INTRODUCTION
The Centre for Isotope Research (CIO) hosts the longest-running radiocarbon (14C) dating unit currently in operation. The first director of the laboratory, Hessel de Vries, was appointed in 1950, and the first 14C dates obtained in 1952 (de Vries and Barendsen Reference de Vries Hl1952). De Vries utilized and made significant developments to the gas proportional counting method of 14C measurement (de Vries and Barendsen Reference de Vries Hl1953). The CIO operated as a conventional facility until the early 1990s, whereupon a 3 MV Tandetron accelerator was obtained from High Voltage Engineering. Over the ensuing decades, the CIO gradually migrated toward making measurements by accelerator mass spectrometry (AMS), and since 2011 has operated exclusively as an AMS laboratory. In 2017, a 200 kV compact accelerator (MICADAS) was purchased from Ionplus AG. The performance of this instrument, as well as updates to routine target preparation and uncertainty propagation, will be discussed in other upcoming articles.
Improvements to the registration and chemical preparation of 14C samples have occurred throughout the entire history of the laboratory. However, a comprehensive overview of such procedures at the CIO has not been produced since Mook and Streurman (Reference Mook and Streurman1983). This paper discusses the protocols now employed for the registration, tracking and pretreatment of samples submitted for 14C dating. Such samples generally relate to palaeo-environmental, archaeological and forensic studies. All post-bomb 14C research applications on aerosols (see Dusek et al. Reference Dusek, Monaco, Prokopiou, Gongriep, Hitzenberger, Meijer and Rockmann2014), atmospheric 14CO2, 14C analysis on water samples, and biogenic carbon fraction determination (among others Palstra and Meijer Reference Palstra and Meijer2014) will not be described in this paper. To cover the array of samples still circumscribed by this overview, each sample material will be dealt with in turn, with specialist approaches discussed separately from routine methods.
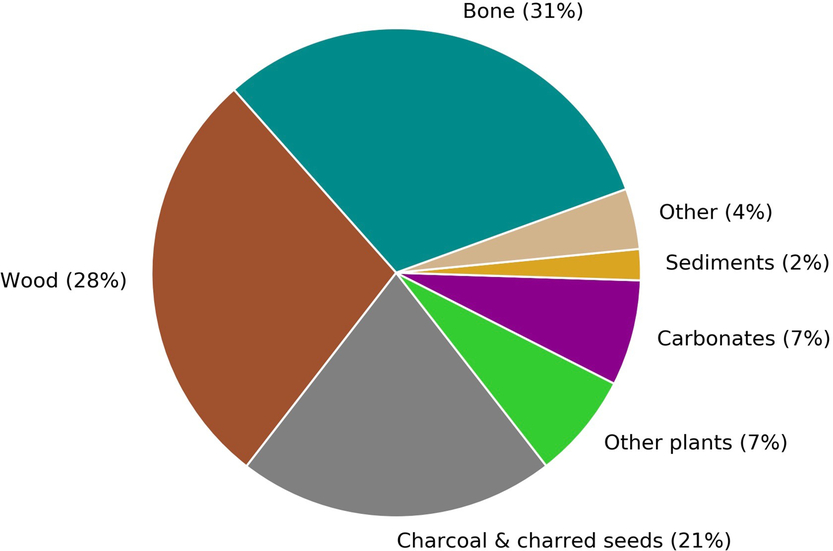
The CIO produces 2500–3000 14C measurements annually, a number that is expected to increase over the coming years as a result of the growth and advancement of the laboratory. About 80% of these measurements are obtained for the purposes of 14C dating. Figure 1 gives a breakdown of the main sample types that will be discussed in this paper.
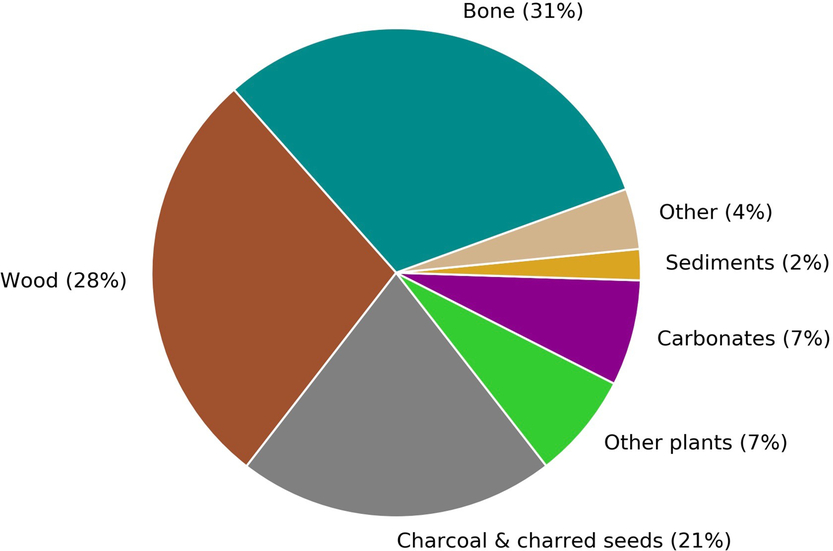
Figure 1 A breakdown by sample type of the materials submitted to Groningen for sample preparation and radiocarbon dating from September 2017 to August 2018. The carbonate group includes both calcined bones and shells.
SAMPLE REGISTRATION
Groningen employs the same template for all sample submissions, which can be found at http://www.rug.nl/research/centre-for-isotope-research/customers/procedure
Each batch of samples is added to a FileMaker Pro™ relational database and assigned a unique Project Number. Every individual sample is then allocated an in-house Sample Number. This is different from our current Laboratory Reference (prefix GrM-), which is a number assigned to an approved radiocarbon result. If an item is pretreated more than once using the same protocol, the same Sample Number is employed. If a different pretreatment method is applied, another Sample Number is issued which is linked to the original. The FileMaker Pro database contains records of all the electronic correspondence with the submitter; the pretreatment procedures applied to each sample; the resultant stable isotope and other supporting data. The current database and its historical predecessors can be interrogated for information on past analyses, including the samples pretreated for gas proportional counting, although details on some of the older records are stored on paper files only. An on-site archive is also maintained of excess unpretreated and pretreated sample material.
Acceptance of Samples for Dating
The CIO has always endeavored to ensure it does not inadvertently accept samples that were acquired illegally or unethically. Our protocols pertaining to this issue were recently revised and strengthened. In essence, requests for 14C dates on artworks, craftworks or decorative objects, which are for commercial purposes only, will not be granted. Materials under strict international regulation, such as ivory, will also not be accepted unless the dates are being sought for the purposes of law enforcement or forensic research. For specific guidance on whether a sample may be submitted for dating, the reader is directed to the departmental website, given above.
MAIN EQUIPMENT
The CIO operates the following instruments and apparatus for 14C dating and stable isotope measurements:
MICADAS (Ionplus AG) 200 kV accelerator mass spectrometer
Gas Interface System (GIS, Ionplus AG) for direct 14CO2 gas measurement on the MICADAS
Carbonate Handling System (CHS, Ionplus AG) coupled to the GIS for carbonate evolution and direct measurement of gaseous 14CO2 on the MICADAS
Elemental analyzer (EA, Elementar Vario Isotope Select™) coupled to the GIS for combustion and direct 14CO2 measurement on the MICADAS
Tuneable infrared laser differential absorption spectrometer (Aerodyne Research) being optimized for high-precision determination of δ13C, δ17O and δ18O in minute fractions of CO2
A second EA (Elementar Vario Isotope Cube™) for combustion of solid and liquid samples
Isotope Ratio Mass spectrometer (IRMS, IsoPrime 100™), coupled to the second EA, for routine δ13C and δ15N measurement
Automated cryogenic collection system (in-house), coupled to the second EA, for trapping the CO2 from combusted solid and liquid samples
Five graphitization manifolds (in-house) each containing ten sample positions
Automated graphite press (in-house)
Combustion manifold (in-house) for the preparation of bulk CO2 standards (liters), such as the International Atomic Energy Agency (IAEA) C7, C8, OX-II and GS-51
SAMPLE PREPARATION
Solid materials are typically taken through pretreatment in batches of around 25 samples. A secondary standard is allocated to each batch and taken in parallel through pretreatment. The secondary standard is, where possible, of the same material type and approximately the same expected age. The stock of secondary standards at the CIO consists of both known-age materials and samples of greater than background age. Full pretreatment duplicates are also regularly implemented (see Quality Assurance, below).
Physical Pretreatment
Solid materials are commonly subjected to some form of physical preparation prior to chemical pretreatment. This usually involves eliminating extraneous soil and particulates from the bulk material by washing in ultrapure water, or careful scraping with a scalpel or metal brush. It is essential that exogenous plant material such as blades of grass, rootlets, or cotton/synthetic fibers from packaging are thoroughly removed before the item is sampled for dating.
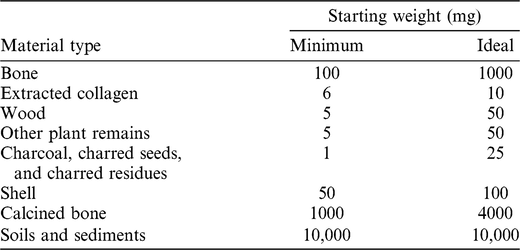
Sampling is governed by the principle of utilizing the minimum amount of material necessary to obtain a successful date. In addition, it is designed to limit damage to characteristic or diagnostic features of both natural and cultural materials, and minimize the aesthetical impact on display pieces. Generally, portions of bone or wood are cut or drilled off using a clean saw or a Dremel 4000™. Large fragments may be crushed with percussion implements to expose greater surface area and enhance the effectiveness of the chemical pretreatment. Flimsy plant samples may also be removed with a scalpel or tweezers. Where possible, plant material is also cut or crushed into small fragments prior to chemical pretreatment. The amounts of starting material required by the CIO for routine 14C dating are listed in Table 1. The smallest samples are often subject to bespoke treatments and, depending on preservation, are less likely to produce optimal dates. In general, the sample sizes described as ideal in Table 1 provide the best chance of obtaining a successful and precise result.
Table 1 Minimum and ideal starting weights (of dry material) for routine samples submitted to Groningen for 14C dating. For minimum-sized samples, submitters should liaise directly with the laboratory.

Chemical Pretreatment
The central goal of 14C chemical pretreatment is to extract from the bulk sample an endogenous fraction that is likely to have remained chemically unmodified during deposition (de Vries and Barendsen Reference de Vries Hl1954; van Klinken and Hedges Reference van Klinken and Hedges1998; Brock et al. Reference Brock, Higham, Ditchfield and Bronk Ramsey2010). This tends to be the comparatively inert structural biopolymers, such as collagen from bone and antler; keratin from fur, horn and nails; and cellulose from plant-based materials. For charcoal and charred seeds, the object is to extract the reduced carbon fraction. Non-polar organic compounds of either synthetic or natural origin such as oils, waxes and resins, glues and adhesives are generally not sought for dating because the material is likely to be either mobile in the environment or asynchronous with the date sought. For routine samples, such contamination usually relates to incidences of curatorial preservation or repair. Where glues or preservations are suspected on any solid sample, they are first subjected to an organic solvent pretreatment (see Dee et al. Reference Dee, Brock, Bowles and Bronk Ramsey2011; Brock et al. Reference Brock, Dee, Hughes, Snoeck, Staff and Bronk Ramsey2018).
Organic Solvent Pretreatment
When required, the CIO implements a Soxhlet extraction procedure to eliminate additives such as glues and preservatives. This generally involves refluxing the sample for several hours in a series of organic solvents. The precise application is determined by the nature of the contaminant and guidance is sought from the relevant literature (Bruhn et al. Reference Bruhn, Duhr, Grootes, Mintrop and Nadeau2001; Dee et al. Reference Dee, Brock, Bowles and Bronk Ramsey2011; Brock et al. Reference Brock, Dee, Hughes, Snoeck, Staff and Bronk Ramsey2018). Generally, the CIO employ the sequence suggested by Bruhn et al. (Reference Bruhn, Duhr, Grootes, Mintrop and Nadeau2001); namely, tetrahydrofuran, chloroform, petroleum ether, acetone and methanol. Routine solvent washes may also be conducted in test-tubes situated in dry-block heaters, with each solvent usually applied for at least 1 hr at 45°C. In all cases, it is essential that any organic (i.e. carbon-containing) solvent be allowed sufficient time, preferably at least 36 hr, to completely evaporate before proceeding to aqueous pretreatment.
Aqueous Pretreatment
The aqueous pretreatment procedures employed by Groningen are very similar to most other academic 14C laboratories (e. g. Brock et al. Reference Brock, Higham, Ditchfield and Bronk Ramsey2010; Dunbar et al. Reference Dunbar, Cook, Naysmith, Tripney and Xu2016; Dumoulin et al. Reference Dumoulin, Comby-Zerbino, Delqué-Količ, Moreau, Caffy, Hain, Perron, Thellier, Setti, Berthier and Beck2017; Steinhof et al. Reference Steinhof, Altenburg and Machts2017). For human, animal and plant-based samples, the core is an acid-base-acid (ABA) procedure designed to eliminate geological carbonates, supramolecular humic substances from the soil, and any atmospheric CO2 absorbed during the alkaline phase. Each step is separated by at least a triplicate rinse with deionized and decarbonized water (referred to henceforth as DW).
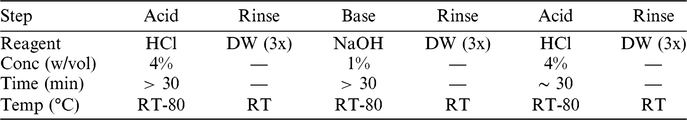
The exact settings employed during aqueous pretreatment (i.e.: concentrations, temperatures, durations) are commonly modified to suit the sample in hand, utilizing the technical experience available at the laboratory. In general, treatments are made milder if the sample is either very small or very fragile. For the ABA steps, in order to minimize exposure to plastic surfaces, all reagents are prepared in glass flasks, and the samples are treated in glass vessels topped by watch-glasses. A general overview of the ABA steps employed at Groningen is given in Table 2.
Table 2 The generic ABA protocol at Groningen. The exact temperatures and concentrations used are tailored to the nature of each sample.
Wood, Seeds, and Uncharred Plant Remains
The preparation of wood, most seeds, and uncharred material in the CIO laboratory is comparatively straightforward. The routine procedure follows the ABA scheme described in Table 2 with the first acid application conducted at 80°C and the second at room temperature. For specialist plant sample projects, non-routine pretreatments are applied, which include further chemical steps.
Holocellulose Extraction (Non-Routine)
If dating of the holocellulose fraction of wood, seeds or other uncharred plant remains is required, and sufficient sample material is available, the following steps are taken. Firstly, the routine ABA protocol is applied, as described above for wood, seeds and uncharred plant samples, but with both acid steps applied at 80°C. Afterwards, an additional aqueous oxidation step (acidified NaClO2, 80°C) is employed as well as a final rinse to neutrality. Wood samples are generally robust enough for a 5% solution of acidified NaClO2 to be employed, but this reagent is weakened for some delicate materials such as paper or food remains.
α-Cellulose Extraction (Non-Routine)
For projects requiring especially high precision, such as tree-rings for calibration or cosmic ray studies (see Hua et al. Reference Hua, Barbetti, Worbes, Head and Levchenko1999; Hogg et al. Reference Hogg, Fifield, Turney, Palmer, Galbraith and Baillie2006; Dee et al. Reference Dee, Pope, Miles, Manning and Miyake2017), the CIO now also applies an α-cellulose wood pretreatment. The method draws strongly on the work of Hoper et al. (Reference Hoper, McCormac, Hogg, Higham and Head1998); Hogg et al. (Reference Hogg, Fifield, Turney, Palmer, Galbraith and Baillie2006, Reference Hogg, Turney, Palmer, Southon, Kromer, Bronk Ramsey, Noronha, Staff, Fenwick, Boswijk, Friedrich, Reynard, Guetter, Wacker and Jones2013) and Staff et al. (Reference Staff, Reynard, Brock and Bronk Ramsey2014). It also follows the same general steps of a physical preparation, optional organic solvent rinse, and an intensified aqueous pretreatment protocol (Figure 2). The reliability of our α-cellulose method is demonstrated in Figure 3. Thus far, 33 different single-year samples have been pretreated in duplicate and 31 results passed the Ward and Wilson (Reference Ward and Wilson1978) test for statistical consistency (see Figure 3).

Figure 2 Images of an alpha-cellulose pretreatment in progress (left) and collagen being demineralized in weak acid in a glass centrifuge vessel topped by a watch glass (right).
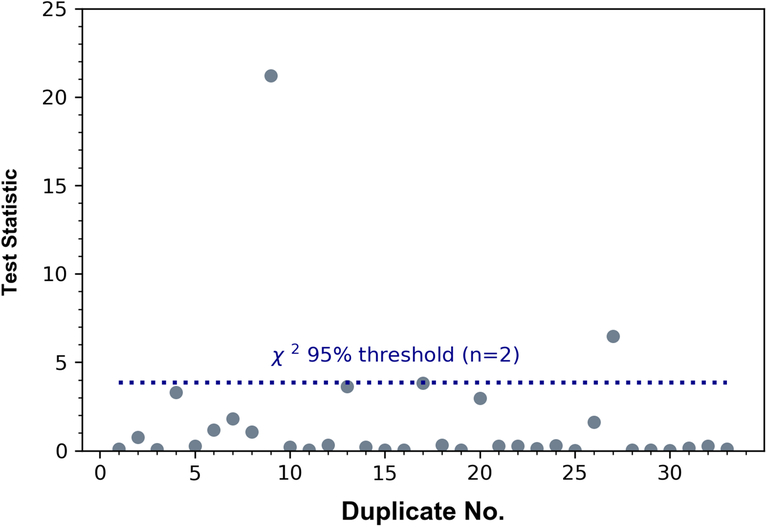
Figure 3 Duplicated pretreatments on dendrochronological tree-rings using the CIO’s new α-cellulose method. By calculating the test statistic (χ2 distribution, n – 1 degrees of freedom, see Ward and Wilson Reference Ward and Wilson1978) the congruence of pairs of results can be assessed. If the t-statistic for each pair is lower than the threshold for statistical consistency (3.84, 95% probability), the two results can be considered indistinguishable. Here, only 2 of the 33 results failed to meet this criterion.
a. Physical Pretreatment
Any extraneous material is removed using compressed air, or by shaving off the outermost surface with a razor blade. For single tree-ring analysis, rings are cleaved using a scalpel or stainless steel blade. The wood fragments are then further cut into as small pieces as possible, to enhance the effectiveness of the pretreatment. If the wood is too dense to be cut with a blade, rings may be ground off using an automatic milling device such as a Dremel 4000. Normally, 50 mg of starting material is sufficient.
b. Organic Solvent Pretreatment
Organic solvent washes are only applied if the tree species is known to be resinous and the risk of the colocation of carbon-containing material is high. Such species include pine, kauri, cedar and sequoia, but exclude oak. The taphonomy of the sample is also taken into consideration. For example, in the case of driftwood, an organic solvent rinse may be considered superfluous. Generally, due to the numbers of samples requiring preparation, it is not practical to employ Soxhlet apparatus, so the samples are placed in test-tubes in a dry-block heater (Fisher Scientific). In any event, recent studies suggest there is no net gain from applying the former method over the latter (MacDonald et al. Reference MacDonald, Chivall, Miles and Bronk Ramsey2019). The routine sequence applied at Groningen is as follows: acetone (45°C, 6 hr); dry thoroughly (>36 hr); DW (45°C, 6 hr); dry overnight.
c. Intensified Aqueous Pretreatment
The aqueous pretreatment commences with a strong acid (HCl, 5.47% w/vol (1.5 M), 80°C, 20 min), followed by triplicate rinses with DW. Then a strong base (NaOH, 17.5% w/vol, 60 min, RT) is applied, during which the reaction vessels are ultrasonicated under a N2 atmosphere. The supernatant fluid is then decanted off and the samples rinsed five times with DW, before strong acid is applied once more (HCl, 5.47% w/vol, 80°C, 20 min). Once a further triplicate rinse has been conducted, the next step involves an aqueous oxidation phase in a dry-block heater (NaClO2, 1.5% w/vol in HCl (0.06M), 80°C). The samples are left for 16 hr, and then a freshly prepared solution in the acidified oxidant is applied for a further 4 hr. Finally, the samples are rinsed 3 times, frozen and freeze-dried (Edwards Modulyo™).
Charcoal, Charred Seeds, and Charred Residues
The preparation of charred plant material is comparatively straightforward. The routine procedure follows the scheme described in Table 2 and has differed little at the CIO since its original development for gas proportional counting by Mook and Streurman (Reference Mook and Streurman1983). Where only small amounts of starting material are available (< 10 mg), it may be deemed permissible for some locations to subject the sample to an acid-only rinse. This course-of-action assumes the humic substances present are contemporary with the reduced carbon fraction. However, this approach is generally only employed for arid environments, such as certain sites in the ancient Near East, where there is considerable evidence the assumption is valid (Wild et al. Reference Wild, Steier, Fischer and Höflmayer2013).
Bone
Two types of pretreatment are applied to bone samples. By far the most common is collagen extraction from whole bone via a modified Longin protocol (Longin Reference Longin1971; Mook and Streurman Reference Mook and Streurman1983). The second is acid digestion of the inorganic (apatite) fraction. The latter method, pioneered at the CIO (Lanting and Brindley Reference Lanting and Brindley1998; Lanting et al. Reference Lanting, Aerts-Bijma and van der Plicht2001), is generally only employed for bones that have been calcined as a result of cremation. Partially charred or burnt bone is generally not suitable for 14C dating (Zazzo and Saliège Reference Zazzo and Saliège2011).
a. Collagen
To extract the collagen fraction from bone samples, fragments are first subject to a mild ABA pretreatment. Powdered bone samples are not suitable for the routine method applied at the CIO. Our method lies between the “chunks” and “gelatinization and ultrafiltration” dichotomy discussed by Sealy et al. (Reference Sealy, Johnson, Richards and Nehlich2014). The bone samples are first decalcified over at least a 24-hr period using mild acid (HCl, 2–4% w/vol, RT). If the material is not soft, the acid solution is refreshed and soft portions removed and stored separately in DW until further preparation. When the solution has stopped bubbling and all the fragments have become soft and pliable, they are rinsed thoroughly with DW. Subsequently, the extract is exposed to NaOH (1%, ∼30 min) to eliminate humic acids, rinsed to neutrality, treated again with acid (HCl, 4% w/vol, 15 min) and then rinsed once more to neutrality. The raw collagen fraction is then denatured to gelatin in acidified DW (pH 3) at 80°C for 18 hr. The dissolved gelatin is then filtered through a 50 μm mesh to eliminate any remaining foreign particulates and thoroughly dried. Finally, the crystalline product is scraped from sides of the glass vessels for weighing out.
b. Calcined Bone
The calcination of bone, from processes such as cremation, results in a closed system frequently making the inorganic fraction reliable for dating (Lanting and Brindley Reference Lanting and Brindley1998; Zazzo and Saliège Reference Zazzo and Saliège2011). At the CIO, calcined bone is first subjected to a weak oxidation (1.5% w/vol, NaClO2) to remove any organic material (48 hr, RT). Afterwards, it is rinsed with DW to neutral pH, and a weak acid applied to eliminate the adsorbed carbonate ions that are most likely to have exchanged with the environment (CH3COOH, 6% w/vol (1 M), 24 hr, RT). The cleaned apatite fraction is then rinsed again, dried overnight at 85°C, and crushed into small pieces. An aliquot (∼ 2 g) of the crushed product is placed in one arm of a Y-shaped glass reaction vessel. A saturated acid solution (H3PO4) is pipetted into the second arm, and the vessel evacuated on a custom-built vacuum manifold. The acid is then mixed with the calcined bone fragments and the vessels placed in a water bath (25°C, 24 hr). The CO2 evolved over this time is cryogenically transferred to a glass flask containing about 15 Sulfix™ particles (WAKO, 8∼20 mesh, mixture of Co3O4 and Ag2O) and stored for 18 hr in a heat block (200°C), to remove sulphurous components. The Sulfix is preheated in oxygen (∼200°C C, overnight) to ensure its purity before each use. Furthermore, the CIO is currently exploring alternative approaches, which would allow Sulfix to be excluded altogether.
Shell
Like calcined bone, the carbonate fraction of shell can reliably be dated by dissolution to CO2. Shells are first etched with acid (HCl, 4%) to remove the outermost surface, rinsed with DW, dried and crushed to powder. The subsequent acid digestion step is similar to that applied to calcined bone. An aliquot (∼20 mg) of the crushed shell is placed in one arm of Y-shaped glass reaction vessel. A saturated acid solution (H3PO4) is pipetted into the second arm, and the vessel evacuated on a vacuum line. The acid is then tipped onto the shell fragments and the glass vessels placed in a water bath (25°C, 24 hr). The CO2 evolved over this period is cryogenically transferred into a glass flask for stable isotope (δ18O and δ13C) analysis and subsequent graphitization and 14C measurement.
Soils and Sediments
Soil samples are rarely suitable for high-precision 14C dating. The main difficulty lies in determining whether the organic fractions obtained come from specific strata in the soil, or from more mobile phases. Nonetheless, in certain circumstances, “bulk” samples from lake sediments or peat bogs may provide useful age estimates. Here, the CIO employ the rudimentary protocol outlined in Table 2, with the reagents usually kept at 80°C. It is preferable to identify specific plant remains (except roots), but if only amorphous organic material is present in particulate form then both the base soluble (humic) and acid and base insoluble (humin) fractions are kept, dried and dated, to provide additional information on the integrity of the soil profile.
Combustion and Graphitization
Aliquots of the pretreatment products are weighed into tin capsules for combustion. The precise quantity weighed out depends on expected carbon content, and thus differs for each sample type. The capsules are combusted in an EA coupled to an IRMS and an automated cryogenic collection system. The IRMS allows δ13C (± 0.15 ‰, VPDB) and δ15N (± 0.30 ‰, Air) values to be determined for all samples submitted for 14C dating. The cryogenic system traps the CO2 released into sealable glass vessels. When the combustion run is complete, the glass vessels are transferred to one of five graphitization manifolds, each of which can take a maximum of ten samples (Aerts-Bijma et al. Reference Aerts-Bijma, van der Plicht and Meijer2001). Four manifolds are used for regular-sized samples (> 0.5 mg C), and the other is a bespoke rig for small samples. Each graphitization position on the manifolds is isolatable and has a T shape, so one “finger” can be cooled whilst the other, a detachable tube, is heated. Prior to use, the detachable tube is loaded with an Fe powder catalyst (∼ 2 mg), except in the case of small sample preparations, where an Fe pellet is used (~ 1.5 mg, de Rooij et al. Reference de Rooij, van der Plicht and Meijer2010). The whole manifold is first evacuated, and each reaction position isolated. The CO2 from each sample is then introduced, and a stoichiometric excess of H2 gas (1: 2.5) added. The hot tube is placed in an oven at 600°C and the cold finger, which draws water vapor from the reaction site, is chilled to between –15 and –20°C using a Peltier device. As the reaction proceeds, graphite forms on the catalyst. Pressure transducers follow the course of the reaction to ensure it proceeds to completion.
Pretreatment Quality Assurance
The CIO employs multiple safeguards for quality control purposes. A secondary (background or known-age) standard is included with each pretreatment batch and taken in parallel through the whole process. Obtaining the correct result for these materials substantiates the ages obtained for the unknown samples. The pretreatment standards we currently utilize include:
Horse bone (Holocene, VIRI intercomparison, Scott et al. Reference Scott, Cook and Naysmith2010)
Owen Buddleia charcoal (Modern, Oxford Radiocarbon Accelerator Unit)
Charred seeds from Jericho (Holocene, in-house)
Dendrochronological tree-rings (Holocene and modern, Dutch Cultural Heritage Agency, Amersfoort, Historic England, London)
Charcoal (Background, Christian-Albrechts-Universitat, Kiel)
Mammoth and rhino bones (Background, in-house)
Carbonate (Local background material, GS-35)
Furthermore, one sample in each batch is split and pretreated in duplicate. This protocol ensures the results are reproducible, irrespective of the sample’s age or depositional history.
In addition to procedural standards, the CIO also employs rejection criteria based on the data acquired during pretreatment. This helps ensure the purity of the pretreatment product, and hence the accuracy of the resultant date. Most yields of less than 0.5 mg or 0.5% (relative to starting weight) are automatically rejected. Collagen samples are failed outright if the C:N ratio on combustion is not between 2.9–3.6. A warning is issued with the 14C result if the C:N ratio is not between 3.1–3.3. Where such cases occur, the cause of the contamination and whether or not it can be remedied is always investigated. For carbonate samples, another key criterion is whether non-condensable gases are observed during the extraction process.
Finally, the CIO also employs many other standards for the purposes of ensuring the accuracy of the laboratory’s IRMS measurements. Data on such references will be reported and assessed in forthcoming complementary articles.
CONCLUSIONS
The last major overview of the chemical pretreatment procedures for 14C dating employed by the CIO was Mook and Streurman (Reference Mook and Streurman1983). Whilst several procedures have remained unchanged since that time, others have been modified, and new ones have been added to the repertoire. This article brings reporting up to date on all of the chemical procedures and processes employed by the 14C dating facility.
ACKNOWLEDGMENTS
M. W. Dee, M. Kuitems, and A. Scifo are supported by a European Research Council Grant (714679, ECHOES).