Nomenclature
- ADF
-
acid detergent fiber
- ANNR
-
artificial neural network regression
- ANN
-
artificial neural network
- BPNN
-
back-propagation neural network
- DA
-
discriminant analysis
- DM
-
dry matter
- ECVA
-
extended canonical variates analysis
- FDA
-
factorial discriminant analysis
- ICA
-
independent component analysis
- iECVA
-
interval extended canonical variates analysis
- iPLS-DA
-
interval partial least-squares discriminant analysis
- iPLSR
-
interval partial least-squares regression
- KNN
-
k-nearest neighbor
- KPCA
-
kernel principal component analysis
- KS
-
Kennard and Stone
- LDA
-
linear discriminant analysis
- LOD
-
limit of detection
- LSD
-
least significance difference
- LS-SVM
-
least-squares support vector machine
- LS-SVMR
-
least-squares support vector machine regression
- LW-PCA
-
locally weighted principal component analysis
- MD
-
Mahalanobis distance
- MDC
-
Mahalanobis distance classifier
- MLMR
-
maximum likelihood multinomial regression
- MLP
-
multilayer perceptron
- MLR
-
multiple linear regression
- MPLS
-
modified partial least-squares
- MPLSR
-
modified partial least-squares regression
- MSE
-
mean squared error
- NDA
-
non-linear discriminant analysis
- NNN
-
non-linear neural networks
- OMD
-
organic matter digestibility
- PCA
-
principal component analysis
- PCR
-
principal component regression
- PLS
-
partial least-squares
- PLS-DA
-
partial least-squares discriminant analysis
- PLSR
-
partial least-squares regression
- QDA
-
quadratic discriminant analysis
- RF
-
random forest
- SAM
-
spectral angle mapper
- SIMCA
-
soft independent modeling class analogy
- SSC
-
soluble sugar content
- SWI
-
single waveband image
- SVDD
-
support vector machine description
- RMSEP
-
root mean square error of prediction
- R p
-
correlation coefficient of prediction
- R
-
coefficient of correlation
- R 2
-
coefficient of determination
- R p 2
-
determination coefficient of prediction
- R c 2
-
determination coefficient of calibration
- SEP
-
standard error of prediction
- RPD
-
ratio prediction to deviation
Introduction
Seed is a living product and must be grown, harvested and processed correctly to maximize its viability and subsequent crop productivity. Seed quality has a profound effect on the development and yield of a crop (Bradbeer, Reference Bradbeer1988). Good seed quality can increase yield significantly. Seed quality depends on the health, physiology, germinability and physical attributes of seeds, including the presence or absence of disease, chemical composition, insect infestation, and the presence or absence of weed seeds or other plant varieties. Quality of seeds and their products is directly or indirectly related to human health; nevertheless, the evaluation of seed quality parameters is a time-consuming process. For example, calculation of the germination percentage commonly requires manual counting and grading of germinating seedlings by experienced technicians. Therefore rapid, simple and accurate detection techniques must be developed for farmers and the agro-industry to ensure quality seed during seeding, growth, harvesting, storage and transport to consumers (Huang et al., Reference Huang, Wang, Zhu, Qin and Huang2015).
The sowing quality of seed is associated with the germination and growth conditions after sowing and depends on seed composition, kernel maturity, insect infestation, diseases, cleanliness and germination ability (Copeland and McDonald, Reference Copeland and McDonald1999). The genetic purity of seeds may be detected by molecular identification, DNA analysis, isotope fingerprinting and mineral element analysis (Bradbeer, Reference Bradbeer1988). Protein electrophoresis, gas chromatography, high-performance liquid chromatography, tetrazolium tests, accelerated ageing and conductivity tests have been employed to evaluate the vigour and germination quality of seeds (Huang et al., Reference Huang, Wang, Zhu, Qin and Huang2015). Most of these chemical and physical techniques exhibit high accuracy and good reliability but have certain limitations, such as their high cost, long time requirements and high operator requirements. With the increasing demand for rapid, non-destructive and reliable techniques for evaluation of seed quality in the modern agro-industry, high-performance techniques must be developed for the evaluation of seed quality. A number of non-destructive testing technologies have been developed for evaluation of seed quality (Huang et al., Reference Huang, Wang, Zhu, Qin and Huang2015). These non-destructive testing technologies are rapid, accurate, reliable and simple methods for assessing the quality of seeds. This review focuses primarily on non-destructive techniques, namely, machine vision, spectroscopy, hyperspectral imaging, electronic nose, soft X-ray imaging and thermal imaging techniques, which have been used to assess seed quality parameters such as chemical composition, genetic purity and classification, disease and insect infestation, as well as vigour and germinability. The emphasis in this review is also placed on insights into the methods and techniques that have been investigated for evaluating seed qualities.
Non-destructive techniques for seed quality assessment
Machine vision
Machine vision, also known as ‘computer vision’ or ‘computer image processing’, is an artificial intelligence technique that simulates human vision (Huang et al., Reference Huang, Wang, Zhu, Qin and Huang2015). This technique is non-destructive, reliable and rapid and has been proven to be an effective and powerful technique for quality evaluation of food and agricultural products, particularly seeds (Hornberg, Reference Hornberg2007). A typical machine vision system consists of four basic components: an illumination system, a sensor or camera, a lens and a computer with frame grabber/digitizer (Fig. 1). Most applications of machine vision address the visible spectrum (380–780 nm) (Gunasekaran et al., Reference Gunasekaran, Paulsen and Shove1985). A machine vision system should be capable of identifying and grading seeds based on image external features, such as size, shape, colour and texture. The superiority, disadvantages and feasibility of different image external features should be simultaneously considered to select the most suitable feature for specific applications. Machine vision has already been used, with varying success, to assess seeds of a range of crop and non-crop species. This review focuses mainly on machine vision techniques that can be used to classify seed varieties, disease detection, identification of seed varieties, etc.

Figure 1. A typical machine vision system
Spectroscopy
Spectroscopy is used to investigate and measure the spectra produced when matter interacts with, or emits, electromagnetic radiation (Huang et al., Reference Huang, Wang, Zhu, Qin and Huang2015). A range of spectroscopic techniques, such as near-infrared- (NIR), mid-infrared- (MIR), fluorescence-, Fourier transform-infrared- (FT-IR) and Raman spectroscopy have been widely and successfully used as sensitive and fast analytical techniques for authentication and quality analysis of a variety of agricultural seeds (Fig. 2). NIR and MIR spectroscopy are based on molecular overtones and combined vibrations. FT-IR spectroscopy is a technique used to record infrared spectra and detect radiation in the MIR region. FT-IR spectroscopy is an information-rich analytical technique, as it provides a greater amount of chemical information regarding the scanned sample than NIR spectroscopy (Lohumi et al., Reference Lohumi, Lee, Lee and Cho2015). Raman spectroscopy is another form of analytical spectroscopy that is suitable for quality and authenticity analysis of agro-food products. This technique can provide specific information needed for identification of sample matrices based on model compounds, such as lipids, proteins and carbohydrates, and is sensitive to minor components (Seo et al., Reference Seo, Ahn, Lee, Park, Mo and Cho2016). This review focuses mainly on spectroscopic techniques that can be used to detect seed quality attributes, such as chemical composition, viability and damage by insects and other causes.

Figure 2. NIR, MIR or FT-IR spectroscopy (left panel) and Raman spectroscopy (right panel). From Seo et al. (Reference Seo, Ahn, Lee, Park, Mo and Cho2016).
Hyperspectral imaging
Hyperspectral imaging has recently emerged as a powerful analytical technique for food quality and authenticity analysis. This technique is used to acquire both spectral and spatial information from an object (Wu and Sun, Reference Wu and Sun2013). A hyperspectral imaging system includes light sources, wavelength dispersion devices and detectors. As the centre of a hyperspectral imaging system, wavelength dispersion devices are used to disperse broadband light into different wavelengths (Fig. 3). The detector collects light, which carries useful information from the wavelength dispersion device and measures the intensity of the light by converting radiation energy into electrical signals (Huang et al., Reference Huang, Wang, Zhu, Qin and Huang2015). Using hyperspectral imaging, sample analysis is convenient and comparatively fast because a large number of samples are analysed at the same time, whereas spectroscopic methods analyse only one sample at a time (Lohumi et al., Reference Lohumi, Lee, Lee and Cho2015). Machine vision and spectroscopy can only provide spatial or spectral information, whereas hyperspectral imaging, which integrates machine vision and spectroscopy advantages, can simultaneously obtain spatial and spectral information by using only one system. In this regard, hyperspectral imaging has been widely used by researchers to evaluate the exterior quality of seeds and predict their internal composition (Mahesh et al., Reference Mahesh, Jayas, Paliwal and White2011a; Zhu et al., Reference Zhu, Wang, Zhang, Huang, Yang, Ma and Wang2011; Huang et al., Reference Huang, Wang, Zhang and Zhu2014).

Figure 3. A typical hyperspectral reflectance/fluorescence imaging system. From Qin et al. (Reference Qin, Chao, Kim and Burks2013).
Thermal imaging
Thermal imaging is a technique for converting the invisible radiation pattern of an object into visible images for feature extraction and analysis without establishing contact with the object. Using this method, the surface temperature of any object can be mapped at a high resolution in two dimensions. The thermal data produced may be used directly or indirectly in many ways (Manickavasagan et al., Reference Manickavasagan, Jayas and White2008). The application of thermal imaging has gained popularity in the agro-food industry in recent years (Vadivambal and Jayas, Reference Vadivambal and Jayas2011). The major advantage of thermal imaging is that it is a non-contact, non-invasive and rapid technique that can be used in online applications (Fig. 4). Thermal cameras are easy to handle and highly accurate temperature measurements are possible (Vadivambal and Jayas, Reference Vadivambal and Jayas2011). Using thermal imaging, it is possible to obtain temperature mapping of any particular region of interest with fast response times, which is not possible with thermocouples or other temperature sensors that can only measure spot data. The repeatability of temperature measurements in thermal imaging is high (Ishimwe et al., Reference Ishimwe, Abutaleb and Ahmed2014). In addition, thermal imaging does not require an illumination source, unlike other imaging systems. Nowadays, thermal imaging has a potential application in many operations involved in agriculture, starting from assessing seed quality, especially in detection of diseases, insects and seedling viability, estimating soil water status, estimating crop water stress, scheduling irrigation, determining disease and pathogen affected plants, estimating fruit yield and evaluating maturity of fruits and vegetables (Chelladurai et al., Reference Chelladurai, Jayas and White2010; Manickavasagan et al., Reference Manickavasagan, Jayas, White and Paliwal2010; Vadivambal and Jayas, Reference Vadivambal and Jayas2011). In spite of the fact that it could be used as a non-contact, non-destructive technique, it has some drawbacks in comparison with other imaging techniques because high resolution thermal imaging is costly and accurate thermal measurements depend on environmental and weather conditions. Thus it may not be possible to develop a universal methodology for its application in seed quality assessment.

Figure 4. A typical thermal imaging system. From Manickavasagan et al. (Reference Manickavasagan, Jayas, White and Paliwal2010).
Soft X-ray imaging
Electromagnetic waves with wavelengths ranging from 1 to 100 nm (and energies of approximately 0.12 to 12 keV) are called soft X-rays. The low penetration power of these waves and their ability to reveal internal density changes make soft X-rays suitable for use in evaluating agricultural products (Neethirajan et al., Reference Neethirajan, Jayas and White2007). Soft X-ray imaging is a well-known technique that takes a few seconds (3–5 s) to produce an X-ray image. Soft X-ray imaging has begun to be used in the seed industry to detect internal voids, defects, insect infestation and insect damage (Karunakaran et al., Reference Karunakaran, Jayas and White2004; Neethirajan et al., Reference Neethirajan, Karunakaran, Symons and Jayas2006; Mathanker et al., Reference Mathanker, Weckler and Bowser2013).
Electronic nose
An electronic nose is an instrument consisting of an array of electronic and chemical sensors with partial specificity and a pattern recognition system that is capable of recognizing simple or complex odours (Wilson and Baietto, Reference Wilson and Baietto2009). These devices typically have arrays of sensors used to detect and distinguish odours precisely in complex samples and at low cost (Zhou et al., Reference Zhou, Wang and Qi2012). Electronic nose devices have been employed in a wide variety of applications, including classification of kernels and microbial pathogen detection.
Quality detection of seeds using non-destructive techniques
Quality assessment of seeds: chemical composition
In recent years, non-destructive sensing techniques, mainly spectroscopy and hyperspectral imaging, have been widely used to determine the internal composition of seeds (Table 1). Previous studies have shown that spectroscopy systems can be applied successfully to determine the protein contents of corn (Armstrong et al., Reference Armstrong, Tallada, Hurburgh, Hildebrand and Specht2011), maize (Baye et al., Reference Baye, Pearson and Settles2006), common beans (Hacisalihoglu et al., Reference Hacisalihoglu, Larbi and Settles2010), rice (Wu and Shi Reference Wu and Shi2004), soybean (Ferreira et al., Reference Ferreira, Galão, Pallone and Poppi2014), peanuts (Wang et al., Reference Wang, Wang, Liu, Liu and Du2012), jatropha (Vaknin et al., Reference Vaknin, Ghanim, Samra, Dvash, Hendelsman, Eisikowitch and Samocha2011), rapeseed (Velasco and Möllers, Reference Velasco and Möllers2002), sunflower (Fassio and Cozzolino, Reference Fassio and Cozzolino2004), canola (Daun et al., Reference Daun, Clear and Williams1994), cotton (Huang et al., Reference Huang, Sha, Rong, Chen, He, Khan and Zhu2013), foxtail millet (Yang et al., Reference Yang, Wang, Zhou, Shuang, Zhu, Li, Li, Liu, Liu and Lu2013), flax, safflower, sesame and palm (Pandord et al., Reference Pandord, Williams and DeMan1988). Previous studies have shown that spectroscopy is highly accurate in protein prediction. The coefficients of determination for prediction (R p 2) of a partial least-squares regression (PLSR) model have been found to be 0.98 for corn (Chen et al., Reference Chen, Ai, Feng, Jia and Song2014), 0.99 for rapeseed (Pandord et al., Reference Pandord, Williams and DeMan1988), 0.96 for cottonseed (Huang et al., Reference Huang, Wan, Zhang and Zhu2013), 0.98 for peanut (Pandord et al., Reference Pandord, Williams and DeMan1988) and 0.91 for soybeans (Ferreira et al., Reference Ferreira, Galão, Pallone and Poppi2014). Spectroscopy has also been used to estimate the fibre content of soybean, corn (Armstrong et al., Reference Armstrong, Tallada, Hurburgh, Hildebrand and Specht2011) and rapeseed (Wittkop et al., Reference Wittkop, Snowdon and Friedt2012; Bala and Singh, Reference Bala and Singh2013;), and the sucrose content of soybean (Choung, Reference Choung2010). However, unsatisfactory results have been reported for carbohydrate determination in maize (Baye and Becker Reference Baye and Becker2004; Tallada et al., Reference Tallada, Palacios-Rojas and Armstrong2009), rice (Wu and Shi Reference Wu and Shi2004), foxtail millet (Chen et al., Reference Chen, Ren, Zhang, Diao and Shen2013) and soybean (Choung Reference Choung2010; Ferreira et al., Reference Ferreira, Pallone and Poppi2013) and made the same conclusions in their study that any changes in the compositional amount among the sample are not translated into differences within the spectra. In recent research, hyperspectral imaging has been used to predict crude protein and crude fat fractions in soybean (Zhu et al., Reference Zhu, Wang, Zhang, Huang, Yang, Ma and Wang2011), protein in wheat (Mahesh et al., Reference Mahesh, Jayas, Paliwal and White2011a) and alpha-amylase activity in wheat (Xing et al., Reference Xing, Van Hung, Symons, Shahin and Hatcher2009, Reference Xing, Symons, Hatcher and Shahin2011). Unsatisfactory prediction results have been obtained in some cases using hyperspectral imaging because of the difficulty of extracting the most important object features for assessing the physical structure and chemical composition of samples. The oil content is an important parameter in the internal quality evaluation of most oilseed crops. Spectroscopy within the range of 400–2500 nm has been widely used to determine oil content in peanuts (Sundaram et al., Reference Sundaram, Kandala, Holser, Butts and Windham2010), maize (Tallada et al., Reference Tallada, Palacios-Rojas and Armstrong2009), safflower (Rudolphi et al., Reference Rudolphi, Becker, Schierholt and von Witzke-Ehbrecht2012), rapeseed (Velasco and Becker, Reference Velasco and Becker1998; Velasco et al., Reference Velasco, Möllers and Becker1999; Petisco et al., Reference Petisco, García-Criado, Vázquez-de-Aldana, de Haro and García-Ciudad2010), sunflower (Pandord et al., Reference Pandord, Williams and DeMan1988; Pérez-Vich et al., Reference Pérez-Vich, Velasco and Fernández-Martínez1998; Fassio and Cozzolino, Reference Fassio and Cozzolino2004), jatropha (Vaknin et al., Reference Vaknin, Ghanim, Samra, Dvash, Hendelsman, Eisikowitch and Samocha2011), canola (Daun et al., Reference Daun, Clear and Williams1994), cotton (Huang et al., Reference Huang, Sha, Rong, Chen, He, Khan and Zhu2013), corn and soybean (Armstrong et al., Reference Armstrong, Tallada, Hurburgh, Hildebrand and Specht2011). The coefficients of determination of the oil prediction model were 0.99, 0.91, 0.98, 0.92, 0.95, 0.98, 0.95, 0.87 and 0.84 for peanut, safflower, rapeseed, sunflower, jatropha, canola, cotton, corn and soybean, respectively. Hyperspectral imaging has also been used to predict the oil and oleic acid concentrations in corn (Weinstock et al., Reference Weinstock, Janni, Hagen and Wright2006). An NIR hyperspectral imaging system (750–1090 nm) was used to predict the oil content in maize and the determination coefficient of the PLSR model for the determination of oil content was found to be 0.75 (Cogdill et al., Reference Cogdill, Hurburgh, Rippke, Bajic, Jones, McClelland, Jensen and Liu2004). The results indicated outstanding performance of the non-destructive technique in the prediction of the internal composition of the seed. Spectroscopy has also been used to determine the fatty acid content of peanuts (Sundaram et al., Reference Sundaram, Kandala, Butts and Windham2010), soybean (Patil et al., Reference Patil, Oak, Taware, Tamhankar and Rao2010), safflower (Rudolphi et al., Reference Rudolphi, Becker, Schierholt and von Witzke-Ehbrecht2012), rapeseed (Kim et al., Reference Kim, Park, Choung and Jang2007), sunflower (Cantarelli et al., Reference Cantarelli, Funes, Marchevsky and Camiña2009), jatropha (Vaknin et al., Reference Vaknin, Ghanim, Samra, Dvash, Hendelsman, Eisikowitch and Samocha2011), canola and flax (Siemens and Daun, Reference Siemens and Daun2005) with high accuracy. The amino acid composition of seeds is also a concern in their quality assessment since high protein content and a rational amino acid composition of seed are a major concern to the plant breeder (Chen et al., Reference Chen, Zhang, Wu and Shi2011). Studies have shown that near-infrared spectroscopy (NIRS) and FT-NIRS can be used successfully in the assessment of amino acid composition in rapeseed (Pandord et al., Reference Pandord, Williams and DeMan1988; Chen et al., Reference Chen, Zhang, Wu and Shi2011), peanuts (Wang et al., Reference Wang, Wang, Liu, Liu and Du2012), rice (Zhang et al., Reference Zhang, Rong, Shi, Wu and Shi2011) and foxtail millet (Yang et al., Reference Yang, Wang, Zhou, Shuang, Zhu, Li, Li, Liu, Liu and Lu2013). An experiment in high-resolution hyperspectral reflectance imagery in the near-infrared region (960–1700 nm) was conducted to predict the amino acid content of fresh soybeans and showed that the best predictions (MSE = 0.305, R = 0.611) were obtained using a non-linear artificial neural network (ANN)-based regression model based on the second-derivative spectra data produced for the nitrogen concentration (Monteiro et al., Reference Monteiro, Minekawa, Kosugi, Akazawa and Oda2007). Spectroscopy has also been used to determine the moisture content of soybean (Pandord et al., Reference Pandord, Williams and DeMan1988; Ferreira et al., Reference Ferreira, Pallone and Poppi2013; Ferreira et al., Reference Ferreira, Galão, Pallone and Poppi2014), sunflower (Pandord et al., Reference Pandord, Williams and DeMan1988; Fassio and Cozzolino, Reference Fassio and Cozzolino2004), peanuts (Sundaram et al., Reference Sundaram, Kandala, Holser, Butts and Windham2010), flax, safflower and cotton (Pandord et al., Reference Pandord, Williams and DeMan1988), as well as the pH of cocoa beans (Sunoj et al., Reference Sunoj, Igathinathane and Visvanathan2016), the mineral contents (K, Mg, Ca and P) of peanuts (Phan-Thien et al., Reference Phan-Thien, Golic, Wright and Lee2011), the seed weight of rapeseed (Velasco et al., Reference Velasco, Möllers and Becker1999), the grain weight of rice and brown rice (Wu and Shi, Reference Wu and Shi2004), the ethanol content of maize (Hao et al., Reference Hao, Thelen and Gao2012), the phenol content of rapeseed (Bala and Singh, Reference Bala and Singh2013) and the polyphenol content of cocoa beans (Sunoj et al., Reference Sunoj, Igathinathane and Visvanathan2016). In recent years, hyperspectral imaging has been used to predict the moisture content of corn (Cogdill et al., Reference Cogdill, Hurburgh, Rippke, Bajic, Jones, McClelland, Jensen and Liu2004; Mahesh et al., Reference Mahesh, Jayas, Paliwal and White2011b) and soybean during drying (Huang et al., Reference Huang, Wang, Zhang and Zhu2014), the sweetness (sucrose, glucose and fructose contents) of soybean (Monteiro et al., Reference Monteiro, Minekawa, Kosugi, Akazawa and Oda2007) and the colour of soybeans during drying (Huang et al., Reference Huang, Wang, Zhang and Zhu2014).
Table 1. Assessment of chemical composition in seeds using different non-destructive techniques

Quality assessment of seeds: insect damage and diseases
Seed damage by insects, fungi or natural causes, such as germination, are an important factor in seed quality during storage and processing. Seed damage is therefore taken seriously by consumers and the food industry. Various non-destructive techniques such as machine vision, spectroscopy, hyperspectral imaging, soft X-ray imaging, electronic nose and thermal imaging have been widely used in the detection of insect damage, insect infestation and diseases in seeds (Table 2). Machine vision has been used together with back-propagation neural networks based on colour features to detect external defects in rice seeds, such as germs, diseases and incompletely closed glumes, with an accuracy of 98.6–99.2% (Cheng et al., Reference Cheng, Ying and Li2006). A machine vision system developed for the detection of damaged wheat kernels based on morphological and textural properties was shown to have a classification accuracy of 91–94% (Delwiche et al., Reference Delwiche, Yang and Graybosch2013). A machine vision system was also used to detect damaged soybeans based on colour features with an accuracy of 99.6% (Shatadal and Tan, Reference Shatadal and Tan2003). Recently, spectroscopy has been used to identify defects in corn (Esteve Agelet et al., Reference Esteve Agelet, Ellis, Duvick, Goggi, Hurburgh and Gardner2012) and soybean (Sirisomboon et al., Reference Sirisomboon, Hashimoto and Tanaka2009). Hyperspectral imaging has been used to detect sprout damage in wheat (Singh et al., Reference Singh, Jayas, Paliwal and White2009a; Xing et al., Reference Xing, Symons, Shahin and Hatcher2010) and to detect sprouting in barley (Arngren et al., Reference Arngren, Hansen, Eriksen, Larsen and Larsen2011). In a recent study, a machine vision system was used to detect diseases and insects for the purpose of quality sorting of areca nuts with an accuracy of 90.9% (Huang, Reference Huang2012). Spectroscopy-based methods have also been used to detect and classify fungus-infected maize (Giacomo and Stefania, Reference Giacomo and Stefania2013), wheat (Soto-Cámara et al., Reference Soto-Cámara, Gaitán-Jurado and Domínguez2012) and soybeans (Wang et al., Reference Wang, Dowell, Ram and Schapaugh2004), to determine the percentage of fungal infection in rice (Sirisomboon et al., Reference Sirisomboon, Putthang and Sirisomboon2013) and to identify the green mottle mosaic virus in cucumber (Lee et al., Reference Lee, Lim and Cho2016). However, this technique has yielded unsatisfactory results for fungal infection determination in rice because the moisture and starch contents in rice affect the overall extent of fungal infection (Sirisomboon et al., Reference Sirisomboon, Putthang and Sirisomboon2013). Numerous studies have been conducted using hyperspectral imaging to detect fungal-infected wheat (Singh et al., Reference Singh, Jayas, Paliwal and White2012) and maize (Del Fiore et al., Reference Del Fiore, Reverberi, Ricelli, Pinzari, Serranti, Fabbri, Bonifazi and Fanelli2010; Williams et al., Reference Williams, Geladi, Britz and Manley2012; Yao et al., Reference Yao, Hruska, Kincaid, Brown, Bhatnagar and Cleveland2013) and to detect bacteria-infected watermelon seeds (Lee et al., Reference Lee, Kim, Song, Oh, Lim, Lee, Kang and Cho2016). One study showed that the electronic nose is a powerful tool for the detection of fungal contamination in wheat; the accuracy obtained using partial least-squares discriminant analysis (PLS-DA) was found to be 85.3% (Paolesse et al., Reference Paolesse, Alimelli, Martinelli, Natale, D'Amico, D'Egidio, Aureli, Ricelli and Fanelli2006). Recently, chlorophyll fluorescence has been used to sort white cabbage seeds, resulting in 97% germination by removing 13.2% of the seeds with very high chlorophyll fluorescence signal from the seed lot (Jalink et al., Reference Jalink, Frandas, Schoor and Bino1998). Similar studies have been conducted to evaluate the seed maturity in cabbage (Dell'Aquila et al., Reference Dell'Aquila, van der Schoor and Jalink2002), tomato (Jalink et al., Reference Jalink, van der Schoor, Birnbaum and Bino1999), barley (Konstantinova et al., Reference Konstantinova, Van Der Schoor, Van Den Bulk and Jalink2002), carrot (Groot et al., Reference Groot, Birnbaum, Rop, Jalink, Forsberg, Kromphardt, Werner and Koch2006) and pepper (Kenanoglu et al., Reference Kenanoglu, Demir and Jalink2013) using chlorophyll fluorescence. Thermal imaging has been used to detect fungal infestations in stored wheat using linear discriminant analysis (LDA) and quadratic discriminant analysis (QDA), with an accuracy of 100% for healthy samples and 96–97% for infected samples (Chelladurai et al., Reference Chelladurai, Jayas and White2010). In a study in which a hyperspectral imaging system (1100–1700 nm) was used to detect aflatoxin B1 (AFB1) contaminants on corn kernels, a PLS-DA was performed, and a minimum classification accuracy of 96.9% was achieved (Kandpal et al., Reference Kandpal, Lee, Kim, Bae and Cho2015). Similar studies have been performed to detect AFB1 contaminants on the surfaces of healthy maize kernels using a short wavelength infrared (SWIR) hyperspectral imaging system (Wang et al., Reference Wang, Heitschmidt, Ni, Windham, Hawkins and Chu2014). The feasibility of short-wave near-infrared hyperspectral (700–1100 nm wavelength range) and digital colour imaging with different statistical discriminant classifiers was investigated for use in the detection of wheat damaged by four different insect species: the rice weevil (Sitophilus oryzae), the lesser grain borer (Rhyzopertha dominica), the rusty grain beetle (Cryptolestes ferrugineus) and the red flour beetle (Tribolium castaneum). Accuracies of 96% were achieved for healthy wheat kernels and 91–100% for insect-damaged wheat kernels (Singh et al., Reference Singh, Jayas, Paliwal and White2010a). Similarly, numerous studies have been performed to detect insect-damaged (Singh et al., Reference Singh, Jayas, Paliwal and White2009a, Reference Singh, Jayas, Paliwal and White2009b, Reference Singh, Jayas, Paliwal and White2010a, Reference Singh, Jayas, Paliwal and White2010b; Serranti et al., Reference Serranti, Cesare and Bonifazi2013) and mildew-damaged (Shahin et al., Reference Shahin, Symons and Hatcher2014) wheat using hyperspectral imaging. Hyperspectral imaging has also been used to detect insect-damaged mung bean (Kaliramesh et al., Reference Kaliramesh, Chelladurai, Jayas, Alagusundaram, White and Fields2013) and insect fragments in semolina (Bhuvaneswari et al., Reference Bhuvaneswari, Fields, White, Sarkar, Singh and Jayas2011) and soybean (Huang et al., Reference Huang, Sha, Rong, Chen, He, Khan and Zhu2013; Chelladurai et al., Reference Chelladurai, Karuppiah, Jayas, Fields and White2014). Soft X-ray imaging technology has been used to detect red flour beetle infestation in wheat. An accuracy of 86% was achieved using textural features with a back-propagation neural network (BPNN) classifier (Karunakaran et al., Reference Karunakaran, Jayas and White2004b). Soft X-ray imaging has also been used to detect internal wheat seed infestation by insects (Karunakaran et al., Reference Karunakaran, Jayas and White2004a) and bug damage in soybean seeds (Pinto et al., Reference Pinto, Cicero, França-Neto and Forti2009). In a recent study, thermal imaging was used to detect insect infestation in wheat with an accuracy of 77.6% for infested seeds and 83% for healthy seeds (Manickavasagan et al., Reference Manickavasagan, Jayas and White2008). A recent study has shown that multispectral imaging can be used for spinach seeds to discriminate uninfected seeds from infected seeds with 80–100% classification rate (Olesen et al., Reference Olesen, Carstensen and Boelt2011).
Table 2. Assessment of insect damages and diseases in seeds using different non-destructive techniques

Quality assessment of seeds: variety identification and classification
Variety identification and classification of seed species using non-destructive techniques has been extensively investigated by researchers worldwide (Table 3). Machine vision has been used to identify four wheat varieties using morphological features and colour features with an accuracy of 95.86%, which suggests that morphological features are more effective than colour features in recognizing wheat varieties (Arefi et al., Reference Arefi, Motlagh and Teimourlou2011). Machine vision has also been used to classify seeds of various species using morphological, colour, textural and wavelet features and to distinguish among species of wheat, barley, oats and rye (Choudhary et al., Reference Choudhary, Paliwal and Jayas2008) and between wheat and barley (Guevara-Hernandez and Gomez-Gil, Reference Guevara-Hernandez and Gomez-Gil2011). Similarly, machine vision has been used to identify nine Iranian wheat seeds based on their varieties, using textural features, with an accuracy of 98.15% (Pourreza et al., Reference Pourreza, Pourreza, Abbaspour-Fard and Sadrnia2012) and to recognize five Chinese corn varieties based on their external features (Chen et al., Reference Chen, Xun, Li and Zhang2010). Machine vision has also been used to identify bean varieties (Venora et al., Reference Venora, Grillo, Ravalli and Cremonini2009), discriminate among wheat grain varieties (Zapotoczny, Reference Zapotoczny2011a, Reference Zapotoczny2011b), identify wheat varieties (Zayas et al., Reference Zayas, Lai and Pomeranz1986; Dubey et al., Reference Dubey, Bhagwat, Shouche and Sainis2006), classify corn (Jingtao et al., Reference Jingtao, Yanyao, Ranbing and Shuli2012; Pazoki et al., Reference Pazoki, Farokhi and Pazoki2013), discriminate among rapeseed varieties (Li et al., Reference Li, Liao, Ou and Jin2007; Kurtulmuş and Ünal Reference Kurtulmuş and Ünal2015), classify pepper seeds (Kurtulmuş et al., Reference Kurtulmuş, Alibaş and Kavdir2016) and classify rice varieties (Rad et al., Reference Rad, Tab and Mollazade2011; Hong et al., Reference Hong, Hai, Lan, Hoang, Hai and Nguyen2015). Accuracy is an important evaluation parameter in variety identification; most of these studies have reported highly accurate results, in the range of 85–100%. In addition, machine vision has been shown to exhibit an overall accuracy of greater than 80% in grading maize (Yi et al., Reference Yi, Junxiong, Wei and Weiguo2007; Wu et al., Reference Wu, Zhang, Song, Li and Lan2013) and soybean (Kılıç et al., Reference Kılıç, Boyacı, Köksel and Küsmenoğlu2007). Recently, an electronic nose was used to distinguish among varieties of wheat seeds with an accuracy of 100% (Zhou et al., Reference Zhou, Wang and Qi2012). Thermal imaging was used in a recent study to identify eight western Canadian wheat varieties. The overall classification accuracies of eight-class model, red-class model (four classes), white-class model (four classes), and pairwise (two-class) model comparisons obtained using a quadratic discriminant method were 76, 87, 79 and 95%, respectively, and those obtained using bootstrap and leave-one-out validation methods were 64, 87, 77 and 91%, respectively (Manickavasagan et al., Reference Manickavasagan, Jayas, White and Paliwal2010). Hyperspectral imaging systems have been used for accurate and reliable discrimination among varieties of maize seeds (Zhang et al., Reference Zhang, Liu, He and Li2012), for classification of four varieties of maize seeds in different years (Huang et al., Reference Huang, Tang, Yang and Zhu2016), for identification of wheat varieties (Choudhary et al., Reference Choudhary, Mahesh, Paliwal and Jayas2009; Zhu et al., Reference Zhu, Wang, Pang, Shan, Wu and Zhao2012), for differentiation of wheat classes grown in western Canada (Mahesh et al., Reference Mahesh, Manickavasagan, Jayas, Paliwal and White2008) and for differentiation among varieties of rice (Kong et al., Reference Kong, Zhang, Liu, Nie and He2013). Some of these applications have achieved a classification accuracy of 100%. Hyperspectral imaging has also been used by several researchers for hardness classification of maize (Williams et al., Reference Williams, Geladi, Fox and Manley2009; McGoverin et al., Reference McGoverin, Engelbrecht, Geladi and Manley2011). Recently, hyperspectral imaging has been used to distinguish among transgenic soybeans (Esteve Agelet et al., Reference Esteve Agelet, Gowen, Hurburgh and O'Donell2012) and rice (Liu et al., Reference Liu, Liu, Lu, Chen, Yang and Zheng2014). Similarly, a NIRS technique has been used to distinguish among herbicide-resistant genetically modified soybean seeds (Lee and Choung, Reference Lee and Choung2011). It has also been demonstrated that multispectral imaging technique can be used to distinguish transgenic- from non-transgenic rice seeds (Liu et al., Reference Liu, Liu, Lu, Chen, Yang and Zheng2014).
Table 3. Assessment of variety identification and classification in seeds using different non-destructive techniques

Quality assessment of seeds: seed viability
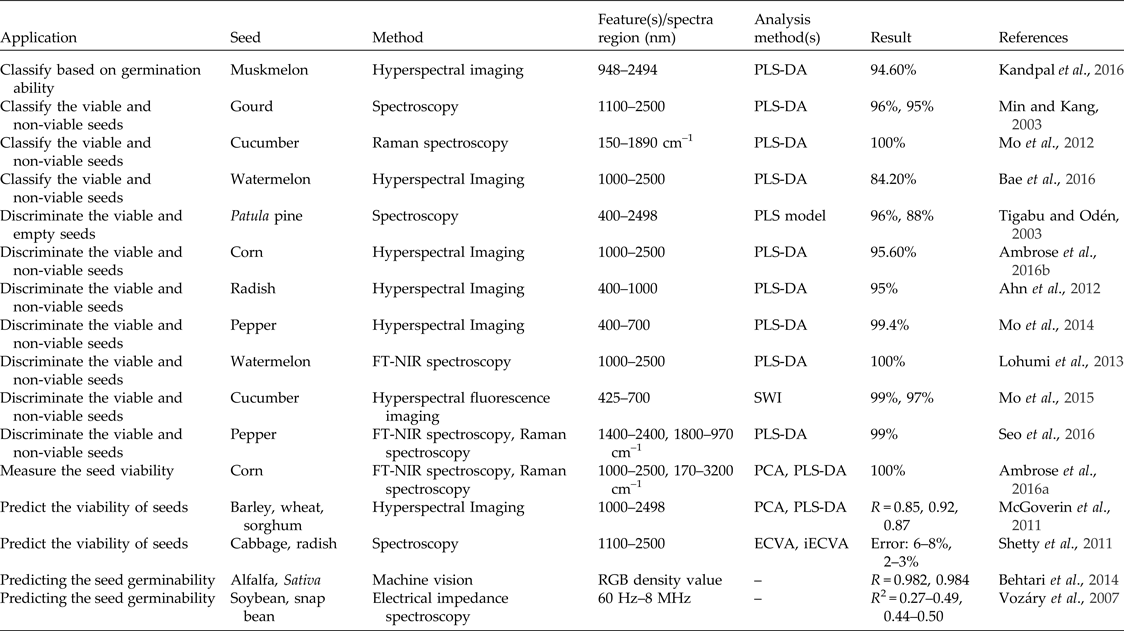
A good-quality seed is one that is capable of germination under various conditions. A non-viable seed is one that fails to germinate even under optimal conditions (Bradbeer, Reference Bradbeer1988). In recent years, non-destructive techniques, mainly spectroscopy and hyperspectral imaging, have been widely used to predict seed viability (Table 4). A machine vision system was used to predict alfalfa and sativa seed germinability using the RGB (red, green, blue) density value with correlation coefficients of 0.982 and 0.984 for alfalfa and sativa, respectively (Behtari et al., Reference Behtari, De Luis and Dabbagh Mohammadi Nasab2014). Researchers have also studied soybean and snap bean seed germinability using electric impedance spectroscopy in the frequency range of 60 Hz to 8 MHz (Vozáry et al., Reference Vozáry, Paine, Kwiatkowski and Taylor2007). Recently, spectroscopy has been used to distinguish viable gourd (Min and Kang, Reference Min and Kang2003), cucumber (Mo et al., Reference Mo, Kang, Lee, Kim, Cho, Lim, Lee and Park2012), patula pine (Tigabu and Odén, Reference Tigabu and Odén2003), watermelon and pepper seeds (Lohumi et al., Reference Lohumi, Mo, Kang, Hong and Cho2013; Seo et al., Reference Seo, Ahn, Lee, Park, Mo and Cho2016) from their non-viable counterparts, to assess corn seed viability (Ambrose et al., Reference Ambrose, Lohumi, Lee and Cho2016) and to predict the viability of cabbage and radish seeds (Shetty et al., Reference Shetty, Min, Gislum, Olesen and Boelt2011). Most of these studies have reported accuracies of more than 90% in viable seed identification. Hyperspectral imaging systems have also been used for accurate and reliable discrimination of viable and non-viable seeds of corn (Ambrose et al., Reference Ambrose, Kandpal, Kim, Lee and Cho2016), radish (Ahn et al., Reference Ahn, Mo, Kang and Cho2012), watermelon (Bae et al., Reference Bae, Seo, Kim, Lohumi, Park and Cho2016) and pepper (Mo et al., Reference Mo, Kim, Lee, Kim, Cho, Lim and Kang2014) with accuracies of 95.6, 95, 84.2 and 99.4%, respectively. Recently, a hyperspectral fluorescence imaging technique was used to extract the fluorescence spectra of cucumber seeds in the 425–700 nm range to discriminate between viable and non-viable cucumber seeds using four types of algorithms. The discrimination accuracies achieved based on the subtraction image, the ratio image and the ratio-subtraction image were 100 and 99.0% for viable and non-viable seeds, respectively (Mo et al., Reference Mo, Kim, Lim, Lee, Kim and Cho2015). Hyperspectral imaging has also been used to classify muskmelon seeds based on germination ability with an accuracy of 94.6%, using a PLS-DA classification algorithm (Kandpal et al., Reference Kandpal, Lohumi, Kim, Kang and Cho2016). Hyperspectral imaging in the range of 1000–2498 nm was able to predict the viability of barley, wheat and sorghum seed with correlation coefficients of 0.85, 0.92 and 0.87, respectively (McGoverin et al., Reference McGoverin, Engelbrecht, Geladi and Manley2011). Recently, multispectral imaging has been demonstrated to be a potential technique to evaluate castor seed viability with 96% correct classification rate at 19 different wavelengths ranging from 375 to 970 nm (Olesen et al., Reference Olesen, Nikneshan, Shrestha, Tadayyon, Deleuran, Boelt and Gislum2015). Other studies have been conducted, using multispectral imaging to examine germination ability and germ length in spinach seeds; with the use of PLS-DA of images of spinach seeds it was possible to classify large spinach seeds from small-sized and medium-sized seeds (Shetty et al., Reference Shetty, Olesen, Gislum, Deleuran and Boelt2012). Infrared thermography has also been used to predict whether a quiescent seed will germinate or die upon water uptake, and the technique was reported to be able to detect imbibition- and germination-associated biophysical and biochemical changes (Kranner et al., Reference Kranner, Kastberger, Hartbauer and Pritchard2010). A similar technique has been used for viability evaluation of lettuce seeds (Kim et al., Reference Kim, Kim, Ahn, Yoo and Cho2013) and to evaluate germination capacity of leguminous plant seeds (Baranowski et al., Reference Baranowski, Mazurek and Walczak2003).
Table 4. Assessment of seed viability using different non-destructive techniques
Summary and future trends
This paper provided an overview of previous studies on seed quality assessment using non-destructive measurement techniques, namely chemical composition (Table 1), insect damage and diseases (Table 2), variety identification and classification (Table 3) and viability (Table 4). Machine vision, spectroscopy, hyperspectral imaging, thermal imaging, electronic nose and soft X-ray imaging are the main techniques to determine seed quality. Among them, spectroscopy and hyperspectral imaging techniques for chemical composition, machine vision, hyperspectral imaging, spectroscopy and soft X-ray imaging for insect and diseases detection, machine vision, thermal imaging and hyperspectral imaging for seed variety identification and classification, and spectroscopy and hyperspectral imaging for viability of seeds has been widely used in research, quality assessment, and for industrial purposes. For this, numerous spectroscopy instruments are commercially available. However, most of the instruments are too expensive to be widely used in practical production. Therefore, one of the main concerns of current researchers is how to decrease the cost while maintaining accuracy of analysis. In contrast, hyperspectral imaging provides both spatial and spectral information and is suitable for both external quality classification and for prediction of internal chemical composition. However, current hyperspectral imaging technology is not widely used compared with spectroscopy. This limitation may be due to the time-consuming process of hyperspectral imaging to generate a hypercube and the large amount of hyperspectral data. As a new technology that has only been studied for over a decade, hyperspectral imaging has a long way to go before it can be moved from laboratories to practical application. Recently, machine vision techniques have been placed as in-line detection and grading systems in actual production. Generally, a complete detection process for machine vision technique includes image acquisition, image processing and analysis, and formulation of decisions. These steps can be accomplished with only one smart camera, considering the increasing development of electronics and microprocessors. Thermal imaging and soft X-ray imaging are of very limited use in seed quality assessment due to high cost, the requirement of a controlled environment as the precision of this instrument fluctuates with environmental condition. The electronic nose technique is commonly used to determine seed quality during storage because it detects chemical interactions between the substrates over the gas sensors and the aromatic compounds. Electronic noses today generally suffer from significant weaknesses which limit their widespread application in seed quality assessment. Their sensing ability is profoundly influenced by ambient factors that are very critical in seed quality assessment. We should address the rapid development of instruments coupled with the improvement of analysis algorithms to help to promote efficient technologies for the seed quality assessment field.
Conclusions
This paper presents an overview of studies that have shown that non-destructive techniques can be used effectively as reliable and accurate tools for the composition prediction, variety identification and classification, quality grading, damage detection, insect infestation detection and viability and germinability prediction of agricultural seeds. These non-destructive techniques are rapid, accurate, reliable and simple tools for quality assessment of seeds. Given the urgent need of the industry for advanced testing methods and rapid development of suitable technologies and instruments, non-destructive techniques exhibit great potential to be dominant methods for quality assessment of seeds.
Acknowledgements
None.
Financial support
This research was partially supported by the Export Strategy Technology Development Program, Ministry of Agriculture, Food and Rural Affairs (MAFRA) and by Golden Seed Project, MAFRA, Ministry of Oceans and Fisheries (MOF), Rural Development Administration (RDA) and Korea Forest Service (KFS), Republic of Korea.
Conflicts of interest
None.









