Stress is one of the most extensively studied adaptive responses over the last century. Operationally, stress is understood as the organism’s response to perceived demands or threats (Levine & Ursin, Reference Levine, Ursin, Brown, Koob and Rivier1991). Exposure to stressful events leads to the activation of autonomic stress systems with the aim of restoring homeostatic levels (Ulrich-Lai & Herman, Reference Ulrich-Lai and Herman2009). Physiologically, this entails an increase in the activity of the sympatho-adrenal-medullary (SAM) and hypothalamus-pituitary-adrenal (HPA) axes. Activation of the SAM axis results in the release of circulating catecholamines, such as adrenaline and noradrenaline, ultimately increasing heart rate or blood pressure (McEwen, Reference McEwen2008; Ulrich-Lai & Herman, Reference Ulrich-Lai and Herman2009). On the other hand, the parallel activation of the better-known HPA axis leads to the release of glucocorticoids into the bloodstream, such as cortisol (Ulrich-Lai & Herman, Reference Ulrich-Lai and Herman2009).
In the field of experimental stress research, two main types of stressors have traditionally been utilized: physical stressors (e.g., physical pain) and psychological stressors (e.g., difficult tasks) (Shields, Reference Shields2020). A variety of stress tools of both natures have emerged over time, including high-difficulty tasks, such as progressive matrices or the Stroop test (Ferreira, Reference Ferreira2019; Gianaros et al., Reference Gianaros, Derbtshire, May, Siegle, Gamalo and Jennings2005). However, due to the need for standardized tools to yield comparable results, protocols specifically designed for stress induction have become prevalent. Among them, two have stood out in the literature: the cold pressor test (CPT) (Lovallo, Reference Lovallo1975), in which the participant must immerse their hand in ice water (physical stressor); and the trier social stress test (TSST) (Kirschbaum et al., Reference Kirschbaum, Pirke and Hellhammer1993), where the participant must undergo a job interview (psychological stressor).
These two stress tools have been the gold standard in acute stress induction. However, it has been observed that the type of stressor utilized activates different systems involved in the stress response. Physical stressors entail activation of the autonomic nervous system (HPA axis) through an immediate bodily reaction that requires activation of the hypothalamus and brainstem (Ulrich-Lai & Herman, Reference Ulrich-Lai and Herman2009). In contrast, psychological stressors activate areas such as the thalamus or frontal lobes, with prefrontal-limbic connections that consequently activate the HPA axis (Dickerson & Kemeny, Reference Dickerson and Kemeny2004). These differences lead to observations that the TSST appears to be more effective in maintaining elevated glucocorticoid levels over a longer period (Schwabe et al., Reference Schwabe, Haddad and Schachinger2008). However, physical stressors seem to have greater ease in activating the SAM axis (typically controlled via blood pressure, e.g., Chrousos, Reference Chrousos2009; Chu et al., Reference Chu, Marwaha, Sanvictores and Ayers2024; Lundberg & Frankenhaeuser, Reference Lundberg and Frankenhaeuser1980). Hence, the higher concentration of glucocorticoids when participants are exposed to the TSST versus the CPT could be related to the nature of the stressor, making both types of stressors relevant for eliciting experimental stress (e.g., Skoluda et al., Reference Skoluda, Strahler, Schlotz, Niederberger, Marques, Fischer, Thoma, Spoerri, Ehlert and Nater2015).
Therefore, subsequent protocols have aimed to integrate both the physical and psychological components of the stressors. A new version of the CPT, the socially evaluated cold pressor test (SECPT) (Schwabe et al., Reference Schwabe, Haddad and Schachinger2008), was created. Although the SECPT has generally yielded better results than the CPT, it does not elicit as high stress responses as the TSST (Schwabe & Wolf, Reference Schwabe and Wolf2009; Smeets, Reference Smeets2011). This led Smeets et al. (Reference Smeets, Cornelisse, Quaedflieg, Meyer, Jelicic and Merckelbach2012) to analyze which components were the most effective in these protocols and merge them into the Maastricht Acute Stress Test (MAST). The MAST combines the immersion of the hand into ice water as the physical stressor and a complex arithmetic task as the psychological stressor. This tool has been validated to elicit robust physiological and psychological stress responses (e.g., Capello & Markus, Reference Capello and Markus2014; Meyer et al., Reference Meyer, Smeets, Giesbrecht, Quaedflieg and Merckelbach2013; Shilton et al., Reference Shilton, Laycock and Crewther2017; Smeets et al., Reference Smeets, Cornelisse, Quaedflieg, Meyer, Jelicic and Merckelbach2012), being more effective in eliciting these responses than both the CPT and the SECPT and having similar benchmark values to the TSST (Smeets et al., Reference Smeets, Cornelisse, Quaedflieg, Meyer, Jelicic and Merckelbach2012).
However, it should be noted that there are many factors influencing stress reactivity, such as psychopathological risk. Thus, higher basal levels of depression and anxiety are associated with increased physiological (Kibler & Ma, Reference Kibler and Ma2004; Light et al., Reference Light, Kothandapani and Allen1998) and psychological stress reactivity (de Rooij et al., Reference de Rooij, Schene, Phillips and Roseboom2010). However, other studies revealed that individuals with higher depressive symptoms exhibit a reduced cardiovascular response following stressor exposure (e.g., Chida & Hamer, Reference Chida and Hamer2008; Phillips et al., Reference Phillips, Hunt, Der and Carroll2011). On the other hand, individuals who perceive the task as controllable appear to have lower stress reactivity (Glass et al., Reference Glass, Reim and Singer1971). Another factor influencing stress reactivity is sex, as some studies have found that men tend to have higher reactivity to acute stress than women (Kirschbaum et al., Reference Kirschbaum, Wüst and Hellhammer1992; Liu et al., Reference Liu, Ein, Peck, Huang, Pruessner and Vickers2017). Furthermore, beyond the need to address all these variables, an element that appears to be consistently overlooked when conducting stress research is the cultural factor. Thus, it has been found that the glucocorticoid response differs among cultures (e.g., Miller & Kirschbaum, Reference Miller and Kirschbaum2019; Souza-Talarico et al., Reference Souza-Talarico, Plusquellec, Lupien, Fiocco and Suchecki2014). Consequently, cultural differences are found in stress appraisal, coping, reactivity, and maintenance (Bernardi et al., Reference Bernardi, Engelbrecht and Jobson2019; Popa et al., Reference Popa, Guillet and Mullet2014; Urizar et al., Reference Urizar, Yim, Kofman, Tilka, Miller, Freche and Johnson2019), as well as pain beliefs and tolerance (Hsieh et al., Reference Hsieh, Tripp, Ji and Sullivan2010; Nayak et al., Reference Nayak, Shiflett, Eshun and Levine2000). A recent meta-analysis by Miller and Kirschbaum (Reference Miller and Kirschbaum2019) evaluated reactivity to the TSST in different cultures, reporting that Spanish people exhibited different levels of stress reactivity than other cultures, such as the Dutch and Australian population, where the MAST has already been validated (Smeets et al., Reference Smeets, Cornelisse, Quaedflieg, Meyer, Jelicic and Merckelbach2012 and Shilton et al., Reference Shilton, Laycock and Crewther2017, respectively).
Therefore, it is important for standardized stress tools to be validated across cultures, ensuring their efficacy beyond the population in which they were initially tested. However, to our knowledge, no validation of the MAST has been conducted in the Spanish population to assess its efficacy in eliciting SAM axis stress responses. The present study aims to validate and evaluate the efficacy of the MAST in eliciting both psychological and physiological stress responses in the Spanish population.
From this main objective, several specific objectives and hypotheses emerge: (i) Analyze sex differences in reactivity to acute stress. It is expected to find a greater stress response in men than in women. (ii) Evaluate differences in stress reactivity depending on baseline levels of depression, anxiety, and stress. While we expect to find greater psychological reactivity among participants with higher baseline psychopathological risk, the hypothesis is uncertain regarding cardiovascular reactivity. (iii) Examine whether the performance index in mental arithmetic of the MAST is related to stress reactivity. We expect to find that higher arithmetic performance is associated with lower reactivity.
Method
Participants
To determine the required sample size, an a-priori power analysis was conducted using G*Power software (version 3.1, Faul et al., Reference Faul, Erdfelder, Lang and Buchner2007), with α set at .05 and 1 − β at .9. A review on stress induction studies indicated that the typically observed effect size is medium to high. Thus, being conservative, for an effect size estimated as medium (ηp2 = .06), is needed a minimum of 88 participants (selecting F tests, repeated-measures ANOVA in G*Power, with four groups [Group: Experimental versus Control × Sex: Men versus Women]). Anticipating potential withdrawals from the experiment, a sample size approximately 30% larger than necessary was recruited.
Therefore, the main sample consisted of a total of 120 participants, who were undergraduate psychology students at the University of Seville that participated in the study for course credits. These data were collected as part of a larger study intended to evaluate the effects of stress on prepulse inhibition of the startle response and cognitive function (Santos-Carrasco and De la Casa, Reference Santos-Carrasco and De la Casa2024). Regarding inclusion criteria, the study included young adult Spanish participants who did not have a current or recent history (last 6 months) of any psychological, neurological, endocrine, or cardiovascular disorder, nor drug consumption (alcohol, psychoactive drugs, and other substances). In addition, it was verified that they did not present a baseline risk score for depression, anxiety, or stress through the DASS-21 scale. Thus, out of the total of 120 participants, one was excluded for presenting hypertension values and 23 were excluded for having high scores in the DASS-21 classified as “Severe” or “Extremely Severe” (following the guidelines of Shields et al., Reference Shields2020).
This left a total sample of 96 participants, who were randomly assigned to two groups: Experimental Group (n = 47; M/W: 18/29) and Control Group (n = 49; M/W = 16/33). The mean age for the groups was similar, with 20.55 years (SD = 3.16) for the Experimental Group and 21.29 years (SD = 4.19) for the Control Group. However, in secondary analyses, where the relationship between depression, anxiety, and stress levels with stress reactivity was examined, the complete sample was considered except for the participant with hypertension (n = 119; Control Group = 60 participants [M/W = 21/39]; Experimental Group = 59 participants [M/W = 21/38]).
Instruments
Depression, anxiety and stress scale (DASS-21)
The Depression, Anxiety, and Stress Scale (DASS-21) developed by Lovibond & Lovibond (Reference Lovibond and Lovibond1995) was administered. This self-administered scale consists of a total of 21 Likert-type items with four options, making it a cost-effective screening tool for detecting depressive, anxious, or stress symptoms (e.g., Ruiz et al., Reference Ruiz, Martín, Falcón and González2017). The Spanish validated version by Daza et al. (Reference Daza, Novy, Stanley and Averill2002) was utilized. This version has demonstrated high levels of internal consistency in both Spanish university and general populations, with Cronbach’s alpha coefficients ranging from 0.90 to 0.95 (Fonseca-Pedrero et al., Reference Fonseca-Pedrero, Paino, Lemos-Giráldez and Muñiz2010; Ruiz et al., Reference Ruiz, Martín, Falcón and González2017). The subscales show alpha values between 0.80 and 0.92 for the depression subscale, 0.73–0.87 for the anxiety subscale, and 0.81 to 0.86 for the stress subscale. In addition, it presents good levels of convergent, discriminant, and internal validity (Daza et al., Reference Daza, Novy, Stanley and Averill2002), with a hierarchical factor structure of three first-order factors (depression, anxiety, and stress) and a second-order factor (emotional symptoms) (e.g., Fonseca-Pedrero et al., Reference Fonseca-Pedrero, Paino, Lemos-Giráldez and Muñiz2010; Ruiz et al., Reference Ruiz, Martín, Falcón and González2017). In the current sample, Cronbach’s alpha coefficients were 0.85, 0.81, 0.62, and 0.68 for the total score of the DASS-21 and the subscales of depression, anxiety, and stress, respectively.
Maastricht acute stress test (MAST)
To induce acute stress, the standardized MAST protocol (Smeets et al., Reference Smeets, Cornelisse, Quaedflieg, Meyer, Jelicic and Merckelbach2012) was used, which has been validated for inducing both physiological and psychological stress (e.g., Moses et al., Reference Moses, Gray, Mischel and Greenwald2023; Shilton et al., Reference Shilton, Laycock and Crewther2017). To adapt the MAST protocol for the Spanish sample, two independent translations were performed by two translators, following the back-translation procedure according to international guidelines and recommendations (Muñiz et al., Reference Muñiz, Elosua and Hambleton2013; Muñiz & Bartram, Reference Muñiz and Bartram2007). Specifically, a bilingual native Spanish–English translator translated the MAST protocol from English to Spanish. Then, a bilingual native English–Spanish translator translated the protocol from Spanish back into English. Following this, the scale was retranslated into Spanish by an independent reviewer using the back-translation procedure. Finally, discrepancies between the original protocol and the back-translated version were resolved by members of the research team. Subsequently, a pilot test was conducted with five Spanish participants (two males, three females; aged 18–27 years) to ensure comprehension of all instructions. After making necessary corrections, the final version of the MAST for the Spanish population was obtained.
Participants in the experimental group underwent the MAST protocol, which starts with a 5 min preparation period where they read instructions on PowerPoint slides. This was followed by a 10 min stress induction phase, combining psychological and physical stress trials. During the psychological trials, participants count backwards from 2043 in steps of 17, and during the physical trials, they immerse their hand in cold water (4–6°C). The experimenter continuously monitors them, providing only negative feedback, and participants believe they are being videotaped. The control group performed a non-stress-related task, alternating between submerging their hand in warm water (35–37°C) and counting aloud from 1 to 25. There was no performance evaluation or videotaping in the control group.
As a novel element compared to previous studies using the MAST, the correct arithmetical responses of participants in the experimental group were registered during the psychological stress trials.
State-trait anxiety inventory (STAI)
To assess trait anxiety at baseline, the STAI was administered in its trait version to screen for participants with elevated anxiety levels. This self-report scale, developed by Spielberger et al. (Reference Spielberger, Gorsuch and Lushene1982), includes the trait anxiety subscale (STAI-T), consisting of 20 Likert-type items with four response options. In addition, to evaluate changes in state anxiety from baseline (prior to MAST) to post-stress, the state anxiety subscale of the STAI (STAI-S) was administered just before and immediately after completing the protocol. This subscale also comprises 20 Likert-type items but focuses on assessing current levels of anxiety.
The Spanish version of the inventory was used (Buela-Casal et al., Reference Buela-Casal, Guillén-Riquelme and Seisdedos Cubero2011). This version has a rescaled scoring (ranging from 0 to 3 points), resulting in a total score ranging from 0 to 60. This version of the STAI demonstrates high internal consistency, with Cronbach’s alpha values ranging from 0.80 to 0.97 for the STAI-S, and between 0.88 and 0.95 for the STAI-T (Fonseca-Pedrero et al., Reference Fonseca-Pedrero, Paino, Sierra-Baigrie, Lennos-Girald and Muniz2012; Guillén Riquelme & Buela Casal, Reference Guillén-Riquelme and Buela Casal2011; Ortuño Sierra et al., Reference Ortuño Sierra, García Velasco, Inchausti, Debbané and Fonseca Pedrero2016). The scale demonstrates good test-retest reliability and evidence supporting its internal structure for both the three-factor (Guillén Riquelme & Buela Casal, 2011) and four-factor models (Fonseca-Pedrero et al., Reference Fonseca-Pedrero, Paino, Sierra-Baigrie, Lennos-Girald and Muniz2012; Ortuño Sierra et al., Reference Ortuño Sierra, García Velasco, Inchausti, Debbané and Fonseca Pedrero2016). It also shows concurrent validity when compared with other anxiety scales such as the IPAT, TMAS, and Burns-A (Ortuño Sierra et al., Reference Ortuño Sierra, García Velasco, Inchausti, Debbané and Fonseca Pedrero2016).
In the current sample, Cronbach’s alpha coefficients were 0.89, 0.89, and 0.92 for the STAI-T, the STAI-S before the MAST and the STAI-S after the MAST, respectively. Temporal stability was calculated using the test-retest reliability coefficient known as intraclass correlation coefficient (ICC, selecting two-way random for absolute agreement of the average measure). To do this, the data from the control group and the experimental group were analyzed separately, as each underwent a different treatment (stress induction via MAST or a stress-free task). In the current sample, the ICC for the STAI-S in the control group was 0.88, and for the experimental group, it was 0.73 (all p’s <.001).
Positive affect and negative affect scales (PANAS)
The PANAS scale (Watson et al., Reference Watson, Clark and Tellegen1988) was also administered before the MAST and immediately after stress induction. This scale assesses both positive and negative affect through two subscales of 10 items each, where participants rate how they currently identify with various positive (positive affect) and negative (negative affect) items on a scale from 1 to 5. The Spanish version of the PANAS (Sandin et al., Reference Sandin, Chorot, Lostao, Joiner, Santed and Valiente1999) was utilized. This version of the PANAS demonstrates high internal consistency, with Cronbach’s alpha values ranging from 0.86 to 0.90 for the positive affect subscale and from 0.84 to 0.91 for the negative affect subscale, both in university students and the general population (e.g., Ortuño-Sierra et al., Reference Ortuño-Sierra, Santarén-Rosell, de Albéniz and Fonseca-Pedrero2015; Sandin et al., Reference Sandin, Chorot, Lostao, Joiner, Santed and Valiente1999). In addition, the scale shows evidence supporting its internal structure for both a two-dimension affect model (Sandin et al., Reference Sandin, Chorot, Lostao, Joiner, Santed and Valiente1999) and a three-dimension model (Ortuño-Sierra et al., Reference Ortuño-Sierra, Santarén-Rosell, de Albéniz and Fonseca-Pedrero2015).
In the current sample, Cronbach’s alpha coefficients were 0.75, 0.82, and 0.89 for the total score of the PANAS, and the subscales of positive and negative affect before the MAST, respectively. After the MAST, the alpha values for these indices were 0.74, 0.85, and 0.90. In addition, temporal stability was calculated through the test–retest reliability coefficient known as ICC (selecting two-way random for absolute agreement of the average measure). To do this, the data from the control group and the experimental group were analyzed separately, as each underwent a different treatment (stress induction via MAST or a stress-free task). In the current sample, the ICC value for positive affect was 0.91, and for negative affect, it was 0.77 for the control group. For the experimental group, the ICC values were 0.79 and 0.66 for the positive and negative affect subscales, respectively (all p’s < .001).
Physiological stress measures
Parameters directly related to the sympathetic-adrenal-medullary (SAM) axis were monitored, as this system provides reliable markers of stress reactivity (e.g., Chrousos, Reference Chrousos2009; Chu et al., Reference Chu, Marwaha, Sanvictores and Ayers2024; Skoluda et al., Reference Skoluda, Strahler, Schlotz, Niederberger, Marques, Fischer, Thoma, Spoerri, Ehlert and Nater2015). Thus, systolic blood pressure (SBP), diastolic blood pressure (DBP), as well as heart rate (HR) were evaluated. These measurements were registered with an automatic oscillometer wrist cuff positioned on the upper participant’s arm (Medisana MTP PLUS) a device already validated in previous studies (Erdem et al., Reference Erdem, Aydogdu and Akpolat2011).
The physiological stress measurements were registered at four consecutive time points for each participant to obtain a comprehensive monitoring of the duration of acute stress at the physiological level. Thus, measurements were extended up to 30 min following exposure to the protocol, following the procedure outlined by Shilton et al. (Reference Shilton, Laycock and Crewther2017). Accordingly, measurements were obtained at baseline before the stress induction protocol [TBase], immediately after completing the MAST [T0], and at 15 [T15] and 35 min [T35] after protocol completion.
Procedure
This study was approved by the Research Ethics Committee of the University of Seville (0559-N23). The experimental sessions were conducted between 9:00 AM and 7:00 PM in an isolated room. Participants were instructed not to consume caffeinated, energy, or sugary drinks, as well as to avoid heavy meals, nicotine, alcohol, or other drugs, and intense physical exercise in the 2 h preceding the study. Upon arrival, participants read the study information sheet, and they voluntarily decided to participate by signing the informed consent form. Subsequently, participants were interviewed to check inclusion criteria, after which they completed the DASS-21, STAI-T, STAI-S, and PANAS scales, followed by the initial measurement of blood pressure and heart rate [TBase]. Afterward, participants were exposed to either the MAST protocol or the non-stress-related control task. Upon completion, physiological and psychological stress measures were reassessed [T0]. Blood pressure and heart rate were reevaluated at 15 [T15] and 35 min [T35] following MAST exposure.
Data analysis
All statistical analyses were conducted using version 29 of SPSS. To determine the significance, alpha was set at 0.05 for all tests. Omega squared (ω 2) was used as the effect size measure in all ANOVA analyses. In addition, the assumption of normality was tested using the Shapiro-Wilk normality test. The Greenhouse–Geisser correction was applied when the sphericity assumption was violated, as verified using Mauchly’s sphericity test. Post-hoc analyses using Bonferroni correction for multiple comparisons were performed. The Bonferroni method was chosen as it is sufficiently conservative and less susceptible to Type I errors due to its limits on alpha inflation (e.g., McHugh, Reference McHugh2011; Midway et al., Reference Midway, Robertson, Flinn and Kaller2020). When the assumption of normality was not met, the corresponding non-parametric test was utilized. There was no missing data.
Results
Demographic and baseline differences
To assess age and sex differences between groups, Mann–Whitney’s tests were used. There were no differences between groups in terms of age (u = 1210.5, p = .65, d = .05) nor sex (u = 1086.5, p = .57, d = .06).
Mean scores on the DASS-21 subscales (depression, anxiety, and stress) and STAI-T are shown in Table 1. A mixed 2 × 2 ANOVA (Group: Experimental versus Control × Sex: Men versus Women) was conducted on DASS-21 subscales and STAI-T scores. The main effect of Group was not significant in any case (all F’s < 1). The main effect of Sex on stress and trait anxiety scores was significant, F(1.92) = 3.97, p = .049, ω 2 = .03, and F(1.92) = 7.77, p = .006, ω 2 = .065, respectively, with women reporting higher levels of both stress and trait anxiety than men. The main effect of sex on depression score was non-significant (p = .215). Only the Group × Sex interaction for the anxiety score was significant, F(1.92) = 11.23, p = .001, ω 2 = .096 (all p’s > .15 for the remaining interactions). Post-hoc comparisons (Bonferroni correction for multiple comparisons, p_bonf < .05) conducted to identify the source of the Group × Sex interaction revealed that women in the control group reported more anxiety than men but the opposite occurred in the experimental group.
Table 1. Mean scores for STAI-T and DASS-21 (depression, anxiety, and stress subscales) before experimental treatment and STAI-S, PANAS+, and PANAS- scores before and after response to the MAST in both groups, stratified by gender. Standard deviations appear between brackets

Physiological and psychological stress responses
Regarding physiological stress responses, preliminary one-way ANOVAs were conducted on baseline SBP, DBP, and HR with Group and Sex as main factors (Groups: Control versus Experimental × Sex: Men versus Women). The main effect of groups was non-significant for all cardiovascular measures (all F’s ≤ 1). However, significant main effects of sex were found for SBP (F(1.92) = 10.57, p = .002, ω 2 = .092) and HR values (F(1.92) = 4.05, p = .047, ω 2 = .031), with women presenting lower SBP and higher HR than men. Neither the main effect of sex for DBP nor any Group × Sex interaction was significant (all p’s > .29). The means, standard deviations, and statistics for all the one-way ANOVA comparisons conducted are given in Table S1 (Supplementary Material).
The mean scores of SBP are displayed in Figure 1. The analyses of SBP were conducted with a 2 × 2 × 4 repeated measures mixed ANOVA (Group: Control versus Experimental × Sex: Men versus Women × Period: TBaseline versus T0 versus T15 versus T35). The main effect of Period was significant, F(3.276) = 4.13, p = .007, ω 2 = .007, revealing a progressive reduction of SBP in both groups. The Period × Group interaction was also significant, F(3.276) = 8.07, p < .001, ω 2 = .017. Post-hoc analyses (Bonferroni correction for multiple comparisons, p_bonf < .05) revealed that the interaction was due to a gradual reduction in SBP for the Control Group, and an increase for the Experimental Group from TBaseline to T0 (immediately after the MAST protocol). The SBP peak in the experimental group at T0 was significantly higher than all other time points (all p’s bonf < .042). The main effect of Group and Sex was also significant, F(1.92) = 10.46, p = .002, ω 2 = .048, and F(1.92) = 21.88, p < .001, ω 2 = .101, respectively. The main effect of Group was due to participants in the Experimental Group showing an overall higher SBP than the Control Group. The main effect of Sex reflects lower SBP values for women than for men at all time points in both groups. Neither the Period × Sex, Group × Sex, nor the three-way interactions were significant (all p’s > .13).

Figure 1. Mean systolic blood pressure values before and after the MAST for Control and Experimental groups, stratified by gender. Error bars represent SEMs.
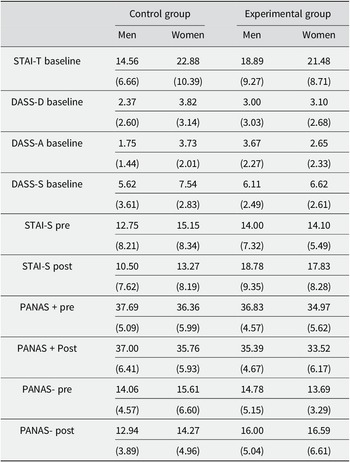
All DBP results obtained by the participants can be observed in Figure 2. A 2 × 2 × 4 repeated-measures mixed ANOVA was conducted on DBP scores (Group: Control versus Experimental × Sex: Men versus Women × Period: TBaseline versus T0 versus T15 versus T35). Mauchly’s test of sphericity indicated a violation of the sphericity assumption for DBP measures, so the Greenhouse-Geisser correction was used. The main effect of Period was significant, F(2.224) = 4.39, p = .009, ω 2 = .009, showing a progressive decrease of DBP in both groups. The Period × Group interaction was also significant, F(2.224) = 6.13, p = .001, ω 2 = .013. Post-hoc analyses (Bonferroni correction for multiple comparisons, p_bonf < .05) revealed that while the Control Group exhibited a gradual reduction in DBP from baseline, participants in the Experimental Group experienced a continuous increase in DBP, peaking after the MAST (T0) with levels significantly higher than those at baseline and T35 (both p’s bonf < .001), but comparable to T15 (p_bonf = 1). The main effect of Group was significant, F(1.92) = 9.09, p = .003, ω 2 = .042. This effect was due to higher DBP in the Experimental compared to the Control Group. Neither the main effect of sex nor the remaining interactions were significant (all p’s > .06).

Figure 2. Mean diastolic blood pressure values before and after the MAST for Control and Experimental groups, stratified by gender. Error bars represent SEMs.
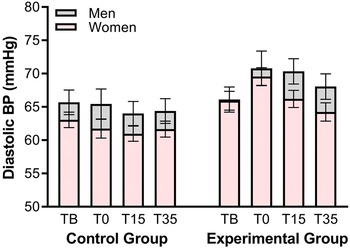
The HR values throughout the experiment can be observed in Figure 3. A 2 × 2 × 4 repeated-measures mixed ANOVA (Group: Control versus Experimental × Sex: Men versus Women × Period: TBaseline versus T0 versus T15 versus T35), with Greenhouse-Geisser correction, was applied to the HR results. The analyses revealed a significant main effect of Period, F(3.252) = 5.15, p = .002, ω 2 = .009, due to a progressive decrease in HR across time points in both groups. No more significant differences appeared (all p’s > .07).

Figure 3. Mean heart rate values and after the MAST for Control and Experimental groups, stratified by gender. Error bars represent SEMs.
Regarding the psychological stress measures, a one-way ANOVA with Group and Sex as main factors (Group: Control versus Experimental × Sex: Men versus Women) was conducted on pre-induction period scores (baseline). Neither the main effects nor any interaction resulted significant (all ps > .31).
All psychological stress measures values are depicted in Table 1 and were submitted to a 2 × 2 × 2 repeated measures mixed ANOVA (Group: Control versus Experimental × Sex: Men versus Women × Period: Pre- versus Post-manipulation). Regarding changes in state anxiety, a main effect of Group was observed, F(1.92) = 4.31, p = .041, ω 2 = .017, with participants in the Experimental Group showing higher anxiety levels than those in the Control Group. The Period × Group interaction was also significant, F(1.92) = 26.71, p < .001, ω 2 = .035. Post-hoc analyses (Bonferroni correction for multiple comparisons, p_bonf < .05) revealed a significant increase in anxiety from pre- to post-MAST period in the Experimental Group, but a reduction in the Control Group. Neither the main effects of Period and Sex, nor any of the interactions were significant (all p’s > .07).
For positive affect scores, the main effect of Period was significant, F(1.92) = 6.09, p = .01, ω 2 = .007, indicating a reduction in positive affect in both groups from the pre- to post-manipulation. All other comparisons were non-significant (all p’s > .17). Regarding negative affect, the Period × Group interaction resulted significant, F(1.92) = 10.92, p = .001, ω 2 = .021. Post-hoc comparisons (Bonferroni correction for multiple comparisons, p_bonf < .05) revealed a significant increase in negative affect from the pre- to post-MAST period for the Experimental but a reduction for the Control Group. The statistics for all the mixed repeated measures ANOVA comparisons conducted are given in Table S2 (Supplementary Material).
Correlations between stress reactivity, arithmetical performance, and psychological measures
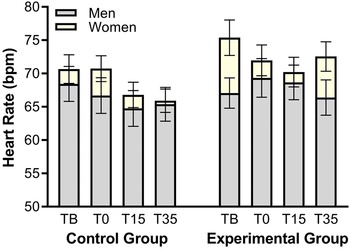
The performance in the mental arithmetic task of the MAST was correlated with stress reactivity measures. These analyses only considered data from participants in the Experimental Group, since the Control Group did not undergo this task. The scores on the four mental arithmetic trials as well as the total performance were correlated with the stress reactivity coefficients. Negative significant correlations were found between the total performance in mental arithmetic and STAI-S reactivity (rho = −.29, p = .043), and between the STAI-S reactivity and performance in mental arithmetic in the first trial (rho = −.39, p = .007). All other correlations were non-significant (all p’s > .05). The graphical representation of all correlations in a heatmap and the scatterplot depicting the relationship between anxious reactivity and total arithmetic performance can be observed in left and right sections of Figure 4, respectively.

Figure 4. Heatmap of correlations between arithmetical performance and stress reactivity coefficients. Statistical differences are represented by * (p ≤ .05) and ** (p ≤ .01) (left section), and Scatterplot of the correlation between total arithmetical performance and state anxiety reactivity (right section). Dotted lines represent the 95% mean confident interval.
Note. Abbreviations: DBP, diastolic blood pressure; HR, heart rate; MA-1, arithmetical performance in the first trial; MA-2, arithmetical performance in the second trial; MA-3, arithmetical performance in the third trial; MA-4, arithmetical performance in the fourth trial; MA-T, total arithmetical performance; PANAS-, negative affect; PANAS+, positive affect; SBP, systolic blood pressure; STAI-S, state anxiety
For the following analyses, the total sample (n = 119) was considered to see if there were differences in stress response based on whether participants had psychopathological risk (“Severe” or “Extremely Severe” scores) or not (“Normal” or “Moderate” scores) based on their scores on the DASS-21. First, scores on the DASS-21 and STAI-T scales were correlated with stress reactivity coefficients and arithmetic performance. None of the correlations were significant (all p’s > .05). Next, repeated-measures ANOVAs (Risk: Psychopathological risk versus Low risk × Period: Pre- versus Post-manipulation) were conducted. No differences were found between subjects in physiological reactivity (all p’s > .53), but differences were found in two of the psychological stress measures. Specifically, participants with higher psychopathological risk exhibited higher levels of state anxiety (F(1.115) = 16.34, p < .001, ω 2 = .062) and negative affect (F(1.115) = 13.54, p < .001, ω 2 = .051) both pre- and post-MAST as compared to the non-risk group. Figure 5 depicts the values obtained by participants with higher and lower psychopathological risk in state anxiety (left section) and negative affect (right section).

Figure 5. Mean values of state anxiety (left section) and negative affect (right section) before and after the MAST for Control and Experimental groups, stratified by the level of psychopathological risk. Error bars represent SEMs.
Discussion
The present study was aimed to validate the MAST in the Spanish population. The results indicated that the experimental group showed an increase in SBP and DBP from baseline to immediately after the MAST. Moreover, an increased psychological stress reactivity was found, as evidenced by the augmented state anxiety and negative affect from baseline to post-MAST in the experimental group. In addition, participants with higher psychopathological risk (depression, anxiety, and stress subscales from the DASS-21) exhibited higher state anxiety and negative affect scores both before and after exposure to the stressor. Finally, a significant correlation was found between math performance and anxiety reactivity, indicating that participants with higher arithmetic performance exhibited lower anxiety reactivity following MAST.
The increases from baseline to post-MAST period observed in the Experimental Group for SBP and DBP are consistent with previous studies using this protocol (e.g., Smeets et al., Reference Smeets, Cornelisse, Quaedflieg, Meyer, Jelicic and Merckelbach2012; Shilton et al., Reference Shilton, Laycock and Crewther2017), supporting its ability to elicit robust autonomic responses. However, there was a reduced HR variability, which in fact may indicate the effective functioning of the homeostatic system whereby HR recovery post-stressor exposure is relatively fast (e.g., Goswami et al., Reference Goswami, Lackner, Papousek, Jezova, Hinghofer-Szalkay and Montani2010; Shilton et al., Reference Shilton, Laycock and Crewther2017; Steptoe et al., Reference Steptoe, Willemsen, Kunz-Ebrecht and Owen2003). As for the sexual differences, it was found that women exhibited lower SBP and higher HR than men at baseline, a sexual discrepancy in cardiovascular indexes that is consistent with previous studies (e.g., Ben-Dov et al., Reference Ben-Dov, Mekler and Bursztyn2008). However, there were no sexual differences in DBP, possibly due to the greater sensitivity of SBP in detecting such differences (e.g., Kajantie & Phillips, Reference Kajantie and Phillips2006). On the other hand, no sexual differences were found in stress reactivity, diverging from findings in studies where higher reactivity is generally observed among men (e.g., Kirschbaum et al., Reference Kirschbaum, Wüst and Hellhammer1992; Kudielka & Kirschbaum, Reference Kudielka and Kirschbaum2005; Liu et al., Reference Liu, Ein, Peck, Huang, Pruessner and Vickers2017). This discrepancy may be due to the lack of control for menstrual cycle hormonal fluctuations or sexual orientation, factors known to influence stress response (Juster et al., Reference Juster, Hatzenbuehler, Mendrek, Pfaus, Smith, Johnson, Lefebvre-Louis, Raymond, Marin, Sindi, Lupien and Pruessner2015; Kirschbaum et al., Reference Kirschbaum, Kudielka, Gaab, Schommer and Hellhammer1999). In addition, it is worth noting that previous studies using the MAST and cardiovascular measures to assess physiological stress reactivity did not evaluate sexual differences, which may suggest the insensitivity of this stressor in eliciting sex-dependent stress responses.
Participants in the Experimental Group showed a significant increase in state anxiety and negative affect from baseline to post-exposure, unlike the Control Group, which showed a decrease. This aligns with studies reporting increased negative states in MAST participants (e.g., Bos et al., Reference Bos, Jacobs van Goethem, Beckers and Kindt2014; Meyer et al., Reference Meyer, Smeets, Giesbrecht, Quaedflieg and Merckelbach2013; Smeets et al., Reference Smeets, Cornelisse, Quaedflieg, Meyer, Jelicic and Merckelbach2012). The reduction in the Control Group likely reflects habituation to the experimental room, previously documented as a potentially threatening situation (e.g., Nash & Heiss, Reference Nash and Heiss1967; Soto et al., Reference Soto, Roberts, Pole, Levenson, Burleson, King and Breland-Noble2012). Both groups experienced a decline in positive affect, possibly due to the monotonous nature of the tasks (e.g., Chin et al., Reference Chin, Markey, Bhargava, Kassam and Loewenstein2017; Rubenking, Reference Rubenking2017). Contrary to our initial hypotheses, no sex differences appeared in psychological stress reactivity, possibly due to the insensitivity of the psychological scales (e.g., Lim et al., Reference Lim, Yun, Choi, Choi, Kwon, Lee and Jang2020; Trofimova, Reference Trofimova2013) or the MAST itself.
To this point, having established the effectiveness of the MAST, we can state that this protocol has been validated in a young-adult Spanish sample. However, given that the nature of this validation inherently considers cultural differences in stress reactivity (e.g., Bernardi et al., Reference Bernardi, Engelbrecht and Jobson2019; Souza-Talarico et al., Reference Souza-Talarico, Plusquellec, Lupien, Fiocco and Suchecki2014; Urizar et al., Reference Urizar, Yim, Kofman, Tilka, Miller, Freche and Johnson2019), it is relevant to discuss whether the results found in the Spanish sample are similar to those found in other cultures. Thus, our results on psychological stress reactivity were similar to that of previous studies (e.g., Smeets et al., Reference Smeets, Cornelisse, Quaedflieg, Meyer, Jelicic and Merckelbach2012; Shilton et al., Reference Shilton, Laycock and Crewther2017; Bos et al., Reference Bos, Jacobs van Goethem, Beckers and Kindt2014), with the impossibility of verifying similarity in terms of the duration of psychological stress since we only registered them at two time points (pre- and post-MAST). As for cardiovascular reactivity, Smeets et al. (Reference Smeets, Cornelisse, Quaedflieg, Meyer, Jelicic and Merckelbach2012) found a significant BP increase from baseline after MAST in Dutch people, and a return to baseline values after 5 min (Smeets et al., Reference Smeets, Cornelisse, Quaedflieg, Meyer, Jelicic and Merckelbach2012). Similarly, a subsequent study, also with Dutch population, found that BP peak persisted until 10 min after MAST exposure (Bos et al., Reference Bos, Jacobs van Goethem, Beckers and Kindt2014). In a sample of Australian population, it appeared an increase in BP from baseline to 20 min after MAST exposure (Shilton et al., Reference Shilton, Laycock and Crewther2017).
A meta-analysis by Miller and Kirschbaum (Reference Miller and Kirschbaum2019) found that Spanish and Dutch populations show lower stress responses after TSST compared to Australians, who exhibit higher reactivity. Our findings align with this, as the cardiovascular stress response in our Experimental Group was short-lived, similar to the Dutch sample, whereas other studies found more sustained responses, like in Australians. Notably, acute stress measured with glucocorticoids (e.g., salivary cortisol -HPA axis-) typically lasts longer than with cardiovascular responses (e.g., SAM axis) (e.g., Hidalgo et al., Reference Hidalgo, Villada and Salvador2020; Mirete et al., Reference Mirete, Molina, Villada, Hidalgo and Salvador2021; Smeets et al., Reference Smeets, Cornelisse, Quaedflieg, Meyer, Jelicic and Merckelbach2012). Therefore, the short duration of cardiovascular stress response reflects the autonomic system’s quick action to restore homeostasis (e.g., Goswami et al., Reference Goswami, Lackner, Papousek, Jezova, Hinghofer-Szalkay and Montani2010; Steptoe et al., Reference Steptoe, Willemsen, Kunz-Ebrecht and Owen2003). The significant differences observed confirm the MAST protocol’s success in generating acute stress. Thus, MAST is effective in eliciting robust autonomic stress responses in the Spanish population, using both physical and psychological stressors to analyze effects on behavior and cognition.
In addition, the results revealed that participants with the highest psychopathological risk exhibited the greatest levels of anxiety and negative affect both before and immediately after the MAST. This supports our hypothesis, as well as replicates previous findings indicating that participants with higher anxiety and depression scores display greater psychological stress reactivity (e.g., de Rooij et al., Reference de Rooij, Schene, Phillips and Roseboom2010). However, we did not find differences between groups in physiological measures, unlike other studies that have reported either an increase (Kibler & Ma, Reference Kibler and Ma2004; Light et al., Reference Light, Kothandapani and Allen1998) or a reduction (Chida & Hamer, Reference Chida and Hamer2008; Phillips et al., Reference Phillips, Hunt, Der and Carroll2011) in cardiovascular stress reactivity. Regarding arithmetic performance, we found that participants with higher performance showed a reduced change in anxiety levels from baseline to the post-MAST period. This is consistent with a key element in stress induction, namely the sense of uncontrollability (e.g., Dickerson & Kemeny, Reference Dickerson and Kemeny2004; Shields, Reference Shields2020). Thus, when participants perceive tasks as controllable, they report lower levels of stress reactivity (e.g., Glass et al., Reference Glass, Reim and Singer1971), suggesting that having control over the arithmetical task would lead to perceiving it as controllable, thereby increasing self-efficacy, which, in turn, is associated with lower stress levels (e.g., Nierop et al., Reference Nierop, Wirtz, Bratsikas, Zimmermann and Ehlert2008). However, these results should be interpreted with caution since, to our knowledge, this is the first time that the arithmetic index from the MAST has been independently analyzed. Moreover, as no baseline assessment of the participants’ arithmetic performance was conducted, it is not possible to determine whether higher arithmetical performance influences stress reactivity, or vice versa
Some possible limitations of our study must be considered. Thus, it could have been relevant to record more time points from MAST protocol presentation for the psychological stress assessment to evaluate changes in subjective stress. Moreover, participants in our study performed some cognitive tasks after the post-MAST measurements. While these tasks were not stressful in nature, they could have induced changes in BP or HR measurements. However, this seems unlikely given that all participants (both in Control and Experimental groups) performed the same tasks, the tasks were fully counterbalanced, and the results of cardiovascular reactivity were similar to previous reports using the MAST. In future studies, it would be interesting to compare the extent to which acute stress reactivity to the stressor is similar to baseline values in individuals affected by pathological acute stress, aiming to evaluate the ecological validity of the MAST. In addition, it is important to note that the relationship between arithmetic performance and stress reactivity is difficult to discern in this study because we do not have a baseline measure of arithmetic performance. Future studies using the MAST should include a baseline arithmetic measure to explore this relationship in depth, considering the possible role of self-efficacy in subjective stress induction. Finally, continuing the research initiated by Miller and Kirschbaum (Reference Miller and Kirschbaum2019) to further validate these experimental stressors across different cultures will enable comparisons of stress reactivity between cultures.
In summary, our study tested the efficacy of the MAST to induce robust acute stress responses in a sample of young Spanish adults. Specifically, participants exposed to the MAST, as opposed to controls, exhibited a significant increase in state anxiety, negative affect, as well as a rise in SBP and DBP. Therefore, we have validated MAST as an effective stress induction protocol in the Spanish population, with some values differing from those reported in other cultures. However, since university students may not be fully representative of the general population in Spain, follow-up studies are recommended to test the generalizability of the results with more diverse samples. Thus, considering the impact of culture on stress reactivity, it is imperative to continue validating this and other stress protocols to analyze how similar or different cultures are under stress.
Supplementary material
To view supplementary material for this article, please visit http://doi.org/10.1017/SJP.2025.4.
Data availability statement
The data and materials that support the findings of this study are available from the corresponding author upon request to comply with the protection of personal data of the participants.
Acknowledgment
We would like to thank Sergio Villa-Consuegra, María de los Ángeles Cintado, Gabriel González and Lucía Cárcel for technical advice and Dr. Tom Smeets for sharing with us the materials of the MAST.
Author contribution
D.S-C.: Conceptualization, data curation, formal analysis, investigation, methodology, writing (original draft, reviewing and editing); L.G.D.l.C.: Conceptualization, formal analysis, funding acquisition, methodology, project administration, supervision, writing (original draft, reviewing and editing).
Funding statement
This research was funded by the Agencia Estatal de Investigación (AEI) of Spain (grant n°: PID2019-107530GB-I00/AEI/10.13039/501100011033) and by the Spanish Ministerio de Ciencia, Innovación y Universidades (PhD grant ref. FPU21/00344).
Competing interests
The authors declare none.