Introduction
Endozoochory, the dispersal of seeds through the gut passage of animals, facilitates the dispersal of seeds from numerous plant species, facilitating the colonization in new habitats, and the expansion of plant populations (McConkey et al., Reference McConkey, Prasad, Corlett, Campos-Arceiz, Brodie, Rogers and Santamaria2012; Baltzinger et al., Reference Baltzinger, Karimi and Shukla2019). The success of the seed dispersal mechanism is influenced by various factors at each stage of the dispersal process (Cain et al., Reference Cain, Milligan and Strand2000; Baltzinger et al., Reference Baltzinger, Karimi and Shukla2019). In each stage of seed dispersal (i.e., from emigration, transfer, and to immigration), seed dispersal vectors contribute to plant dispersal in terms of both quantity and quality components (Schupp, Reference Schupp1993; van Leeuwen et al., Reference van Leeuwen, Villar, Mendoza Sagrera, Green, Bakker, Soons, Galetti, Jansen, Nolet and Santamaría2022). Seed dispersal effectiveness depends on the quantity of seeds dispersed by animals and whether these seeds can germinate and establish in new habitats (Schupp et al., Reference Schupp, Jordano and Gómez2010). For endozoochory by ungulates, seed dispersal effectiveness has been widely examined in each stage, from ingestion of seeds, transportation of seeds through the animal gut, and to the dissemination stage (Baltzinger et al., Reference Baltzinger, Karimi and Shukla2019).
During the dissemination stage, seeds deposited through endozoochory encounter diverse environmental conditions (i.e., deposit quality) that significantly impact their survival and establishment (Baltzinger et al., Reference Baltzinger, Karimi and Shukla2019). These conditions include moisture level changes following the fecal desiccation, fecal types, feeding regime of animals, nutrient availability and seedling competition within fecal deposits (e.g., Milotić and Hoffmann, Reference Milotić and Hoffmann2016b, Reference Milotić and Hoffmann2017; Baltzinger et al., Reference Baltzinger, Karimi and Shukla2019; Karimi et al., Reference Karimi, Hemami, Esfahani and Baltzinger2020). Animal feces can serve as potential nutrient source containing elements such as nitrogen and potassium (Sakadevan et al., Reference Sakadevan, Mackay and Hedley1993; Williams and Haynes, Reference Williams and Haynes1995; Dai, Reference Dai2000). Simultaneously, animal feces may contain harmful compounds that prevent seed germination (Marambe et al., Reference Marambe, Nagaoka and Ando1993; Meyer and Witmer, Reference Meyer and Witmer1998). The effects of fecal matter on seed germination and plant growth vary depending on the specific plant and animal species involved due to their fecal morphological types and digestion (Milotić and Hoffmann, Reference Milotić and Hoffmann2016b; Guevara-Torres and Facelli, Reference Guevara-Torres and Facelli2023; Ramos et al., Reference Ramos, Campos, Cona and Giordano2024). These vector animal species-specific results emphasize the need of testing the response to each factor, which affects the successful results of endozoochorous seed dispersal. Understanding these effects is crucial for comprehending the dynamics of endozoochorous seed dispersal and early seedling establishment.
Hydropotes inermis argyropus, commonly known as the Korean water deer, is the most prevalent ungulate species on the Korean peninsula. This deer play a significant role in the dispersal of various plant species, especially forbs and those originating from open habitats (Lee et al., Reference Lee, Shin, Ahn, Kim, Kim and Lee2021, Reference Lee, Ryu and Lee2022). A study by Park and Lee (Reference Park and Lee2014) examined the influence of Korean water deer feces on the soil condition and growth of Zea mays seedlings. The authors found that the amount of feces added significantly affected the growth of Zea mays seedlings in nutrient-poor soil. This finding suggests that the fecal components of the deer can provide nutrients to the soil for plants to grow. However, at the same time, animal feces may also contain phytotoxic elements that could impede seed germination (e.g., frugivorous birds, Meyer and Witmer (Reference Meyer and Witmer1998); cattle and horse, (Milotić and Hoffmann, Reference Milotić and Hoffmann2016b, Reference Milotić and Hoffmann2017); cattle, chicken and pig, Marambe et al. (Reference Marambe, Nagaoka and Ando1993)), and still little is known about how the presence of the Korean water deer feces affects the germination and growth of plant species dispersed through the deer endozoochory. Given the relevance of endozoochory, it is imperative to systematically investigate and analyze the specific effects of feces on seed germination within this ecological context.
The objective of this study was to assess the effects of feces itself on seed germination and the early growth of seeds dispersed by the Korean water deer endozoochory through germination tests. Specifically, we investigated whether the presence of feces have an effect on (i) seed germination of species known to be dispersed through endozoochory and (ii) on the early growth of seedlings of 12 plant species previously reported to germinate from the feces of Korean water deer (Lee and Lee, Reference Lee and Lee2020; Lee et al., Reference Lee, Ryu and Lee2022).
Materials and methods
Seed preparation
Based on previous studies (Lee and Lee, Reference Lee and Lee2020; Lee et al., Reference Lee, Ryu and Lee2022), we selected 12 plant species that have been observed to germinate from the feces of the Korean water deer (Table 1). These species had previously been observed to germinate from the feces of the Korean water deer. The seeds of selected plant species were obtained for research purposes from the National Institute of Biological Resources. For species nomenclature, we followed a database managed by the National Institute of Biological Resources (https://species.nibr.go.kr/) and to determine whether each target species was an alien plant in South Korea, we referred to Korea National Arboretum (Reference Korea National Arboretum2019).
Table 1. The list of plant species tested for germination, and their growth form
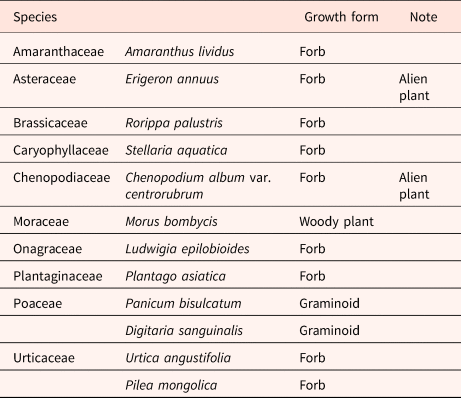
The species that are categorized as alien plants are followed by Korea National Arboretum (Reference Korea National Arboretum2019).
Feces preparation
For the experiments, the feces of the Korean water deer were collected from Taehwa Research Forest and Civilian Control Zone, where previous sampling had been conducted (Lee and Lee, Reference Lee and Lee2020; Lee, et al. Reference Lee, Ryu and Lee2022). The fecal pellet groups were individually stored in zipper bags, transported to the laboratory, and cleaned on their outer surface. Subsequently, the fecal pellets were allowed to air-dry at room temperature. To mitigate the potential effects of different sampling seasons and sites, all collected fecal samples were combined. The feces were ground to avoid possible contamination from external seeds and to ensure a consistent fecal amount could be used in the experiments.
Experimental setup
For the experimental setup, above mentioned 12 plant species were tested for germination under conditions of with (feces) and without the feces (control) of the Korean water deer. For each species, five seeds were sown in each pot, resulting in 10 replications for each treatment (with and without feces), and thus a total of 240 pots were tested. The pots (150 mm in diameter and 135 mm in height) were filled with commercial soil (Baroker, Seoul Bio Co., Ltd., Eumseong, Korea). In each pot with the treatment condition of adding the feces of Korean water deer, 5 g of air-dried and ground feces was added. All seeds were directly sown on the surface of feces or soil to facilitate light availability. The experiment was carried out at the temperature-controlled greenhouse facility at Seoul National University. The temperature of the greenhouse was set at 23°C during the experiments. The pots were watered on a regular basis and kept moist. During the experimental period, the pots were randomly allocated and redistributed every other day. The number of seedlings that germinated for each species (emergence rate) in each treatment was checked 60 days after the initial day of sowing, and the height of each seedling was measured.
Data analysis
To investigate the effects of fecal presence on the seedling emergence rate in each species, a Generalized Linear Model (GLM) of Poisson distribution was applied. To compare the effects of fecal presence on seedling height, the data were validated for homogeneity and homoscedasticity prior to analysis, and the t-test and Wilcoxon rank-sum tests were used. Visualization of the data was conducted using ggplot2 (Wickham, Reference Wickham2016) package. All analyses were conducted in R version 4.2.1 (R Core Team, 2022).
Results
Number of seedlings emerged
All 12 tested plant species germinated. Across all treatments (control and feces) and species, a median of one seed germinated per pot sown with five seeds. For all 12 species tested, the comparison of the seeds that were sown on the feces had no significantly different emergence rate from that of seeds that were sown in soil (P > 0.05; GLM model fitted with Poisson distribution; Fig. 1). The results showed that overall, the feces had no significant impact on the germination rate of any species. Detailed statistics can be found in the Supplementary Table 1.
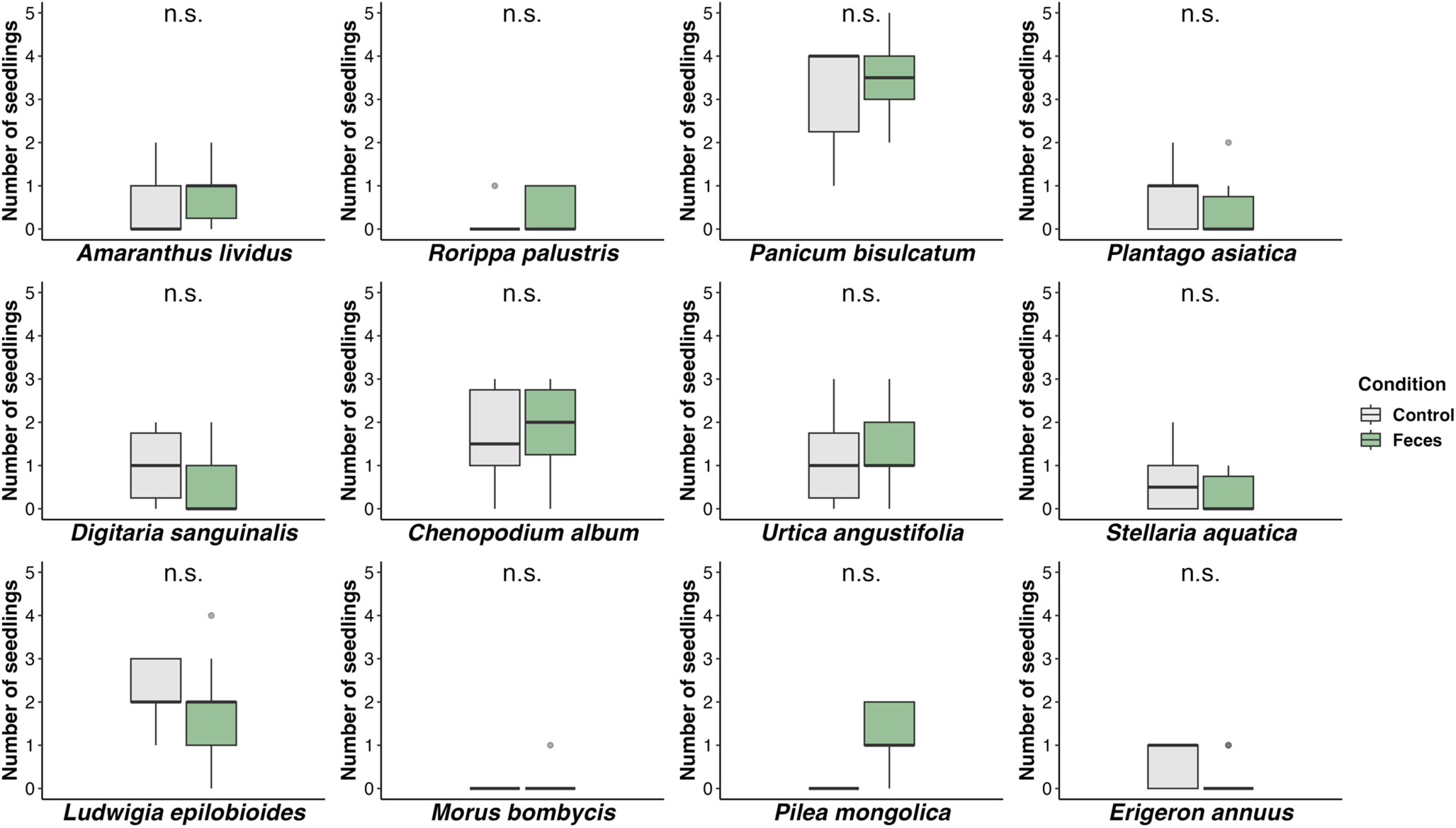
Figure 1. The number of seedlings of each plant species germinated in a condition with feces (Feces) and without feces (Control) of the Korean water deer. n.s. denotes for not significant (P > 0.05) based on the GLM model with Poisson distribution.
Seedling height
On the final day of the germination test, we measured the height of each seedling that germinated. We found that the height of all species that germinated showed no differences from that of control seedlings that were sown on the soil for 11 out of 12 plant species tested (P > 0.05; t-test; Fig. 2; Supplementary Table 2). In the case of Urtica angustifolia sown on the ground feces showed higher seedling height compared to that of the control condition (t-statistic = 2.657; d.f. = 12.138, P = 0.021; t-test).
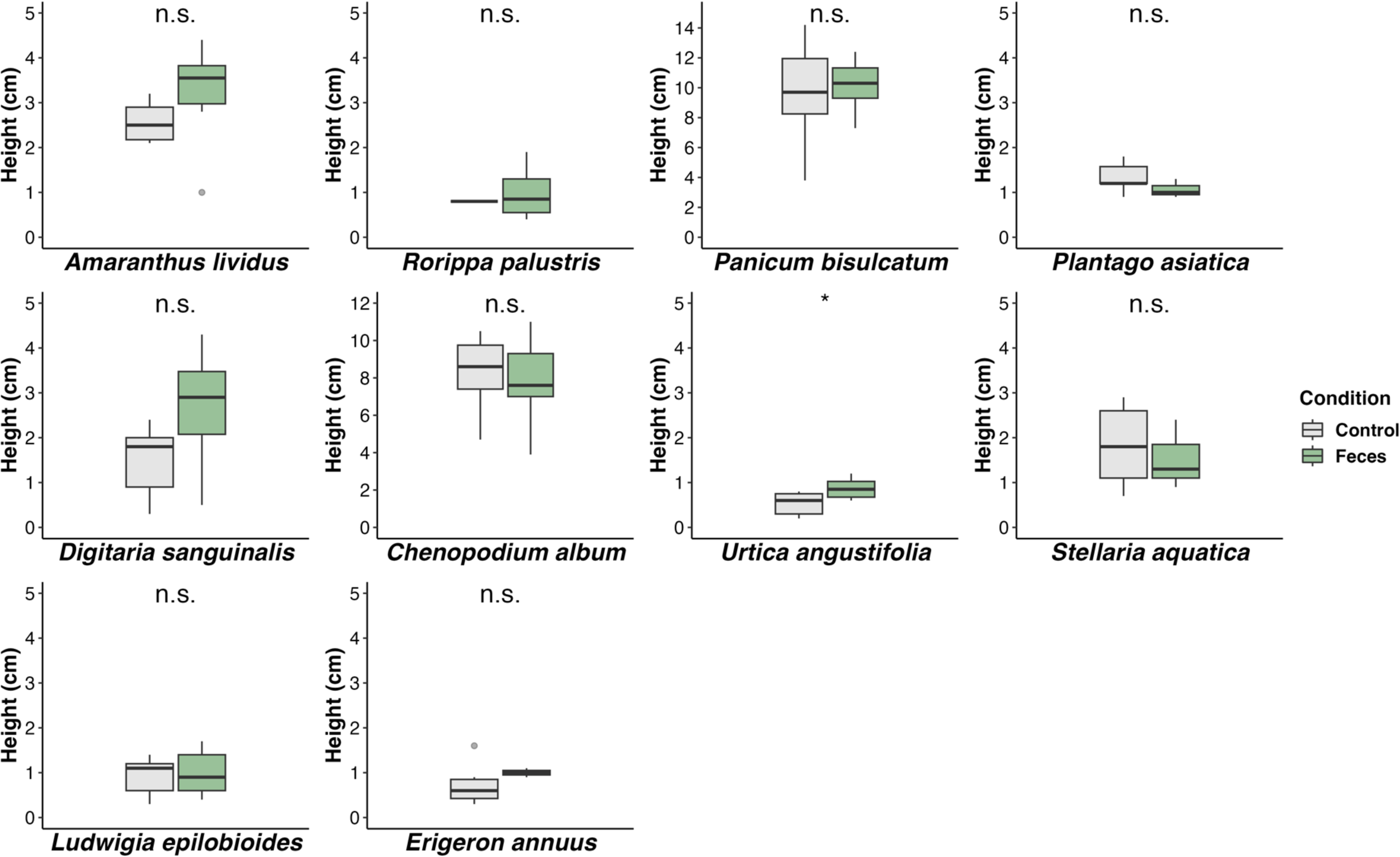
Figure 2. Seedling height of each plant species germinated in a condition with feces (Feces) and without feces (Control) of the Korean water deer. n.s. denotes for not significant and * denotes for significant (P < 0.05) following the t-test.
Discussion
Understanding the effects of feces on seed germination and seedling growth enables us to comprehend the costs and benefits associated with the entire process of endozoochorous seed dispersal. This study aimed to investigate the influence of Korean water deer feces itself on seed germination and initial seedling growth of plant species dispersed through Korean water deer endozoochory. Among the 12 species studied, there were no significant differences in seed performance between those sown with feces and those sown without feces, and no differences in seedling growth for 11 of these species, except for U. angustifolia. Our findings indicate that negligible impact of feces per se on seed germination and initial seedling growth in the context of the Korean water deer endozoochory.
Previous studies suggest that the response of plant species depends on the plant species and physiology of animal species involved (e.g., ruminant and hindgut-fermenting). The results of this study could be associated with the gastrointestinal physiology of Korean water deer, a ruminant species. While the effects of feces on seed germination differed among plant species, certain species exhibited lower germination rates when exposed to feces (Milotić and Hoffmann, Reference Milotić and Hoffmann2016b). These results are also affected by the fermented gut characteristics of cows and horses (Milotić and Hoffmann, Reference Milotić and Hoffmann2016b). Additionally, in a study examining Neltuma flexuosa seed germination and seedling growth with feces from cow, horse and mara, the results varied depending on the animal species (Ramos et al., Reference Ramos, Campos, Cona and Giordano2024). Horse and mara feces showed reduced germination rates, while cow feces had no discernible effects. Regarding seedling growth, the native and wild mara species showed negligible effects, whereas domestic cow and horse species exhibited increased seedling growth compared to normal conditions. Moreover, the effects of native herbivore kangaroo and domestic sheep on different plant species also varied, promoting the growth of aboveground biomass in wallaby grass and reducing wild oat aboveground biomass, depending on the animal species involved (Guevara-Torres and Facelli, Reference Guevara-Torres and Facelli2023). Therefore, it is essential to analyze and compare the results specific to both animal and plant species, to understand the ecological correlation between seed dispersal through endozoochory and associated animals and plants. Furthermore, it is crucial to consider not only the seed germination rate but also the timing of seed germination. Previous research has shown that feces can delay seed germination (Meyer and Florence, Reference Meyer and Florence1996; Ramos Font et al., Reference Ramos Font, González Rebollar and Robles Cruz2015; Milotić and Hoffmann, Reference Milotić and Hoffmann2016b). Additionally, certain species exhibited low germination rates, suggesting that their germination requirements may not have been met, including mortality shortly after germination. For example, Morus bombycis showed a particularly low germination rate.
Overall seedling height showed no significant differences with the addition of feces. However, for U. angustifolia, the addition of feces showed higher growth (Fig. 2). It is important to note that the low germination rate for some species could have obscured the results, which could reduce the statistical power. In our study, we only assessed the early growth of the seedlings, which did not allow for the evaluation of the longer-term effects of feces on seeds of species dispersed through endozoochory. However, a study by Milotić and Hoffmann (Reference Milotić and Hoffmann2016a) demonstrated that over an extended growth period, plants with feces showed higher biomass production compared to those without feces. Related to this, Park and Lee (Reference Park and Lee2014) tested the effects of the Korean water deer feces on the growth of Zea mays, demonstrating that the addition of feces resulted in greater plant material production than in plants without feces in nutrient-poor soil. Although we found negligible effects on early seedling stages, seed dispersal through the Korean water deer endozoochory may have positive consequences by promoting the growth of plant species in the longer term, as the fecal components can release the potential beneficial nutritions such as nitrogen, potassium, phosphorus (Rowarth et al., Reference Rowarth, Gillingham, Tillman and Syers1985; McDowell, Reference McDowell2006; Park and Lee, Reference Park and Lee2014; Park et al., Reference Park, Chun and Lee2015). Moreover, this pattern could also be related to the time it takes for nutrients to be released from fecal materials through physical breakdown and mineralization processes (Rowarth et al., Reference Rowarth, Gillingham, Tillman and Syers1985; Eichberg et al., Reference Eichberg, Storm and Schwabe2007).
According to our previous feeding experiments of the Korean water deer, testing the effects of gut passage on seed germination, the seeds consumed by water deer exhibited a recovery rate of less than 30% across all tested species (Lee et al., Reference Lee, Shin, Ahn, Kim, Kim and Lee2021). It is evident that a considerable proportion of seeds undergo consumption during gut passage, which can be related to the seed traits including seed morphology (Mouissie et al., Reference Mouissie, Van Der Veen, Veen and Van Diggelen2005; Lee et al., Reference Lee, Shin, Ahn, Kim, Kim and Lee2021). The low recovery rate of ingested plant seeds, combined with potential seedling competition, could significantly impact the overall survival and dispersal success of seeds through endozoochory. Based on the current results and our former research on gut passage effects (Lee et al., Reference Lee, Shin, Ahn, Kim, Kim and Lee2021), the deer is not an effective endozoochorous seed disperser. According to a meta-analysis study (Torres et al., Reference Torres, Castano and Carranza-Quiceno2020), the deer family (Cervidae) is not an effective seed dispersal vector for endozoochory, showing that gut passage by deers has no significant effects on seed germination. Furthermore, despite our efforts to assess the impact of Korean water deer feces on seed germination and seedling growth, a thorough experimental design using gut-passed seeds is essential to evaluate the effectiveness of endozoochorous seed dispersal, as the gut passage may alter the chemical and mechanical aspects of seeds (Samuels and Levey, Reference Samuels and Levey2005).
Endozoochorous seed dispersal is a comprehensive process, involving ingestion, defecation, and post-dispersal events. As the overall success of emergence and fruiting in real situations has low probabilities (Eichberg et al., Reference Eichberg, Storm and Schwabe2007), final seedling emergence in real deposition events and fruiting stage also needs to be tested. After deposition, the feces are likely to undergo changes in their nutritional components and moisture (Holter, Reference Holter1991, Reference Holter2016). Moreover, we placed the seeds on the top parts of ground feces; however, in real deposition events, the water deer defecate the seeds in the structure of small fecal pellet groups, which may impose additional physical and biological barriers to seed germination. The feces type of deer is usually fecal pellet groups defecated with several small fecal pellets and is prone to desiccate after deposition (Welch, Reference Welch1985; Eichberg, et al. Reference Eichberg, Storm and Schwabe2007; Milotić and Hoffmann, Reference Milotić and Hoffmann2016b), affecting seed germination due to moisture loss.
After deposition through endozoochory, seeds encounter not only environmental factors such as habitat deposition site, weather, and moisture (Dickinson and Craig, Reference Dickinson and Craig1990), but also, biological components including secondary seed removal by dung beetles (Milotić et al., Reference Milotić, Baltzinger, Eichberg, Eycott, Heurich, Müller, Noriega, Menendez, Stadler and Ádám2019; Urrea-Galeano et al., Reference Urrea-Galeano, Andresen, Coates, Mora Ardila, Díaz Rojas and Ramos-Fernández2019) and seedling competitions (Milotić and Hoffmann, Reference Milotić and Hoffmann2017), and each stage determining the success of endozoochorous seed dispersal. Furthermore, in outdoor environments, the seed germination rate would be decreased due to the fast desiccation in the outdoor environments, and the germination conditions in the outdoor environments compared to greenhouse results in a lower germination rate as well (Milotić and Hoffmann, Reference Milotić and Hoffmann2016b; Karimi et al., Reference Karimi, Hemami, Esfahani and Baltzinger2020). Furthermore, we sowed five seeds to avoid possible intra-species competition; however, in our former Korean water deer results, the highest density showed 161 seedlings per fecal pellet group (Lee and Lee, Reference Lee and Lee2020), suggesting possible higher competition among seedlings. Intra-species competition may have different effects on seedling dispersal patterns as well (Milotić and Hoffmann, Reference Milotić and Hoffmann2017). Therefore, the comprehensive understanding of endozoochorous seed dispersal processes by the Korean water deer needs to be thoroughly investigated from ingestion to defecation to comprehensively understand the costs, benefits, and overall effectiveness.
Acknowledgements
We thank the members of the Plant Ecology Laboratory of Seoul National University for their help with the experiments. We appreciate anonymous reviewers for their constructive comments on the manuscript.
Funding statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No.2017R1A2B4006761).
Competing interests
The authors have no relevant financial or non-financial interests to disclose.






