Knowledge of how the prevalence and severity of adverse effects vary for different antipsychotics allows clinicians to reduce the occurrence of these effects. We review the range of adverse effects associated with antipsychotics and their clinical impact, and give an overview of the various sources of data on adverse effects and their relative strengths and weaknesses. Potential problems in interpreting the evidence base are considered and the importance of the patients' perspective emphasised. We conclude with an examination of total discontinuation rates as a global measure of effectiveness that incorporates both tolerability and efficacy.
RANGE AND CLINICAL IMPACT OF ADVERSE EFFECTS
Antipsychotics are associated with a wide range of potential adverse effects (Appendix 1) which can affect the patient in several ways (Fig. 1). For example the stiffness, slowness of movement and tremor of antipsychotic-induced parkinsonism (Reference Dursun, Haddad and BarnesDursun et al, 2004) can make it difficult for a patient to write, fasten buttons and tie shoelaces, leading to reduced quality of life. The blank ‘mask-like’ expression, tremor, stooped posture, drooling and abnormalities of gait (including lack of arm swing) are easily observable by others and mark the patient out as ‘different’, hence contributing to stigma. When severe the festinant gait may result in falls and injury, particularly hip fracture in older patients. Patients who recognise the link between these problems and antipsychotic medication may miss out doses or stop medication totally.
Appendix 1 Adverse effects of antipsychotics
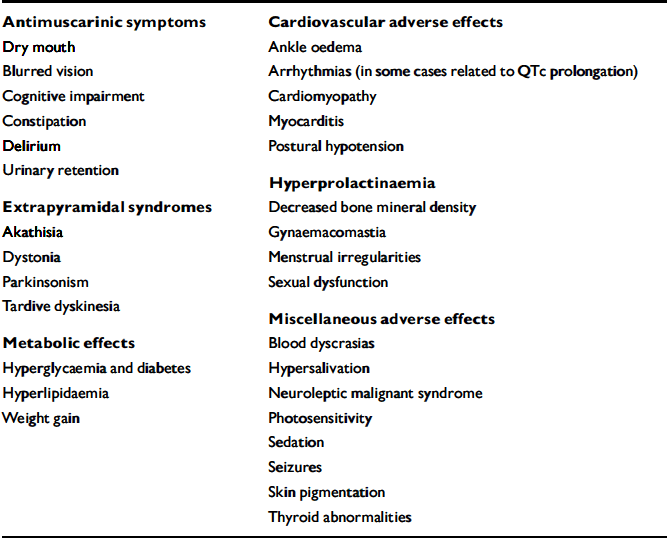
| Antimuscarinic symptoms | Cardiovascular adverse effects |
| Dry mouth | Ankle oedema |
| Blurred vision | Arrhythmias (in some cases related to QTc prolongation) |
| Cognitive impairment | Cardiomyopathy |
| Constipation | Myocarditis |
| Delirium | Postural hypotension |
| Urinary retention | |
| Hyperprolactinaemia | |
| Extrapyramidal syndromes | Decreased bone mineral density |
| Akathisia | Gynaemacomastia |
| Dystonia | Menstrual irregularities |
| Parkinsonism | Sexual dysfunction |
| Tardive dyskinesia | |
| Miscellaneous adverse effects | |
| Metabolic effects | Blood dyscrasias |
| Hyperglycaemia and diabetes | Hypersalivation |
| Hyperlipidaemia | Neuroleptic malignant syndrome |
| Weight gain | Photosensitivity |
| Sedation | |
| Seizures | |
| Skin pigmentation | |
| Thyroid abnormalities |
Many patients who adhere poorly to medication do not inform their clinical team of this and some go to great lengths to hide their non-adherence (covert non-adherence). Poor adherence during acute treatment of psychosis leads to chronic symptoms whereas poor adherence after remission increases the risk of relapse. Both may have serious consequences, including self-harm, aggression and readmission to hospital. When clinician and patient are aware of adverse effects, treatment can be adjusted to minimise the problems (e.g. dose reduction of the antipsychotic, prescription of an anti-Parkinsonian agent or a switch to an alternative antipsychotic with less propensity to cause the adverse effect).
SOURCES OF DATA
Data on adverse effects are available from a range of sources. These include randomised controlled trials (RCTs), naturalistic studies, part-marketing surveillance, and non-randomised and open trials. Open and non-randomised trials are methodologically inferior to double-blind randomised controlled trials but nevertheless contribute to the evidence base. All data sources can be considered as being pieces of a jigsaw; the full picture of drug tolerability is only evident when all the pieces are put together.
Randomised controlled trials
Strengths
Double-blind randomised trials are regarded as the gold standard level of evidence for the following reasons.
-
(a) Randomisation reduces the risk of bias in baseline characteristics and so makes it more probable that differences in outcome reflect differences between the treatments being investigated.
-
(b) Comparative data can be obtained against either placebo or one or more comparator drugs. Placebo data are particularly helpful in identifying the baseline rate of adverse effects independent of treatment with an active drug. Many potential adverse drug effects (e.g. weight gain, sexual dysfunction, onset of diabetes) are multifactorial and occur in the general population.
-
(c) Prospective assessment allows accurate measurement of adverse effects. This may involve the use of standardised rating scales (Table 1).
-
(d) Patient and rater bias are eliminated by masking.
Table 1 Examples of ratings scales used to assess side-effects of antipsychotics
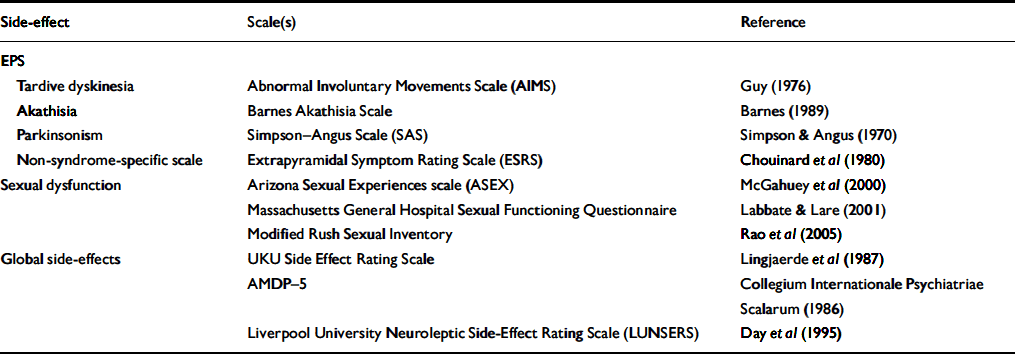
| Side-effect | Scale(s) | Reference |
|---|---|---|
| EPS | ||
| Tardive dyskinesia | Abnormal Involuntary Movements Scale (AIMS) | Guy (Reference Guy1976) |
| Akathisia | Barnes Akathisia Scale | Barnes (Reference Barnes1989) |
| Parkinsonism | Simpson-Angus Scale (SAS) | Simpson & Angus (Reference Simpson and Angus1970) |
| Non-syndrome-specific scale | Extrapyramidal Symptom Rating Scale (ESRS) | Chouinard et al (Reference Chouinard, Ross-Chouinard and Annable1980) |
| Sexual dysfunction | Arizona Sexual Experiences scale (ASEX) | McGahuey et al (Reference McGahuey, Gelenburg and Laukes2000) |
| Massachusetts General Hospital Sexual Functioning Questionnaire | Labbate & Lare (Reference Labbate and Lare2001) | |
| Modified Rush Sexual Inventory | Rao et al (Reference Rao, Zajecka and Skubiak2005) | |
| Global side-effects | UKU Side Effect Rating Scale | Lingjaerde et al (Reference Lingjaerde, Ahlfors and Bech1987) |
| AMDP-5 | Collegium Internationale Psychiatriae Scalarum (1986) | |
| Liverpool University Neuroleptic Side-Effect Rating Scale (LUNSERS) | Day et al (Reference Day, Wood and Dewey1995) |
EPS, extrapyramidal side-effects; AMDP-5, Association for Methodology and Documentation in Psychiatry Adverse Event Questionnaire
In practice these advantages are not always as clear-cut as they seem. For example, relatively few trials assess the success of masking and when they do the methods used, analysis and reporting of the results are inconsistent (Reference Boutron, Estellat and RavaudBoutron et al, 2005). A review of papers claiming to be RCTs, published in the British Journal of Psychiatry and the American Journal of Psychiatry, showed that reporting of the method of randomisation was uncommon (Reference Ogundipe, Boardman and MastersonOgundipe et al, 1999). The authors concluded that the RCT status of some of the papers must therefore be in doubt.
Fig. 1 Clinical impact of adverse effects.
Weaknesses
Although RCTs can allow accurate information on the incidence and prevalence of adverse effects to be gathered, most trials of antipsychotics have relatively small samples and are short term, lasting 4–8 weeks. Such studies may underestimate early-onset side-effects that are uncommon and cannot provide data on side-effects that develop in the medium and long term. For example, amenorrhoea is an adverse effect of antipsychotics that reflects hyperprolactinaemia (Reference Wieck and HaddadWieck & Haddad, 2003). In the Schizophrenia Outpatient Health Outcome (SOHO) study the baseline prevalence was approximately 33% of women (Reference Haro and Salvador-CarullaHaro & Salvador-Carulla, 2006). Definitions of amenorrhoea differ; if it is defined as three consecutive missed episodes of menstruation then it will be impossible to detect in a drug trial of less than 12 weeks' duration. The inability of short-term trials to provide data on long-term tolerability, including weight gain, sexual functioning and metabolic parameters, is a major weakness, as in clinical practice antipsychotics are often prescribed to patients for several years or even decades. This drawback has been partly addressed by two recently published RCTs with relatively long follow-up periods: the Cost–Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS) in the UK (Reference Jones, Barnes and DaviesJones et al, 2006), which followed patients for 1 year, and phase 1 of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study in the USA (Reference Lieberman, Stroup and McEvoyLieberman et al, 2005), which followed patients for 18 months. Nevertheless neither study is long enough to accurately assess the risk of tardive dyskinesia. Prospective studies of conventional antipsychotics indicate a cumulative incidence of tardive dyskinesia of approximately 20% over 5 years of treatment (Reference Morgenstern and GlazerMorgenstern & Glazer 1993).
The protocols of most RCTs exclude patients with significant comorbid medical conditions. Consequently the tolerability of drugs in people with physical illness (for example those with hepatic and renal impairment) is often unknown prior to licensing. Some trials may also underestimate tolerability because there may be incentives for patients to remain in the trial that do not operate in clinical practice.
Naturalistic studies
Naturalistic studies, including pharmaco-epidemiological studies, have the advantage of assessing ‘real world’ patients. Pharmaco-epidemiological studies can have very large samples, enabling relatively rare adverse effects to be investigated. Both incidence and prevalence data can be generated. These studies are limited to data recorded on computerised record systems and the absence of relevant data may prevent adjustment for potential confounding factors. Furthermore, the lack of randomisation limits attribution of causality. Data regarding the safety of drugs in pregnancy derive from post-marketing surveillance and naturalistic studies because pregnant women are invariably excluded from RCTs.
Post-marketing surveillance
Post-marketing surveillance is an essential component of assessing drug safety and tolerability, and often provides the first evidence of adverse effects that are rare or confined to particular at-risk groups. Remoxipride was an antipsychotic marketed in the late 1980s. Trials indicated similar efficacy to haloperidol for treating positive and negative symptoms but with less risk of extrapyramidal side-effects. Following its introduction in Europe a significant number of cases of aplastic anaemia were reported (as many as 1 in 10 000). Remoxipride was withdrawn in 1993 (Reference Fung, Thornton and MybeckFung et al, 2001). Pimozide is a conventional antipsychotic. Between 1971 and 1995, 16 deaths and 24 cases of serious cardiac events were reported to the Committee for the Safety of Medicines. This led to the following recommendations: (a) patients prescribed pimozide should undergo a baseline electrocardiogram (ECG) followed by annual ECGs; (b) if the QTc interval is prolonged, treatment needs to be closely supervised or withdrawn; and (c) pimozide should not be prescribed in conjunction with other drugs that prolong the QTc interval (Reference Haddad and AndersonHaddad & Anderson, 2002).
Post-marketing surveillance includes prescription event monitoring (Reference MannMann, 1998) and reports of adverse drug reactions (Reference GoughGough, 2005). Various national and international regulatory bodies provide systems for post-marketing surveillance, an example being the UK yellow card system for reporting adverse drug reactions. Post-marketing surveillance is also conducted by pharmaceutical companies or by independent research companies employed by them. The potential conflict of interest inherent in manufacturers collecting, evaluating and reporting post-marketing data on their own products has been the subject of recent discussion (Reference Fontanarosa, Rennie and DeAngelisFontanarosa et al, 2004). This point apart, post-marketing surveillance has several weaknesses: it relies on voluntary participation; underreporting is widespread; submitted reports may be of poor quality with inadequate detail; and the ability to confirm causality is limited. Incomplete numerator data on events and unreliable denominator data make it difficult to calculate rates of adverse events.
The withdrawal of drugs for safety reasons demonstrates that licensing is not a guarantee of safety and highlights the importance of the continuing assessment of tolerability and safety from further studies and post-marketing surveillance. Between 1960 and 1999 121 prescription drugs were withdrawn from worldwide markets for safety reasons (Reference Fung, Thornton and MybeckFung et al, 2001). Drugs that act on the central nervous system were the most common category withdrawn; in a more detailed breakdown by drug class antidepressants were ranked fifth (7.4%). The top safety reasons for withdrawal among all drugs were hepatic (26.2%), haematological (10.5%), cardiovascular (8.7%), dermatological (6.3%) and carcinogenic issues (6.3%). The median time on the market for products where this information was available was 5.4 years, with approximately one-third being withdrawn within the first 2 years of initial marketing.
PROBLEMS IN INTERPRETING TOLERABILITY DATA
The researcher or clinician is faced with several problems when evaluating the literature on adverse effects of antipsychotics (Appendix 2).
Appendix 2 Potential problems in interpreting tolerability data
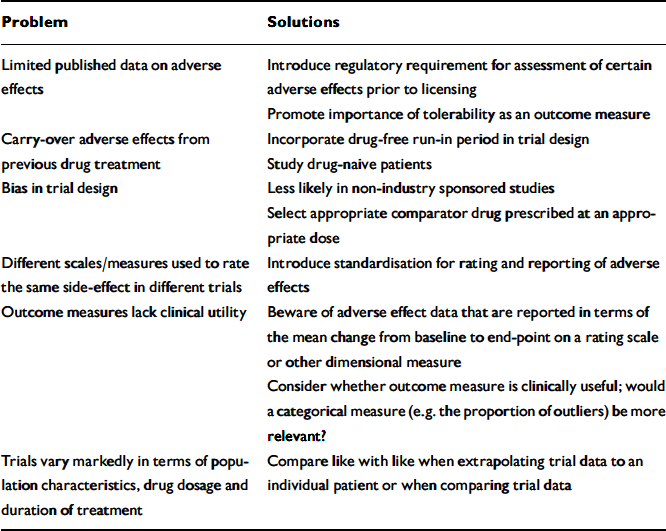
| Problem | Solutions |
|---|---|
| Limited published data on adverse effects | Introduce regulatory requirement for assessment of certain adverse effects prior to licensing |
| Promote importance of tolerability as an outcome measure | |
| Carry-over adverse effects from previous drug treatment | Incorporate drug-free run-in period in trial design |
| Study drug-naive patients | |
| Bias in trial design | Less likely in non-industry sponsored studies |
| Select appropriate comparator drug prescribed at an appropriate dose | |
| Different scales/measures used to rate the same side-effect in different trials | Introduce standardisation for rating and reporting of adverse effects |
| Outcome measures lack clinical utility | Beware of adverse effect data that are reported in terms of the mean change from baseline to end-point on a rating scale or other dimensional measure |
| Consider whether outcome measure is clinically useful; would a categorical measure (e.g. the proportion of outliers) be more relevant? | |
| Trials vary markedly in terms of population characteristics, drug dosage and duration of treatment | Compare like with like when extrapolating trial data to an individual patient or when comparing trial data |
Limited data
Many papers that report RCTs of antipsychotics provide little data on adverse effects and concentrate on efficacy. Where such data are provided they are often limited, for example until recently most trials of antipsychotics did not include any measures of glucose and lipid regulation.
Drug carry-over effects
A second problem is that most trials evaluate patients with chronic psychosis who must discontinue a previous antipsychotic before starting the trial. This makes drug carry-over effects inevitable. For example, the potential for weight gain associated with a particular antipsychotic is underestimated, as patients are likely to have gained weight during previous antipsychotic treatment, thus minimising their potential for further weight gain (Reference HaddadHaddad, 2005). Assessing patients with first-onset psychosis who are drug naive overcomes this problem, but enrolling such patients into trials is notoriously difficult and such RCTs are rare.
Bias in trial design
Industry-sponsored trials are more likely to report results that favour the sponsor's compound than are independent studies (Reference Ahmer, Arya and AndersonAhmer et al, 2005). Possible explanations include publication bias and bias in trial design. An example of the latter is that most RCTs of atypical antipsychotics employ haloperidol as the active comparator. Among the conventional antipsychotics, haloperidol is associated with a high incidence of extrapyramidal side-effects (EPS) and so it is not surprising that these studies generally report an advantage in relation to EPS for the atypical agents, an advantage that remains in meta-analyses (Reference Geddes, Freemantle and HarrisonGeddes et al, 2000; Reference Bagnall, Jones and GinnellyBagnall et al, 2003). In contrast, RCTs that have a low-potency conventional antipsychotic as comparator show no significant difference in the incidence of EPS for atypical antipsychotics other than clozapine (Reference Leucht, Wahlbeck and HamannLeucht et al, 2003; Reference Lieberman, Stroup and McEvoyLieberman et al, 2005).
Comparison between trials
It is often necessary to compare data on adverse effects between trials. For example, the relatively few head-to-head RCTs of atypical antipsychotics make cross-study comparisons, despite their methodological pitfalls, a necessity. Furthermore, as estimates of the prevalence/severity of an adverse effect for any given drug will vary between trials, an adjusted value is often required. Meta-analysis is commonly used to allow data from different studies to be pooled and compared, but this approach is often not possible when analysing data on adverse effects because of varying methodologies used to assess such effects. For example, there are several scales to measure sexual function (Table 1). Parkinsonian symptoms are usually assessed using the Simpson–Angus Scale (Reference Simpson and AngusSimpson & Angus, 1970), but some studies report the proportion of patients prescribed an anticholingeric drug, a clinical proxy for parkinsonism. Even when the same rating scale or measure is used, the outcome may be expressed in different ways. Parkinsonian symptoms may be reported as mean change in score on the Simpson–Angus Scale from baseline to end-point or as the number of patients with scores above a specified cut-off. Similarly, measures of weight change during a study include mean change in kilograms, the percentage of patients with increments of weight change (e.g. 0–5 kg, 5–10 kg, etc.) and the number of patients with an arbitrary measure of significant weight gain, (e.g. an increase of more than 7% of baseline weight).
Outcome measures that lack clinical utility
Many studies present data on adverse effects in terms of the mean change in an outcome measure (e.g. a rating scale or the blood concentration of a compound). Often this has no clinical utility to the clinician or patient. For example, reporting the mean change in serum prolactin during the course of a trial is far less relevant than reporting the proportion of patients with a prolactin level above the upper limit of normal at the start and end of the study. Even more useful is the proportion of these patients who also have symptoms consistent with hyperprolactinaemia (i.e. the proportion with biochemical plus clinical hyperprolactinaemia). Similarly, mean weight change is less useful than knowing the proportion of patients with specific increments of weight change.
Comparing like with like
When trial data are reviewed to aid the treatment of a specific patient (e.g. to assist selection of an antipsychotic drug), it is important to ensure that the trials reviewed deal with patients with similar characteristics to the patient being treated and use similar drug dosages to those likely to be used clinically. For example, data on adverse effects gathered from trials in patients with chronic schizophrenia cannot be reliably applied to drug-naive patients, as the latter are more sensitive to a range of adverse effects. Similarly, premenopausal women are more prone to develop antipsychotic-induced hyperprolactinaemia than postmenopausal women. Consequently it would be misleading to extrapolate data on prolactin-related side-effects from an RCT that included a high proportion of postmenopausal women to the treatment of a premenopausal patient. When trials are combined in a meta-analysis one should consider whether differences between the trials in terms of populations studied, drug dosage and the duration of treatment invalidate the approach. Most adverse effects are dose related but the relationship between the prevalence of an adverse effect and duration of drug treatment varies depending on the effect being considered. For example, akathisia is particularly common in the first week after starting an antipsychotic or increasing the dose whereas tardive dyskinesia usually only appears after several months or years of treatment (Reference Dursun, Haddad and BarnesDursun et al, 2004).
PATIENT PERSPECTIVE
Until recently research on adverse effects was largely concerned with quantifying symptoms rather than determining their impact on patients. Recently there has been increasing interest in the subjective view of patients to treatment, including antipsychotic medication (Reference Voruganti, Cortese and OyewumiVoruganti et al, 2000; Reference Angermeyer, Loffler and MullerAngermeyer et al, 2001; Reference Hasler, Moergeli and BachmannHasler et al, 2004). There are several overlapping domains, including subjective satisfaction with treatment, subjective quality of life and subjective response to treatment.
Satisfaction with treatment
Patient satisfaction with treatment is influenced by multiple factors and not just symptom reduction (Reference Hasler, Moergeli and BachmannHasler et al, 2004). Factors that predicted dissatisfaction with care in a large European study included unemployment, more severe psychopathology and a high rate of hospital admission (Reference Thornicroft, Tansella and BeckerThornicroft et al, 2004). Other reasons for dissatisfaction include lack of involvement in treatment planning or decision-making, lack of involvement with treatment options, drug side-effects and lack of information about these. In a UK survey of callers to a national mental health telephone helpline, distressing side-effects were strongly correlated with low treatment satisfaction (Reference Fakhoury, Wright and WallaceFakhoury et al, 2001). In this survey the most distressing side-effects reported (percentage of patients with the side-effect who reported it as distressing) were weight gain (73%), depression (67%), insomnia (66%), difficulty thinking/concentrating (63%), sedation (59%) and sexual dysfunction (58%). Men were more likely to report sexual dysfunction as distressing and women more likely to report weight gain as distressing. Several studies indicate that adverse effects of antipsychotics are often not diagnosed or treated (e.g. Reference Mitra and HaddadMitra & Haddad, 2007) and that psychiatrists tend to underestimate the distress that they cause (e.g. Reference Day, Kinderman and BentallDay et al, 1998).
Subjective quality of life
Many factors influence a patient's view of their quality of life, including positive and negative symptoms, depression, cognitive impairment, hospitalisation and perceived support (Reference Thornicroft, Tansella and BeckerThornicroft et al, 2004). Several studies have reported that quality of life is higher in patients treated with atypical antipsychotics than in those treated with conventional antipsychotics (Reference Franz, Lis and PluddemannFranz et al, 1997). However, in the CATIE study, the largest independent randomised double-blind study in schizophrenia research, there were no significant differences in psychosocial functioning (assessed using the Quality of Life Scale; Reference Heinrichs, Hanlon and CarpenterHeinrichs et al, 1984) between those treated with atypical drugs and those treated with perphenazine, a conventional drug; all treatment groups showed modest improvement (Reference Swartz, Perkins and StroupSwartz et al, 2007). This is consistent with the CUtLASS study (Reference Jones, Barnes and DaviesJones et al, 2006), which found no difference in quality of life scores between patients prescribed typical and atypical antipsychotics.
Subjective response to treatment
The Drug Attitude Inventory (DAI; Reference Hogan, Awad and EastwoodHogan et al, 1983) is an established tool that assesses acceptability and subjective tolerability (subjective response) of medication. Factors that influence subjective response include insight, previous experience of medication, health beliefs and the quality of the therapeutic relationship. In one study patients on atypical antipsychotics reported a more positive subjective response and a lower prevalence of dysphoria than those on typical antipsychotics (Reference Voruganti, Cortese and OyewumiVoruganti et al, 2000). Subjective response, as assessed by DAI score, is strongly correlated with adherence (Reference Awad and HoganAwad & Hogan, 1994). However, adherence is influenced by many other factors, including the quality of the therapeutic relationship between the patient and physician or keyworker (Reference Frank and GundersonFrank & Gunderson, 1990).
DISCONTINUATIONS OWING TO INTOLERABILITY
When interpreting trials there is often a tendency to consider individual side-effects in isolation (e.g. weight gain, EPS, hyperprolactinaemia, etc.) In reality patients often experience several adverse effects, and whereas each on its own may be minor together they may be a major burden. One measure of overall tolerability is the proportion of patients who stop treatment and cite side-effects as the cause. Although intolerability is a major cause of antipsychotic drug discontinuation in schizophrenia it often accounts for fewer discontinuations than lack of efficacy (Reference Lieberman, Stroup and McEvoyLieberman et al, 2005; Reference Kinon, Liu-Seifert and AdamsKinon et al, 2006; Reference Haro, Suarez and NovickHaro et al, 2007). For example in phase I of the CATIE study patients were randomised double-blind to one of five antipsychotics and followed for up to 18 months. In four of the five drug cohorts more patients stopped treatment for lack of efficacy than for intolerability (Reference Lieberman, Stroup and McEvoyLieberman et al, 2005; Fig. 2). In the naturalistic SOHO study the percentage of patients discontinuing treatment over 3 years because of lack of efficacy exceeded those discontinuing treatment for intolerability in all drug cohorts (Reference Haro, Suarez and NovickHaro et al, 2007). This was also the case in a meta-analysis of four RCTs of olanzapine in schizophrenia (Reference Kinon, Liu-Seifert and AdamsKinon et al, 2006).
These results are consistent with a concept mapping study that investigated medication adherence in people with schizophrenia (Reference Kikkert, Schene and KoeterKikkert et al, 2006). Based on interviews with people with schizophrenia, carers and health professionals, ten clinically relevant clusters were identified that affected medication adherence. Medication efficacy was rated by patients and carers as the most important cluster affecting adherence, but professionals rated this as significantly less important, ranking it fifth out of the ten clusters. Conversely patients and carers placed side-effects relatively low compared with positive aspects of medication, whereas professionals prioritised side-effects as the second most important cluster. So, compared with patients and carers, professionals overestimate the importance of adverse effects for adherence and underestimate the importance of efficacy.
TOTAL DISCONTINUATION RATES: GLOBAL MEASURE OF EFFECTIVENESS
Clinicians and patients need to balance adverse effects against the effectiveness of a drug in treating the psychiatric illness. If a patient obtains significant benefit from a drug they may be willing to put up with considerable adverse effects (as demonstrated with clozapine). Adverse effects are common with clozapine and regular monitoring of the full blood count is mandatory throughout treatment, owing to the risk of neutropaenia. Nevertheless patients often accept the adverse effects, presumably because clozapine provides a level of symptom control for their treatment-resistant illness that was not achieved with previous antipsychotics. In problem-centred interviews with patients discharged from hospital on clozapine a wide range of side-effects were reported, including fatigue or sedation (56%), lack of motivation (21%), hypersalivation (21%), anticholinergic effects (16%), weight gain (15%) and orthostatic hypotension (11%) (Reference Angermeyer, Loffler and MullerAngermeyer et al, 2001). Despite this nearly one-third of patients stated that they felt better as a result of clozapine and almost half expected a worsening of their mental state if they stopped the medication.
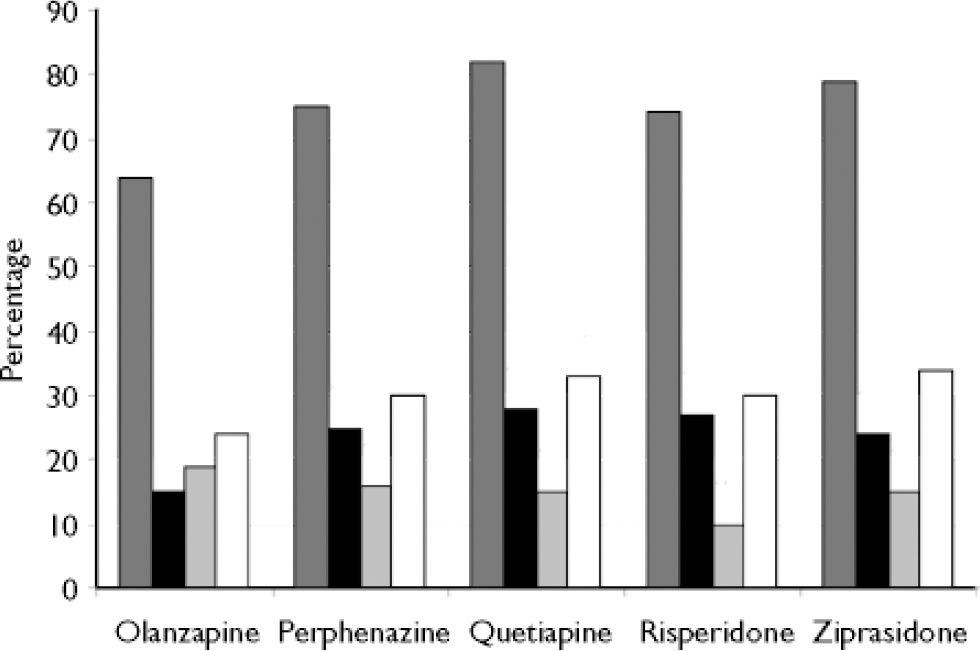
Fig. 2 Percentage of patients discontinuing medication for various reasons in phase I of CATIE study. ![]() , Total discontinuations; ▪, discontinuations owing to lack of efficacy;
, Total discontinuations; ▪, discontinuations owing to lack of efficacy; ![]() , discontinuations owing to intolerability; □, discontinuations owing to patient's decision (data from Reference Lieberman, Stroup and McEvoyLieberman et al, 2005).
, discontinuations owing to intolerability; □, discontinuations owing to patient's decision (data from Reference Lieberman, Stroup and McEvoyLieberman et al, 2005).
Thus it is helpful to have a global measure of the effectiveness of a drug that combines both tolerability and effectiveness in treating symptoms. One way to achieve this is to record the total discontinuation rate on the drug at a given time point or the time to discontinuation for any reason. As stopping medication in a trial is a joint decision made by patient and clinician, this outcome measure also has the advantage of incorporating the patient's and the clinician's views.
Discontinuation of treatment for any reason was the primary outcome measure in the CATIE study (Reference Lieberman, Stroup and McEvoyLieberman et al, 2005). The results of phase I of the study illustrate the importance of balancing efficacy and tolerability. Of the five antipsychotics in phase I, olanzapine was associated with the highest percentage of patients stopping treatment because of intolerability but the lowest percentage stopping treatment for lack of efficacy (Leiberman et al, 2005). When discontinuations owing to lack of efficacy and intolerability were combined with discontinuations for other reasons then the total discontinuation rate for each of the five antipsychotics was lowest with olanzapine (Fig. 2). The high total discontinuation rates seen with all drugs in the CATIE study might partly reflect the double-blind design (Reference Haddad and DursunHaddad & Dursun, 2006).
The total discontinuation rate has also been used as the outcome measure in several naturalistic studies (Reference Hodgson, Belgamwar and Al-tawarahHodgson et al, 2005; Reference Tiihonen, Walhbeck and LonnqvistTiihonen et al, 2006). Of particular note is the study by Tiihonen et al (Reference Tiihonen, Walhbeck and Lonnqvist2006) in which a nationwide cohort of 2230 consecutive adults hospitalised in Finland for the first time with a diagnosis of schizophrenia or schizoaffective disorder were followed prospectively. Total rates of discontinuation, adjusted for the effect of confounders, were determined for the ten most commonly used antipsychotics and compared with haloperidol. Initial treatment with clozapine, perphenazine depot and olanzapine were associated with the lowest total discontinuation rates, and in all three cases these were significantly less than the rate associated with haloperidol. Significant differences were also seen between antipsychotics in the rates of readmission, with clozapine, perphenazine depot and olanzapine all being associated with significantly lower readmission rates than haloperidol.
CONCLUSIONS
Data on adverse effects are available from a range of sources, including randomised controlled trials, post-marketing surveillance and naturalistic studies. All sources of data carry their own advantages and disadvantages. The best overview of adverse effects comes from considering all sources together. There is inconsistent reporting of adverse effects across studies and many outcome measures lack clinical meaning. Future research would benefit greatly if standardisation for the reporting of adverse effects could be reached. The impact of side-effects on patients has not been sufficiently studied. It is important that the patient's subjective experience, in which adverse effects have a role, are considered in the assessment of a drug. Although adverse effects are an important outcome, with many antipsychotics they account for less treatment discontinuation than lack of efficacy; this finding has been noted in naturalistic studies and in RCTs. Total discontinuation rates provide a useful global outcome measure that incorporates tolerability and efficacy and patient and clinician viewpoints. In clinical practice, patients should be informed of common side-effects prior to treatment and monitored for their occurrence during treatment.








eLetters
No eLetters have been published for this article.