Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Bradford, Kent J.
and
Somasco, Oscar A.
1994.
Water relations of lettuce seed thermoinhibition. I. Priming and endosperm effects on base water potential.
Seed Science Research,
Vol. 4,
Issue. 1,
p.
1.
Dahal, Peetambar
and
Bradford, Kent J.
1994.
Hydrothermal time analysis of tomato seed germination at suboptimal temperature and reduced water potential.
Seed Science Research,
Vol. 4,
Issue. 2,
p.
71.
Christensen, Maren
Meyer, Susan E.
and
Allen, Phil S.
1996.
A hydrothermal time model of seed after-ripening in Bromus tectorum L..
Seed Science Research,
Vol. 6,
Issue. 4,
p.
155.
Bradford, K. J.
1997.
Basic and Applied Aspects of Seed Biology.
Vol. 30,
Issue. ,
p.
349.
Cantliffe, Daniel J.
Sung, Yu
and
Nascimento, Warley M.
1999.
Horticultural Reviews.
p.
229.
Meyer, Susan E.
Debaene-Gill, Susan B.
and
Allen, Phil S.
2000.
Using hydrothermal time concepts to model seed germination response to temperature, dormancy loss, and priming effects in Elymus elymoides.
Seed Science Research,
Vol. 10,
Issue. 3,
p.
213.
Matilla, Angel J.
2000.
Ethylene in seed formation and germination.
Seed Science Research,
Vol. 10,
Issue. 2,
p.
111.
Alvarado, V.
and
Bradford, K. J.
2002.
A hydrothermal time model explains the cardinal temperatures for seed germination.
Plant, Cell & Environment,
Vol. 25,
Issue. 8,
p.
1061.
Hills, P.N.
and
van Staden, J.
2003.
Thermoinhibition of seed germination.
South African Journal of Botany,
Vol. 69,
Issue. 4,
p.
455.
Nascimento, Warley Marcos
2003.
Ethylene and lettuce seed germination.
Scientia Agricola,
Vol. 60,
Issue. 3,
p.
601.
Batlla, Diego
and
Benech-Arnold, Roberto Luis
2004.
A predictive model for dormancy loss in Polygonum aviculare L. seeds based on changes in population hydrotime parameters.
Seed Science Research,
Vol. 14,
Issue. 3,
p.
277.
Nascimento, Warley Marcos
Cantliffe, Daniel James
and
Huber, Donald John
2004.
Ethylene evolution and endo-beta-mannanase activity during lettuce seed germination at high temperature.
Scientia Agricola,
Vol. 61,
Issue. 2,
p.
156.
Alvarado, Veria
and
Bradford, Kent J.
2005.
Hydrothermal time analysis of seed dormancy in true (botanical) potato seeds.
Seed Science Research,
Vol. 15,
Issue. 2,
p.
77.
Huarte, Roberto
and
Benech-Arnold, Roberto L.
2005.
Incubation under fluctuating temperatures reduces mean base water potential for seed germination in several non-cultivated species.
Seed Science Research,
Vol. 15,
Issue. 2,
p.
89.
Kpczyska, Ewa
Pikna-Grochala, Justyna
and
Kpczyski, Jan
2006.
Hormonal regulation of tomato seed germination at a supraoptimal temperature.
Acta Physiologiae Plantarum,
Vol. 28,
Issue. 3,
p.
225.
Meyer, Susan E.
and
Allen, Phil S.
2009.
Predicting seed dormancy loss and germination timing for Bromus tectorum in a semi-arid environment using hydrothermal time models.
Seed Science Research,
Vol. 19,
Issue. 4,
p.
225.
Schwember, Andrés R.
and
Bradford, Kent J.
2010.
A genetic locus and gene expression patterns associated with the priming effect on lettuce seed germination at elevated temperatures.
Plant Molecular Biology,
Vol. 73,
Issue. 1-2,
p.
105.
Dong, Tingting
Tong, Jianhua
Xiao, Langtao
Cheng, Hongyan
and
Song, Songquan
2012.
Nitrate, abscisic acid and gibberellin interactions on the thermoinhibition of lettuce seed germination.
Plant Growth Regulation,
Vol. 66,
Issue. 2,
p.
191.
Deng, Z.
and
Song, S.
2012.
Sodium nitroprusside, ferricyanide, nitrite and nitrate decrease the thermo-dormancy of lettuce seed germination in a nitric oxide-dependent manner in light.
South African Journal of Botany,
Vol. 78,
Issue. ,
p.
139.
Huo, Heqiang
Dahal, Peetambar
Kunusoth, Keshavulu
McCallum, Claire M.
and
Bradford, Kent J.
2013.
Expression of9-cis-EPOXYCAROTENOID DIOXYGENASE4Is Essential for Thermoinhibition of Lettuce Seed Germination but Not for Seed Development or Stress Tolerance .
The Plant Cell,
Vol. 25,
Issue. 3,
p.
884.
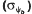 and hydrotime constants (θH) estimated by the model were used to predict the germination rates at reduced ψ and supraoptimal temperatures in the presence or absence of ACC. The distribution of ψb values among seeds in the population (ψb(g)) increased (became more positive) as the imbibition temperature increased, indicating a greater sensitivity of seeds to reduced ψ at thermoinhibitory temperatures. Slitting the seeds at the cotyledonary end to disrupt the integrity of the endosperm extended the high temperature range for germination and maintained lower ψb(g) distributions compared with intact seeds as temperature increased. ACC (10 mm) in the imbibition solution raised the upper temperature limit for germination by about 2°C, and also lowered ψb(g) values by 0.1 to 0.2 MPa. The effect of ACC on ψb(g) was more pronounced at higher imbibition temperatures and in slit seeds. ACC (via conversion to ethylene) extends the high temperature limit for lettuce seed germination by acting in the embryo to maintain a lower ψ threshold for the initiation of growth as temperature increases. Evidence from other species as well suggests that this may be a general mechanism for the action of ethylene in promoting seed germination.
and hydrotime constants (θH) estimated by the model were used to predict the germination rates at reduced ψ and supraoptimal temperatures in the presence or absence of ACC. The distribution of ψb values among seeds in the population (ψb(g)) increased (became more positive) as the imbibition temperature increased, indicating a greater sensitivity of seeds to reduced ψ at thermoinhibitory temperatures. Slitting the seeds at the cotyledonary end to disrupt the integrity of the endosperm extended the high temperature range for germination and maintained lower ψb(g) distributions compared with intact seeds as temperature increased. ACC (10 mm) in the imbibition solution raised the upper temperature limit for germination by about 2°C, and also lowered ψb(g) values by 0.1 to 0.2 MPa. The effect of ACC on ψb(g) was more pronounced at higher imbibition temperatures and in slit seeds. ACC (via conversion to ethylene) extends the high temperature limit for lettuce seed germination by acting in the embryo to maintain a lower ψ threshold for the initiation of growth as temperature increases. Evidence from other species as well suggests that this may be a general mechanism for the action of ethylene in promoting seed germination.