
The archaeological fats known as bog butter that we previously used for the method validation (Casanova et al. Reference Casanova, Knowles, Williams, Crump and Evershed2018) are commonly recovered from peat bogs in Ireland and Scotland (Earwood Reference Earwood1997; Synnott and Downey Reference Synnott and Downey2004; Synnott Reference Synnott2010, Reference Synnott2014). Their characteristic lipid profile is that of degraded animal fats with high abundances of free C14:0, C16:0 and C18:0 FAs and low abundances of hydroxy fatty acids (Evershed et al. Reference Evershed, Dudd, Copley, Berstan, Stott, Mottram, Buckley and Crossman2002; Berstan et al. Reference Berstan, Dudd, Copley, Morgan, Quye and Evershed2004; Cronin et al. Reference Cronin, Downey, Synnott, McSweeney, Kelly, Cahill, Ross and Stanton2007; Smyth et al. Reference Smyth, Berstan, Casanova, McCormick, Mulhall, Sikora, Synnott and Evershed2019). While the origin of most of the Irish specimens analyzed was securely identified as ruminant dairy fats (Smyth et al. Reference Smyth, Berstan, Casanova, McCormick, Mulhall, Sikora, Synnott and Evershed2019), probably originating from cattle milk (Mattiangeli et al. Reference Mattiangeli, Cooke, Ó’Maoldúin, Sikora, Mulhall, Bradley and Teasdale2020), Scottish examples have both a ruminant dairy and ruminant adipose origin (Berstan et al. Reference Berstan, Dudd, Copley, Morgan, Quye and Evershed2004). Approximately 500 finds of bog butter are on record as having been recovered from peat bogs in Ireland. With around 50 radiocarbon dates previously determined for these, they range in date from the Bronze Age to the post-Medieval era (Downey et al. Reference Downey, Synnott, Kelly and Stanton2006; Synnott and Sikora Reference Synnott and Sikora2018; Smyth et al. Reference Smyth, Berstan, Casanova, McCormick, Mulhall, Sikora, Synnott and Evershed2019). Some bog butter specimens were dated using both bulk and compound-specific determinations and statistically indistinguishable results were obtained using both approaches (Casanova et al. Reference Casanova, Knowles, Williams, Crump and Evershed2018). These archaeological and historical bog butter finds therefore represent ideal materials from which to generate new radiocarbon standards due to their nature, age and size. These materials would be particularly suited for CSRA of FAs, extracted from archaeological, historical or environmental matrices.
We present herein (i) the selection process used to identify two bog butter finds to be prepared as radiocarbon processing standards, (ii) their cleaning, (iii) their homogenization, and finally, (iv) their homogeneity assessment by lipid composition, stable carbon isotope and radiocarbon analyses.
MATERIALS AND METHODS
Sampling
Following a rigorous selection process by the National Museum of Ireland (NMI), nine samples of bog butter were selected to assess their suitability for use as standards. In order to determine this, samples ranging in size from 20 to 50 mg were taken from beneath the surface of the bog butter mass. This was carried out under sterile conditions using a clean scalpel and the samples were subsequently stored in glass vials. Sampling and handling of the bog butter samples was performed using nitrile gloves to prevent contamination with skin lipids. Solvent washed scalpels and tweezers were used at all times. All sampling and handling of the bog butter finds was carried out under the supervision of and with licence from the National Museum of Ireland (Licences to Alter and Export Nos 6630; 6717; Licence to Alter No 6669). Following the selection of the two bog butter specimens to be used as the standards (see results section), between 400–500 g of each was sampled using solvent washed scalpels and tweezers. These were stored in covered aluminium trays with fitted lids then put in sealed plastic bags to prevent any contamination during sampling and transfer to Bristol.
Cleaning and Homogenization of the Bog Butter Standards
All steps were performed using gloves, in a fume hood and all surfaces covered with clean aluminium foil. Cleaning and homogenization methods were tested on 1–2 g of the bog butter specimens before being performed on the remaining bulk samples. The bog butter specimens were freeze-dried for 72 hr to remove residual water and were then separated into sub-samples of approximately 40 g, which were transferred to large culture tubes (i.d. 5 cm). These sub-samples were then melted at 70–75oC on a heating block and then centrifuged (3000 rpm, 5 min) while molten to achieve separation of fats from visible contaminants (denser than the fats). The melting and centrifuging process was repeated until all the fat (pale yellow colored oil melt) separated from the contaminants (black colored particulates). Once cooled, the fats and contaminants were removed from the tubes using a spatula and physically separated using a scalpel. The contaminants with adhering fat were re-melted and re-centrifuged until no more separation of particulates from the fats was achievable. The various particle-free bog butter samples were then combined and melted in a large beaker on a hot plate at 70–75oC and stirred with a glass agitator to ensure homogeneity. The liquid fats from the two bog butter specimens were poured into separate trays covered with aluminium foil (to < 0.5 cm thickness) and allowed to cool. Once solidified, the bog butter samples were sliced into 8 pieces of around 5 × 8 cm for storage. Any loose material which remained in the beaker and tray was collected and transferred to glass vials for storage.
Lipid Biomarker Analyses
Lipid extraction and methylation were performed on ˜2 mg of the bog butter samples, following established procedures (Correa-Ascencio and Evershed Reference Correa-Ascencio and Evershed2014). Bog butter samples were treated with H2SO4/MeOH (4%, v/v, 5 mL, 70oC, 1 hr). Ultrapure (MilliQ™) water (2 mL) was added and lipids were extracted using n-hexane (4 × 3 mL). After mixing, the n-hexane fractions were transferred to a 3.5 mL vial and blown down under a gentle stream of N2. An aliquot (1/4) of the total lipid extract (TLE) was blown down and diluted using an appropriate amount of n-hexane before analyses by gas chromatography (GC), GC-mass spectrometry (GC-MS) and GC-combustion-Isotope ratio mass spectrometry (GC-C-IRMS).
GC analyses were performed on an Agilent Technologies 7890A instrument fitted with a flame ionization detector. TLEs (1 µL) were injected into a non-polar column (DB 1 stationary phase, 15 m × 0.32 mm i.d., 0.1 μm thickness, Agilent Technologies) at a constant flow (He, 10 mL.min–1). The temperature program started at 50ºC for 2 min then increased to 350ºC at a rate of 10ºC.min–1 and held for 10 min. GC-MS analyses were performed on a Finnigan Trace GC coupled with a Finnigan Trace MS quadrupole. TLEs (1 µL) were injected into a non-polar column (DB 1 stationary phase, 50 m × 0.32 mm i.d., 0.17 µm thickness, Agilent Technologies). The temperature program was identical to GC analyses, but with a maximum temperature of 300ºC. The MS used an electron ionization (EI) mode operating at 70eV. The total ion current (TIC) was acquired over the range m/z 50-650 Da. GC-C-IRMS analyses were performed on an Agilent technologies 7890A coupled with an IsoPrime GC5 combustion interface and an IsoPrime 100 MS. TLEs were injected in a similar column and using a similar temperature program as for GC-MS, but with a starting temperature of 40ºC. The MS used an EI mode operating at 70eV and had three Faraday cups collecting masses m/z 44, 45, and 46.
Radiocarbon Analyses
Bulk radiocarbon dating of the bog butter specimens (1 mg of C) was performed by direct combustion and graphitization of ˜1.2 mg of butter in a Vario cube Elemental Analyser (EA) linked to an Ion Plus Automated Graphitisation Equipment 3 (AGE3). For CSRA analyses (200–300 µg of C per isolated compound), ˜3 mg of lipids were methylated as described above, then isolated following the pcGC procedure described in Casanova et al. (Reference Casanova, Knowles, Williams, Crump and Evershed2018). Briefly, the C16:0 and C18:0 solutions (the FA with lowest abundance was at a concentration of ˜5 µg C µL–1) were injected (1 µL × 40 injections) into a DB1 column (Rxi-1ms, 30 m × 0.53 mm i.d., 1.5 µm thickness, Restek) in a Hewlett-Packard series II GC coupled via a transfer line (heated at 310ºC) to a Gerstel preparative fraction collector (heated at 310ºC). The C16:0 and C18:0 FAMEs were isolated into individual solventless traps based on their retention time. The glass wool containing the isolated FAMEs was transferred directly into an Al capsule for combustion and CO2 reduction into graphite on activated iron catalyst (2 hr, 580ºC) using an EA-AGE3 system. Size-matched standards and blanks were analyzed alongside every batch. The AMS measurements were performed on the Bris-MICADAS accelerator at the Bristol Radiocarbon Accelerator Mass Spectrometry Facility. Radiocarbon dates determined for FAMEs were corrected for the derivative C using a mass balance and combined as described previously (Casanova et al. Reference Casanova, Knowles, Williams, Crump and Evershed2018, Reference Casanova, Knowles, Bayliss, Dunne, Barański, Denaire, Lefranc, di Lernia, Roffet-Salque, Smyth, Barclay, Gillard, Claßen, Coles, Illet, Jeunesse, Krueger, Marciniak, Minnitt, Rotunno, van de Velde, van Wijk, Cotton, Daykin and Evershed2020a). The statistical consistency of radiocarbon measurements was evaluated at the 5% level using a χ2 test (Ward and Wilson Reference Ward and Wilson1978), and the values combined as the weighted mean as described in Casanova et al. (Reference Casanova, Knowles, Bayliss, Dunne, Barański, Denaire, Lefranc, di Lernia, Roffet-Salque, Smyth, Barclay, Gillard, Claßen, Coles, Illet, Jeunesse, Krueger, Marciniak, Minnitt, Rotunno, van de Velde, van Wijk, Cotton, Daykin and Evershed2020a).
RESULTS
Preselection of the Bog Butter Standards
The criteria used for identifying suitable bog butter finds for use as radiocarbon standards were (1) bog butter specimens large enough to allow up to 500 g to be sampled with minimal impact on their historical or cultural value and their overall integrity; (2) specimens that survived in several pieces (either post-excavation, at the time of discovery, or at the time of reporting) and not deemed suitable for museum display; (3) absence of conservation treatments involving organic-based products; (4) absence of modern contamination deriving from the excavation and storage (e.g. plasticizers from plastic bags); (5) lipid distribution with approximately equal abundances of C16:0 and C18:0 FAs (i.e. ratio C16:0/C18:0 as close as possible to 1) to enable the same masses of each fatty acid to be extracted and isolated during CSRA; (6) lipid distribution with relatively low abundances of C18:1 FA or other compounds which could partially coelute with the C18:0 (or C16:0) FA; and (7) in cases where several specimens fulfil the above criteria, samples with significantly different dates were considered desirable.
Bog butter specimens contained in vessels were oftentimes subject to wax treatment in the past for the purposes of consolidation of the vessel. This process rendered these particular specimens unsuitable for our current purpose (criterion 3). From the remaining specimens, we identified nine of large size (> 10 kg; criterion 1) and in several pieces (criterion 2) as being the potential candidates for use as standards (Table 1). Three of them (IB4, IB20, IB29) had been previously analyzed and were not re-sampled (Smyth et al. Reference Smyth, Berstan, Casanova, McCormick, Mulhall, Sikora, Synnott and Evershed2019). At this stage, two different areas of each of the remaining bog butter specimens were sub-sampled for both lipid and radiocarbon analyses.
Table 1 Bog butter specimens preselected, bulk conventional 14C age, C16:0/C18:0 peak area ratio and stable carbon isotope values.

The lipid analyses by GC and GC-MS revealed all nine sampled specimens to be free of obvious contamination from storage or restoration work (criteria 3, 4). The C14:0, C16:0 and C18:0 FAs were found to be dominant in the TLE of all the specimens. Some of the bog butter specimens also contained hydroxy fatty acids (OHFAs; the 10-C18:0 OHFA being dominant), biomarkers of adipocere formation (Smyth et al. Reference Smyth, Berstan, Casanova, McCormick, Mulhall, Sikora, Synnott and Evershed2019), in appreciable abundances. Only five specimens (IB4, IB29, IB33, IB37, IB38) showed a peak area ratio of C16:0/C18:0 FAs below 2 (Table 1; criterion 5). Sample IB37, however, displayed significantly different C16:0/C18:0 FA peak area ratios between the two sub-samples with one at 1.6 and the other at 2.7, suggesting its lipid composition was inhomogeneous. Two specimens (IB29, IB34) showed the presence of C18:1 FA at similar abundance to the C18:0 FA, and thus were not suitable (criterion 6). The δ13C values on the C16:0 and C18:0 FAs measured by GC-C-IRMS of six specimens (IB4, IB20, IB29, IB34, IB36, IB38) plotted firmly in the reference range of ruminant dairy animals (Copley et al. Reference Copley, Berstan, Dudd, Docherty, Mukherjee, Straker, Payne and Evershed2003) and two others (IB33, IB35) at the edge of this reference range confirming that all of these specimens were dairy fats (Table 1, Figure 1a). Sample IB37, however, displayed δ13C16:0/18:0 values outside the reference range of ruminant animals with one sub-sample plotting in the dairy region (Δ13C < –3.1‰) and the other in the ruminant adipose region (Δ13C > –3.1‰), thus being of uncertain origin.

Figure 1 (a) δ13C18:0 values plotted against δ13C16:0 values and (b) calibrated bulk radiocarbon dates (calibrated using OxCal v4.4.2 and the IntCal20 terrestrial calibration curve; Reimer et al. Reference Reimer, Austin, Bard, Bayliss, Blackwell, Ramsey, Butzin, Cheng, Edwards, Friedrich, Grootes, Guilderson, Hajdas, Heaton, Hogg, Hughen, Kromer, Manning, Muscheler, Palmer, Pearson, van der Plicht, Reimer, Richards, Scott, Southon, Turney, Wacker, Adolphi, Büntgen, Capano, Fahrni, Fogtmann-Schulz, Friedrich, Köhler, Kudsk, Miyake, Olsen, Reinig, Sakamoto, Sookdeo and Talamo2020) for the nine preselected bog butter specimens.
The bulk radiocarbon dates on the sub-samples for bog butter specimens IB33 to IB38 inclusive provided statistically consistent measurements. The radiocarbon dates identified one specimen (IB33) as dating to the Early Bronze Age, five (IB4, IB34 to IB37 inclusive) dating to the Iron Age, one (IB20) of medieval date and two (IB29, IB38) of post-medieval date (Table 1, Figure 1b).
Only three specimens, therefore, matched all the selection criteria. Specimen IB4 has a C16:0/C18:0 ratio of 1.85 and dates to 1924 ± 26 BP, IB33 has an average ratio of 1.55 and dates to 3765 ± 26 BP, finally, IB38 has an average ratio of 1.25 and dates to 330 ± 18 BP. Specimens IB33 and IB38 were selected as those bog butter specimens to be used as radiocarbon standards as they exhibited the smallest C16:0/C18:0 ratios (criterion 5). These specimens also display very distinct radiocarbon ages providing one standard in the range of prehistoric pottery vessels and another in the range of historical vessels (criterion 7).
Cleaning and Homogenization
We sampled 495.37 g of IB33 and 426.12 g of IB38 for the creation of radiocarbon standards (Figure 2 a1-2, b1-2). During the initial freeze-drying step, 17.0% of the mass of IB33, and 3.3% of the mass of IB38, were lost as water. After the removal of visible contaminants (animal hair, pieces of ancient wooden container and peat particles, stored afterwards in separate glass vials) using the melting and centrifuging process, 377.6 g (76.6% of the initial mass) of IB33 and 406.6 g (95.4% of the initial mass) of IB38 remained for homogenization (Figure 2 a3, b3).

Figure 2 Pictures of the main steps to generate homogenous radiocarbon standards from (a) IB33 and (b) IB38 bog butter finds: 1. Bog butter specimens from the NMI collections (left) and sampling with tweezers and scalpel removing obvious contaminants (e.g. pieces of wooden container) (right). 2. Sampled masses for cleaning. 3. Masses collected after removal of visible contaminants by melting and centrifuging (left), isolated peat-like contaminant materials (middle), isolated wooden materials and animal hair (right). 4. Homogenization by melting in a large beaker (left), pouring in a tray covered in foil (middle) and cooled bog butters as thin tablets (right). 5. Bog butter standards divided into eight pieces (left) and remaining material collected from the beaker and after slicing (right).
Once homogenized, cooled and solidified (Figure 2 a4, b4), the bog butter specimens were sliced into eight plates with total masses of 372.4 g (75.0% of the initial mass) and 400.6 g (94.0% of the initial mass) for IB33 and IB38, respectively (Figure 2 a5, b5). The remaining loose material account for 2.5 g (0.4% of the initial mass) and 2.9 g (0.7% of the initial mass) of IB33 and IB38, respectively. In total, 373.9 g (75.5% of the initial mass) of IB33 and 403.5 g (94.7% of the initial mass) of IB38 were homogenized and constitute the amounts of the new radiocarbon standards available. The different plates are now stored individually wrapped in foil in aluminium trays to avoid potential contamination during future handling of the standards.
Homogeneity Assessment
To assess the homogeneity in terms of lipid composition and radiocarbon age, the eight plates and the two vials of material were analyzed, resulting in a total of 10 analyses per bog butter specimen.
The lipid analyses (Table 2, Figure 3 a,b) revealed a C16:0/C18:0 peak area ratio average of 1.62 ± 0.03 (SD) with extreme values of 1.59 and 1.67 for IB33 and an average of 1.29 ± 0.05 (SD) with extreme values of 1.26 and 1.41 for IB38. These values are close to the original ratios and suggest the lipid profiles were not altered during the preparation procedure and the bog butter standards are homogeneous. The δ13C16:0/18:0 values from the different pieces are statistically indistinguishable as the means were found to be δ13C16:0 = −31.5 ± 0.1‰ and δ13C18:0 = −35.6 ± 0.1‰ (T’=0.4, T’(5%) = 16.9, ν = 9 and T’ = 0.5, T’(5%) = 16.9, ν = 9, respectively) for IB33 and δ13C16:0 = −29.3 ± 0.1‰ and δ13C18:0 = −33.6 ± 0.2‰ (T’ = 0.3, T’(5%) = 16.9, ν = 9 and T’ = 0.9, T’(5%) = 16.9, ν = 9, respectively) for IB38 (Figure 2c). These results further support the homogeneity of the two bog butter standards.
Table 2 Results of homogenization for IB33 and IB38. Mass of pieces, ratio of C16:0/C18:0, stable carbon isotope analyses (analytical error is 0.5‰ and averages are reported), bulk and CSRA measurements reported as the conventional radiocarbon age. *Measurement excluded (outliers or C16:0 and C18:0 ages non-statistically identical).
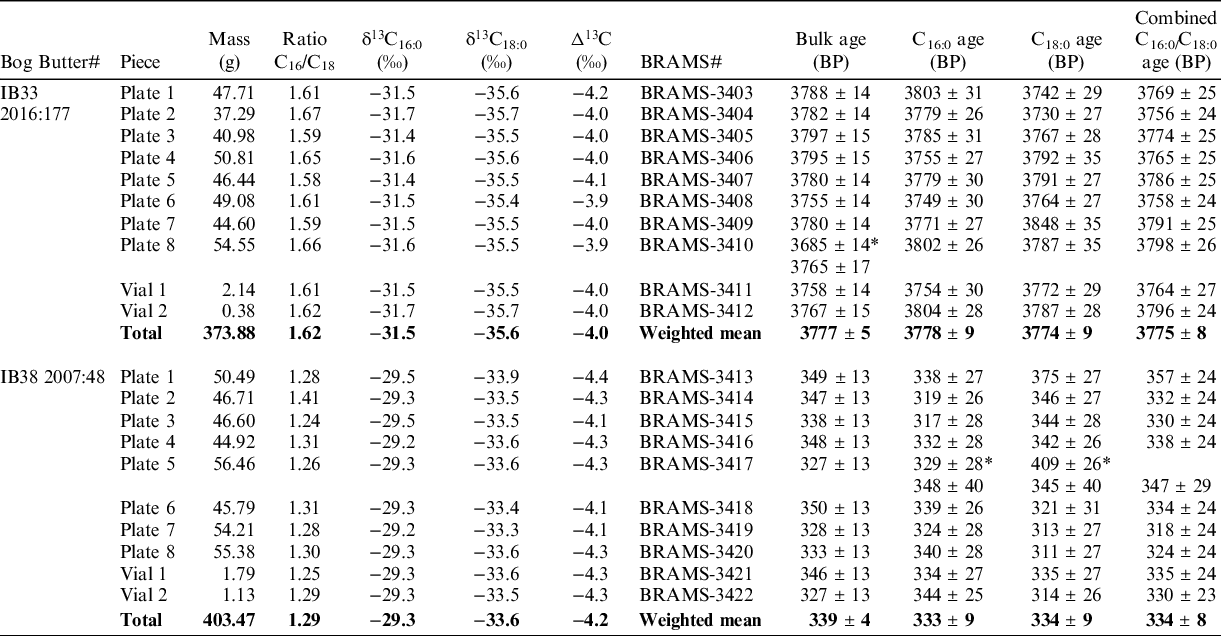

Figure 3 GC lipid profile of (a) IB33 (plate 1) and (b) IB38 (plate 1) after homogenization. (c) δ13C18:0 values plotted against δ13C16:0 values after homogenization.
Both bulk and CSRA determinations were performed to assess the homogeneity in radiocarbon ages of the subsamples (Table 2). Our quality assurance criteria for the CSRA approach is to obtain statistically consistent ages at the 5% level between the two fatty acids (Casanova et al. Reference Casanova, Knowles, Williams, Crump and Evershed2018, Reference Casanova, Knowles, Bayliss, Dunne, Barański, Denaire, Lefranc, di Lernia, Roffet-Salque, Smyth, Barclay, Gillard, Claßen, Coles, Illet, Jeunesse, Krueger, Marciniak, Minnitt, Rotunno, van de Velde, van Wijk, Cotton, Daykin and Evershed2020a).
For bulk measurements on IB33, one measurement (plate 8) was detected as a statistical outlier and was re-measured. With the new date on plate 8, the bulk dates are statistically identical at the 5% level (T’ = 8.7, T’(5%) = 16.9, ν = 9) and give a weighted mean of 3777 ± 5 BP. For CSRA measurements, the C16:0 and C18:0 FA radiocarbon ages pass the statistical consistency test at the 5% level for all of the 10 plates and vials. The measurements on individual fatty acids are statistically consistent at the 5% level (T’ = 14.3, T’(5%) = 30.1, ν = 19) and give a weighted mean of 3776 ± 6 BP. If the C16:0 and C18:0 FA ages are combined for each piece prior to calculating their weighted means (as would usually be the case when reporting an age of a pottery vessel), the results are also statistically consistent (T’ = 3.8, T’(5%) = 16.9, ν = 9) and their weighted mean is 3775 ± 8 BP. For this bog butter specimen, the CSRA weighted mean age (both using individual and combined C16:0 and C18:0 FA measurements) is statistically indistinguishable to that obtained on bulk material (T’ = 0.0, T’(5%) = 3.8, ν = 1 for both). Taken all together, individual bulk- and CSRA measurements for the butter IB33-2016:177 are statistically indistinguishable whether using the individual (T’ = 23, T’(5%) = 42.6, ν = 29) or combined (T’ = 12.5, T’(5%) = 30.1, ν = 19) ages of the C16:0 and C18:0 FAs. The weighted mean was found to be 3777 ± 4 BP in both cases.
The bulk measurements on IB38 are statistically indistinguishable at the 5% level (T’ = 5.1, T’(5%) = 16.9, ν = 9) and give a weighted mean of 339 ± 4 BP. For CSRA measurements on IB38, the C16:0 and C18:0 FA radiocarbon ages pass the statistical consistency test at the 5% level for nine plates and vials. The CSRA of Plate 5 provided inconsistent measurements, the C18:0 FA radiocarbon age likely being erroneous due to its older age and was re-measured. The second CSRA measurements on this plate are statistically consistent and are used in the remainder of the analyses. The measurements on individual C16:0 and C18:0 FAs are statistically consistent at the 5% level (T’ = 6.4, T’(5%) = 28.9, ν = 18) and their weighted mean is 334 ± 6 BP. If the C16:0 and C18:0 FAs for each piece are combined (as would usually be the case when reporting an age of a pottery vessel), the results are also statistically consistent (T’ = 1.8, T’(5%) = 16.9, ν = 9) and give a weighted mean of 334 ± 8 BP. The CSRA weighted means are statistically indistinguishable from the those obtained on bulk measurements ((T’ = 0.5, T’(5%) = 3.8, ν = 1 and T’ = 0.5, T’(5%) = 3.8, ν = 1 and T’ = 0.3, T’(5%) = 3.8, ν = 1 and T’ = 0.5, T’(5%) = 3.8, ν = 1 and, for the individual and combined measurements of FAs, respectively). Taken all together, individual bulk and CSRA ages determined for the bog butter specimen IB38 are statistically indistinguishable whether we use the individual (T’ = 12, T’(5%) = 42.6, ν = 29) or combined (T’ = 7.2, T’(5%) = 30.1, ν = 19) ages of the two FAs. The weighted mean was found to be 338 ± 3 BP in both cases.
These results demonstrate that the prepared bog butter specimens are homogeneous in age whether we use a bulk or a compound-specific approach. The results also confirm that no exogenous carbon is introduced during the pcGC protocol in any quantity that would significantly affect the CSRA determinations.
CONCLUSION
We have identified and selected two ideal bog butter specimens to produce radiocarbon standards. The specimens were cleaned from contaminants and homogenized. They have been proven to be homogenous in their lipid composition, stable carbon isotope ratios and radiocarbon ages and are, therefore, suitable for use as quality control standards.
These two new bog butter standards are ideally suited for use in the compound-specific radiocarbon analyses of FAs, particularly the C16:0 and C18:0 FAs, isolated from archaeological matrices such as pottery vessels, human/animal remains or other fat-based commodities, e.g. cosmetics. At the BRAMS facility, we now routinely use these processing-standards in batches of unknown age samples of fatty acids (currently alongside our pseudo-processing standards as described in the introduction).
We dated the two bog butter standards to 3777 ± 4 BP (IB33) and 338 ± 3 BP (IB38). These samples will be subjected to an intercomparison exercise with other laboratories equipped with or having access to compound-specific capabilities and AMS instruments in order to provide robust consensus values for these two new standards. Subject to the outcome of this exercise, the ultimate intention will be to make these two new standards available to the wider radiocarbon community under license and by permission of the National Museum of Ireland.
ACKNOWLEDGMENTS
We acknowledge the European Research Council for funding a Proof of Concept grant (LipDat, H2020 ERC-2018-PoC/812917) to RPE and financing EC’s post-doctoral contract. We acknowledge C. Smith, Conservation Dept., National Museum of Ireland for facilitating access to the bog butter specimens and for supervising the entire sampling process. We thank the NERC (Ref. CC010) and NEIF (www.isotopesuk.org) for funding and maintenance of GC/MS and GC-C-IRMS instruments used in this work. All samples for 14C dating were prepared and analyzed at the BRAMS facility and we acknowledge NERC, BBSRC, and the University of Bristol for capital funding for the facility.







