Depression is a chronic disorder and requires long-term treatment. Reference Andrews1 A number of antidepressant drugs are effective in treating depressive episodes and preventing relapse. However, antidepressants cause adverse reactions. Tolerability determines whether an effective dose is administered and whether people continue treatment after the symptoms improve. Reference Frank and Judge2,Reference Lingam and Scott3 To inform clinicians' and individuals' decisions on short- and long-term treatment, both effectiveness and adverse reactions need to be recorded. Several instruments have been developed to measure adverse reactions to psychotropic medication, including the Systematic Assessment for Treatment Emergent Side Effects (SAFTEE) Reference Levine and Schooler4 and the UKU Side Effect Rating Scale. Reference Lingjaerde, Ahlfors, Bech, Dencker and Elgen5 However, widespread use has been hampered by their length, complexity and demands on clinicians' time. The SAFTEE and the UKU have been primarily designed for and validated in people using antipsychotic medication. The measurement of adverse reactions to antidepressants remains relatively underdeveloped. Reference Jordan, Knight and Pointon6 Self-report measures of adverse reactions have been tested in individuals on antipsychotic medication and have been found reliable except for the assessment of movement disorder. Reference Lambert, Cock, Alcock, Kelly and Conley7,Reference Lindstrom, Lewander, Malm, Malt, Lublin and Ahlfors8 As movement disorder is uncommon among people on antidepressant medication, self-report measures of adverse reactions may be suitable for antidepressants. We report on the use of a short self-report measure of adverse reactions in a large study comparing two antidepressants: a selective serotonin reuptake inhibitor (SSRI) and a tricyclic antidepressant with predominantly noradrenaline-reuptake-inhibiting effects. We explore the interface between symptoms of depression and adverse reactions to establish which symptoms are related to the use of each antidepressant. Repeated assessments were used to establish which adverse reactions persist or habituate with continued treatment. Finally, we tested whether adverse reactions predict discontinuation of antidepressants.
Method
Sample and study design
The Genome-Based Therapeutic Drugs for Depression (GENDEP) project is a part-randomised multicentre open-label pharmacogenetic study with two active pharmacological treatment arms. Reference Uher, Maier, Hauser, Marušič, Schmael and Mors9 It was designed to establish clinical and genetic determinants of therapeutic response and adverse reactions to two antidepressants with contrasting primary modes of action: nortriptyline (a tricyclic antidepressant with strong affinity for the noradrenaline transporter) and escitalopram (an SSRI). Eight hundred and eleven adults of White European parentage diagnosed with ICD–10 10 /DSM–IV 11 unipolar major depression of at least moderate severity Reference Wing, Sartorius and Ustin12 were recruited in eight European countries between July 2004 and December 2007. Personal or family history of bipolar disorder or schizophrenia constituted exclusion criteria. The study was approved by ethics boards in all participating centres. All participants provided a written consent after the procedures were fully explained. GENDEP is registered at EudraCT (EudraCT 2004-001723-38, http://eudract.emea.europa.eu) and ISRCTN (ISRCTN03693000, http://www.controlled-trials.com).
Participants included 297 men and 514 women between 19 and 72 years old (mean age 42.5 (s.d. = 11.8)). The average participant was in her second episode of moderately severe depression and scored 28.7 (s.d. = 6.7) on the Montgomery–Åsberg Depression Rating Scale (MADRS) Reference Montgomery and Åsberg13 at baseline. Participants with no contraindications were randomly allocated to receive nortriptyline (n = 235) or escitalopram (n = 233) for 12 weeks. People with contraindications for one of the drugs were allocated non-randomly to the other antidepressant: 225 to escitalopram and 118 to nortriptyline. Escitalopram was initiated at 10 mg daily and increased to a target dose of 15 mg daily within the first 2 weeks and could be further increased to 20 mg and 30 mg daily. Nortriptyline was initiated at 50 mg daily and titrated to a target dose of 100 mg daily within the first 2 weeks and could be further increased to 150 mg and 200 mg daily. Other psychotropic medication was not allowed with the exception of occasional use of hypnotics. Adherence was monitored weekly by self-report and plasma levels of antidepressants were measured at week 8. Of the 811 participants, 628 (77%) completed 8 weeks and 527 (65%) completed 12 weeks on the originally allocated antidepressant. Individuals treated with escitalopram and nortriptyline improved to a similar degree on traditional measures of depressive symptoms. Reference Uher, Maier, Hauser, Marušič, Schmael and Mors9 A detailed description of the GENDEP sample and outcomes is available elsewhere. Reference Uher, Maier, Hauser, Marušič, Schmael and Mors9
Measures
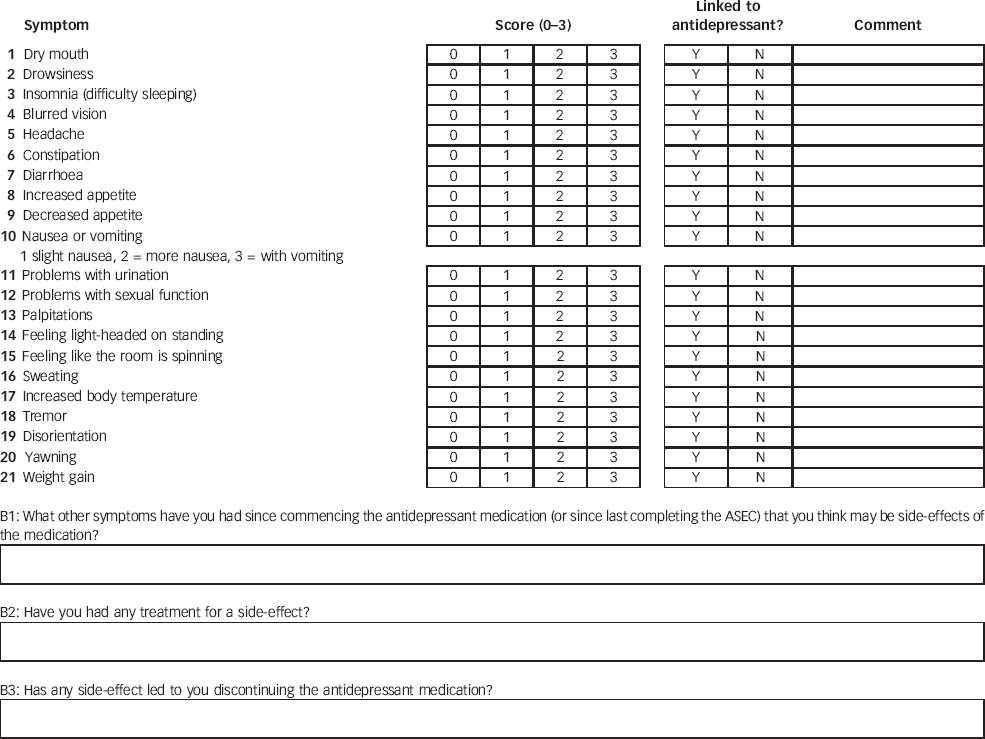
The Antidepressant Side-Effect Checklist (ASEC) was constructed by K.J.A. and A.F. as a self-report instrument to measure 21 adverse reactions to antidepressants: dry mouth, drowsiness, difficulty sleeping (insomnia), blurred vision, headache, constipation, diarrhoea, increased appetite, decreased appetite, nausea or vomiting, problems with urination, problems with sexual function, palpitations, feeling light-headed on standing (orthostatic dizziness), feeling like the room is spinning round (vertigo), sweating, increased body temperature, tremor, disorientation, yawning, and weight gain (see Appendix). This list of adverse reactions was compiled from a review of the literature, Reference Demyttenaere, Albert, Mesters, Dewe, De and Sangeleer14–Reference Trindade, Menon, Topfer and Coloma18 and comprised effects reported for antidepressants and not included in previous measures, such as yawning. Reference Gutierrez-Alvarez19 For each item, the participants rated the severity of the specified symptom on a four-point scale (0 absent; 1 mild; 2 moderate; 3 severe) and specified whether a symptom (if present) was likely to be a side-effect of the antidepressant drug (yes or no). A space for comment was provided next to each item. Optional free-text entries gave an opportunity to list other complaints and explain the impact of adverse reactions. Each study participant was asked to complete the ASEC at baseline, before commencing the study medication, then weekly for the duration of the study and at a follow-up 6 months after starting the medication.
The UKU Side Effect Rating Scale (Udvalg for Kliniske Undersoegelser: Committee for Clinical Investigations) is a comprehensive measure of psychological and physical adverse reactions to psychotropic drugs. Reference Lingjaerde, Ahlfors, Bech, Dencker and Elgen5 It is scored by a trained health professional in a semi-structured interview with the individual and taking into account any additional sources of information. For 48 specific psychological, neurological, autonomic, sexual, dermatological and other symptoms, the UKU records the presence and severity of the symptom (0 no or doubtful; 1 mild; 2 moderate; 3 severe; anchors specified for each symptom) and the likelihood of causal relationship to the psychotropic medication (0 improbable; 1 possible; 2 probable). Fourteen UKU items are similar to items of the ASEC. In the GENDEP study, the UKU scale was administered in face-to-face sessions by psychiatrists at baseline (before commencement of study medication), at study weeks 8 and 12 and at the 6-month follow-up. The original English and Danish versions were used as developed by the scale authors and translated to the other six languages, with back-translation to English checked and differences resolved in consensus meetings. The scale completion took 10–30 minutes. All raters underwent training following the UKU manual, Reference Lingjaerde, Ahlfors, Bech, Dencker and Elgen5 rated 10 recorded interviews and achieved interrater reliability of >0.9 (kappa).
Depression severity was measured weekly with three established scales: the clinician-rated 10-item MADRS, Reference Montgomery and Åsberg13 the 17-item Hamilton Rating Scale for Depression (HRSD–17), Reference Hamilton20 and the self-report 21-item Beck Depression Inventory (BDI). Reference Beck, Ward, Mendelson, Mock and Erbaugh21 A psychometric analysis found that depressive symptoms could be better described by three symptom dimensions derived by categorical item factor analysis: observed mood, cognitive symptoms and neurovegetative symptoms. Reference Uher, Farmer, Maier, Rietschel, Hauser and Marusic22 The dimensional scores are obtained based on a graded-response item-response theory model fitted to non-overlapping sets of items and using the previously reported item parameters Reference Uher, Farmer, Maier, Rietschel, Hauser and Marusic22 in MULTILOG–7 for Windows (Scientific Software International, Inc.; www.ssicentral.com). The neurovegetative symptoms dimension comprises insomnia, poor appetite, weight loss and decreased libido and overlaps significantly with the content of adverse effects rating scales. The observed mood dimension comprises clinician-rated items assessing core mood symptoms, anxiety and activity and has no content overlap with ASEC. Therefore, the observed mood score was used as a covariate to control for the severity of depressed mood in the analyses of adverse reactions.
Statistical analysis
The agreement between ASEC and UKU was tested in 2846 ratings where both UKU and ASEC were available for participants during treatment. For the 14 items with corresponding content in the two instruments, the agreement between ASEC and UKU was quantified using the kappa coefficient with quadratic weights, Reference Cohen23 which is equivalent to an intraclass correlation. Reference Fleiss and Cohen24 The internal structure of the ASEC was explored using standard psychometric analysis, including item-total correlation, average inter-item covariance and Cronbach's alpha. Categorical item factor analysis was performed using the robust weighted least squares estimation Reference Flora and Curran25 in Mplus 5.1 for Windows (Muthen & Muthen; www.StatModel.com). Reference Muthen and Muthen26 The value of summing items into a single score (scalability) was assessed by Mokken analysis, which returns the Loevinger coefficient of homogeneity. The Loevinger coefficient is interpreted according to published recommendations: 0.30 to 0.39 weak scale; 0.40 to 0.49 an acceptable scale; 0.50 or higher a strong scale. Reference Mokken27
As relatively few individuals reported suffering from the complaints to a moderate or severe degree, data on individual adverse reactions were collapsed into dichotomous variables (present/absent) for further analyses.
To address the question of whether specific symptoms are more common during antidepressant treatment than in medication-free depression, we compared reports of each complaint between the medication-free baseline and later assessments during antidepressant treatment in the same individuals (n = 641) using a logistic regression with robust estimator of variance to control for multiple assessments within an individual. This estimation returns standard errors that are robust to within-individual correlation, thus relaxing the assumption that observations be independent. Reference Kent28 The total scale score was treated as a continuous variable and analysed using linear mixed models with hierarchical random effects of individual and centre of recruitment as previously reported. Reference Uher, Maier, Hauser, Marušič, Schmael and Mors9
To test the hypothesis that the two antidepressants have distinct adverse effect profiles, we compared individual complaints between participants randomly allocated to escitalopram and nortriptyline while they were on the randomly allocated medication. The comparisons were performed using logistic models with robust standard errors to allow multiple observations within an individual. Reference Kent28
Effects of antidepressant dose, severity of depression and study week were assessed using likelihood ratio tests with robust standard errors to compare nested multiple logistic regression models Reference Kent28 for individual complaints and mixed linear models Reference Uher, Maier, Hauser, Marušič, Schmael and Mors9 for the total score.
The predictive value of adverse reactions was tested with these as predictors of time to discontinuation of initially allocated antidepressant in a survival Cox proportional hazard model. Reference Hosmer and Lemeshow29 These models were run first with adverse effect as a single predictor and then repeated with severity of depressed mood as a covariate, as the severity of depressed mood was a significant predictor of discontinuation Reference Uher, Maier, Hauser, Marušič, Schmael and Mors9 and correlated with measures of adverse reactions.
Results
Data completeness
There were 8545 ASEC ratings (mean 11.2 ratings per participant) and 2837 UKU ratings (3.7 ratings per participant) available for analysis. The ASEC data were largely complete, with only 0.5% of item-wise values missing. There was a higher rate of missing values in the longer UKU (4.2%) with most missing values in specific questions on sexual adverse effects and more missing values for women (4.9%) than for men (2.9%).
Agreement between self-rated ASEC and clinician-rated UKU
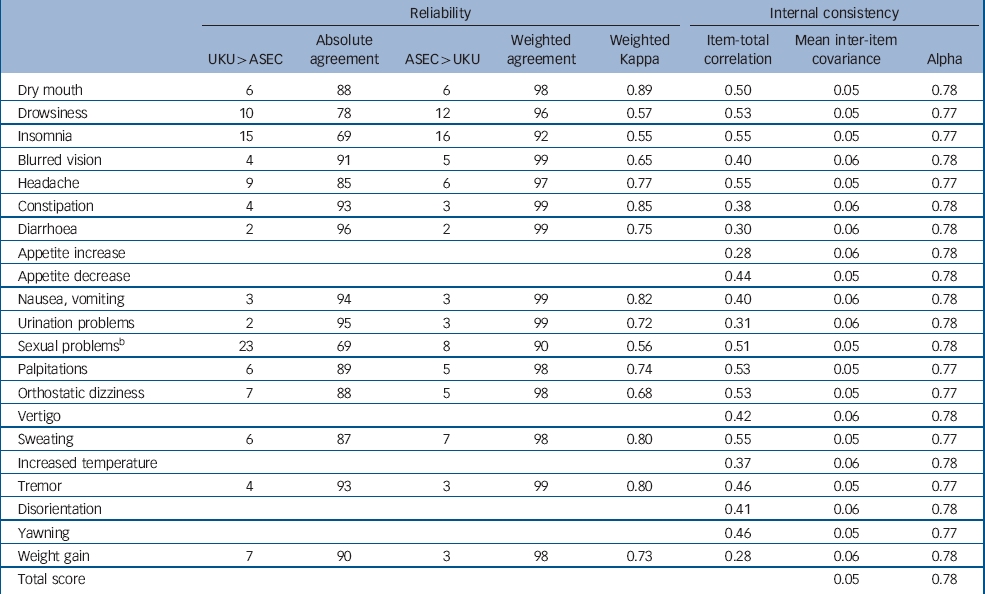
For the 14 items with close equivalents on both scales, the agreement between the self-rated ASEC and the interviewer-rated UKU was good, with kappas ranging from 0.55 for insomnia to 0.89 for dry mouth (Table 1). There was no bias for either ASEC or UKU ratings being more severe, as disagreements were equally distributed in both directions (Table 1). The ASEC question on sexual problems did not have a single-item equivalent on the UKU, but agreement between ASEC and a positive rating on any of the five relevant UKU items (diminished sexual desire, erectile dysfunction, ejaculatory dysfunction, orgasmic dysfunction, dry vagina) was 0.56. The correlation between summed total scores on the ASEC and UKU was 0.63 (95% CI 0.61–0.66).
Table 1 Reliability and internal consistency of the Antidepressant Side-Effect Checklist (ASEC)a
| Reliability | Internal consistency | |||||||
|---|---|---|---|---|---|---|---|---|
| UKU > ASEC | Absolute agreement | ASEC > UKU | Weighted agreement | Weighted Kappa | Item-total correlation | Mean inter-item covariance | Alpha | |
| Dry mouth | 6 | 88 | 6 | 98 | 0.89 | 0.50 | 0.05 | 0.78 |
| Drowsiness | 10 | 78 | 12 | 96 | 0.57 | 0.53 | 0.05 | 0.77 |
| Insomnia | 15 | 69 | 16 | 92 | 0.55 | 0.55 | 0.05 | 0.77 |
| Blurred vision | 4 | 91 | 5 | 99 | 0.65 | 0.40 | 0.06 | 0.78 |
| Headache | 9 | 85 | 6 | 97 | 0.77 | 0.55 | 0.05 | 0.77 |
| Constipation | 4 | 93 | 3 | 99 | 0.85 | 0.38 | 0.06 | 0.78 |
| Diarrhoea | 2 | 96 | 2 | 99 | 0.75 | 0.30 | 0.06 | 0.78 |
| Appetite increase | 0.28 | 0.06 | 0.78 | |||||
| Appetite decrease | 0.44 | 0.05 | 0.78 | |||||
| Nausea, vomiting | 3 | 94 | 3 | 99 | 0.82 | 0.40 | 0.06 | 0.78 |
| Urination problems | 2 | 95 | 3 | 99 | 0.72 | 0.31 | 0.06 | 0.78 |
| Sexual problemsb | 23 | 69 | 8 | 90 | 0.56 | 0.51 | 0.05 | 0.78 |
| Palpitations | 6 | 89 | 5 | 98 | 0.74 | 0.53 | 0.05 | 0.77 |
| Orthostatic dizziness | 7 | 88 | 5 | 98 | 0.68 | 0.53 | 0.05 | 0.77 |
| Vertigo | 0.42 | 0.06 | 0.78 | |||||
| Sweating | 6 | 87 | 7 | 98 | 0.80 | 0.55 | 0.05 | 0.77 |
| Increased temperature | 0.37 | 0.06 | 0.78 | |||||
| Tremor | 4 | 93 | 3 | 99 | 0.80 | 0.46 | 0.05 | 0.77 |
| Disorientation | 0.41 | 0.06 | 0.78 | |||||
| Yawning | 0.46 | 0.05 | 0.77 | |||||
| Weight gain | 7 | 90 | 3 | 98 | 0.73 | 0.28 | 0.06 | 0.78 |
| Total score | 0.05 | 0.78 | ||||||
Internal structure of ASEC
All ASEC items were positively related to the total score (Table 1). The item-test correlations for most ASEC items were between 0.4 and 0.55. Lower item-total correlations were found for diarrhoea, increased appetite, urination problems and weight gain. The average inter-item covariance was 0.05 and Cronbach's alpha was 0.78. A Mokken analysis returned a Loevingen coefficient of 0.20, indicating weak scalability. These indices suggest a lower internal consistency than would be expected for a unidimensional scale measuring a single construct. However, a factor analysis of categorical items showed one dominant factor with a high ratio of first-to-second eigenvalue, few significant loadings on a second factor and frequent cross-loadings, indicating that division into multiple subscales would not improve measurement. Therefore, the ASEC, similar to other measures of adverse reactions, is best conceived of as an index composed of causal indicators, rather than an internally consistent unidimensional scale. Reference Streiner30 The internal structure of such indices may be less transferable between populations. Therefore, we report analyses separately for each item. In addition, we use the total score as an approximate index of all reported adverse reactions.
Frequencies of complaints and drug comparisons
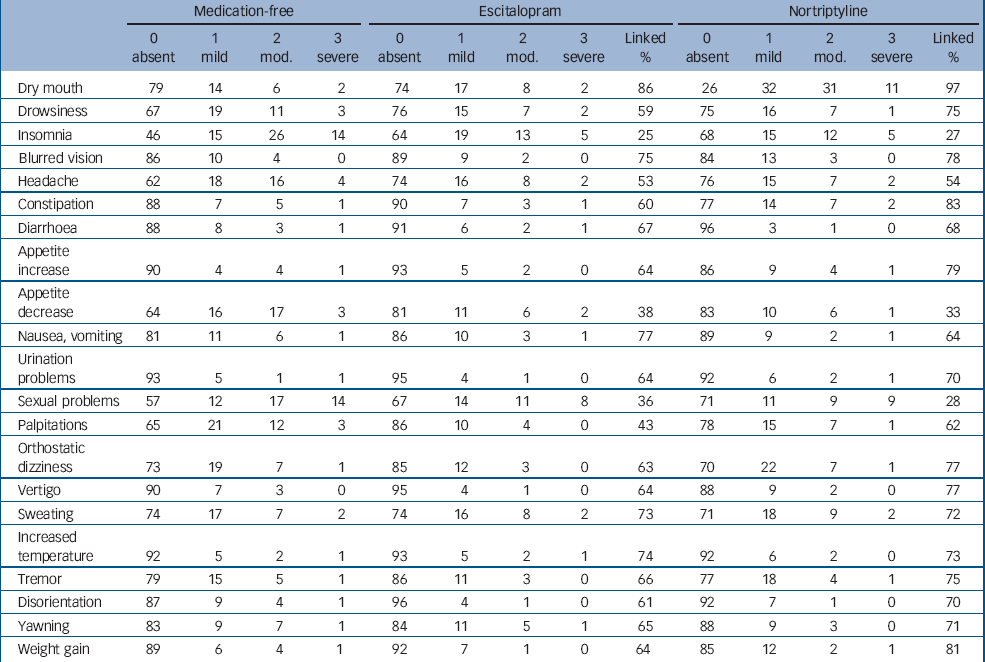
In Table 2, the frequencies of endorsing each potential adverse effect are given separately for individuals who were not taking any antidepressant medication at the time of rating (‘medication-free’), individuals taking escitalopram and those taking nortriptyline. The most frequently reported complaint was dryness of mouth, which was experienced by 74% of individuals while taking nortriptyline. Some complaints commonly considered to be adverse reactions to medication, e.g. drowsiness, headache, problems with sexual function and palpitations, were actually more common in untreated individuals than in those taking either antidepressant. Among individuals taking an antidepressant, the attribution of subjective complaints to the antidepressant medication (‘% linked’) varied from around 30% for sexual problems to 97% for dry mouth on nortriptyline. Insomnia, decreased appetite and problems with sexual function were attributed to either escitalopram or nortriptyline in less than 50% of cases. The UKU items not covered in ASEC were either rarely endorsed (e.g. neurological and dermatological complaints) or overlapped with symptoms of depression (e.g. concentration, fatigability, tension and emotional indifference). Therefore, further analyses focused on the more complete ASEC.
Table 2 Frequencies of endorsement on the Antidepressant Side-Effect Checklista
| Medication-free | Escitalopram | Nortriptyline | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 absent | 1 mild | 2 mod. | 3 severe | 0 absent | 1 mild | 2 mod. | 3 severe | Linked % | 0 absent | 1 mild | 2 mod. | 3 severe | Linked % | |
| Dry mouth | 79 | 14 | 6 | 2 | 74 | 17 | 8 | 2 | 86 | 26 | 32 | 31 | 11 | 97 |
| Drowsiness | 67 | 19 | 11 | 3 | 76 | 15 | 7 | 2 | 59 | 75 | 16 | 7 | 1 | 75 |
| Insomnia | 46 | 15 | 26 | 14 | 64 | 19 | 13 | 5 | 25 | 68 | 15 | 12 | 5 | 27 |
| Blurred vision | 86 | 10 | 4 | 0 | 89 | 9 | 2 | 0 | 75 | 84 | 13 | 3 | 0 | 78 |
| Headache | 62 | 18 | 16 | 4 | 74 | 16 | 8 | 2 | 53 | 76 | 15 | 7 | 2 | 54 |
| Constipation | 88 | 7 | 5 | 1 | 90 | 7 | 3 | 1 | 60 | 77 | 14 | 7 | 2 | 83 |
| Diarrhoea | 88 | 8 | 3 | 1 | 91 | 6 | 2 | 1 | 67 | 96 | 3 | 1 | 0 | 68 |
| Appetite increase | 90 | 4 | 4 | 1 | 93 | 5 | 2 | 0 | 64 | 86 | 9 | 4 | 1 | 79 |
| Appetite decrease | 64 | 16 | 17 | 3 | 81 | 11 | 6 | 2 | 38 | 83 | 10 | 6 | 1 | 33 |
| Nausea, vomiting | 81 | 11 | 6 | 1 | 86 | 10 | 3 | 1 | 77 | 89 | 9 | 2 | 1 | 64 |
| Urination problems | 93 | 5 | 1 | 1 | 95 | 4 | 1 | 0 | 64 | 92 | 6 | 2 | 1 | 70 |
| Sexual problems | 57 | 12 | 17 | 14 | 67 | 14 | 11 | 8 | 36 | 71 | 11 | 9 | 9 | 28 |
| Palpitations | 65 | 21 | 12 | 3 | 86 | 10 | 4 | 0 | 43 | 78 | 15 | 7 | 1 | 62 |
| Orthostatic dizziness | 73 | 19 | 7 | 1 | 85 | 12 | 3 | 0 | 63 | 70 | 22 | 7 | 1 | 77 |
| Vertigo | 90 | 7 | 3 | 0 | 95 | 4 | 1 | 0 | 64 | 88 | 9 | 2 | 0 | 77 |
| Sweating | 74 | 17 | 7 | 2 | 74 | 16 | 8 | 2 | 73 | 71 | 18 | 9 | 2 | 72 |
| Increased temperature | 92 | 5 | 2 | 1 | 93 | 5 | 2 | 1 | 74 | 92 | 6 | 2 | 0 | 73 |
| Tremor | 79 | 15 | 5 | 1 | 86 | 11 | 3 | 0 | 66 | 77 | 18 | 4 | 1 | 75 |
| Disorientation | 87 | 9 | 4 | 1 | 96 | 4 | 1 | 0 | 61 | 92 | 7 | 1 | 0 | 70 |
| Yawning | 83 | 9 | 7 | 1 | 84 | 11 | 5 | 1 | 65 | 88 | 9 | 3 | 0 | 71 |
| Weight gain | 89 | 6 | 4 | 1 | 92 | 7 | 1 | 0 | 64 | 85 | 12 | 2 | 1 | 81 |
In individuals who were not taking any antidepressant at baseline, we compared ratings during the medication-free status with follow-ups during antidepressant treatment to establish whether treatment with antidepressants led to specific adverse reactions. This analysis included 372 escitalopram-treated and 262 nortriptyline-treated individuals. Table 3 shows the odds ratios (OR) of experiencing an adverse effect for each antidepressant compared with medication-free status in the same participants. The only complaint that was significantly more frequent during treatment with escitalopram compared with antidepressant-free baseline was dryness of mouth (OR = 1.46, 95% CI 1.09–1.95). On the other hand, 9 of the 21 complaints were significantly less frequent during treatment with escitalopram than when antidepressant-free, with odds ratios ranging from 0.37 for palpitations to 0.63 for headache. Treatment with nortriptyline was associated with a dramatic increase in dryness of mouth (OR = 9.04) and smaller increases in constipation, increased appetite and weight gain. Ten of the 21 complaints were reduced during nortriptyline treatment compared with the antidepressant-free baseline.
Table 3 Comparisons between escitalopram, nortriptyline and medication-free statea

| Escitalopram v. medication-free | Nortriptyline v. medication-free | Nortriptyline v. escitalopram | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | Lower | Upper | P | OR | Lower | Upper | P | OR | Lower | Upper | P | |
| Dry mouth | 1.46 b | 1.09 | 1.95 | 0.010 | 9.04 | 6.49 | 12.60 | <0.001 | 11.53 | 8.20 | 16.22 | <0.001 |
| Drowsiness | 0.74 | 0.58 | 0.94 | 0.015 | 0.60 | 0.44 | 0.80 | 0.001 | 1.12 | 0.84 | 1.50 | 0.443 |
| Insomnia | 0.50 | 0.41 | 0.61 | <0.001 | 0.30 | 0.23 | 0.39 | <0.001 | 0.73 | 0.54 | 0.97 | 0.033 |
| Blurred vision | 0.79 | 0.57 | 1.09 | 0.148 | 1.31 | 0.91 | 1.90 | 0.152 | 1.73 | 1.20 | 2.49 | 0.003 |
| Headache | 0.63 | 0.50 | 0.79 | <0.001 | 0.42 | 0.32 | 0.55 | <0.001 | 0.83 | 0.63 | 1.09 | 0.184 |
| Constipation | 0.86 | 0.61 | 1.19 | 0.359 | 1.95 | 1.32 | 2.89 | 0.001 | 2.63 | 1.83 | 3.78 | <0.001 |
| Diarrhoea | 0.86 | 0.59 | 1.25 | 0.430 | 0.23 | 0.15 | 0.35 | <0.001 | 0.38 | 0.24 | 0.59 | <0.001 |
| Appetite increase | 0.71 | 0.48 | 1.06 | 0.096 | 1.67 | 1.01 | 2.76 | 0.045 | 2.33 | 1.60 | 3.39 | <0.001 |
| Appetite decrease | 0.44 | 0.36 | 0.55 | <0.001 | 0.29 | 0.22 | 0.39 | <0.001 | 0.78 | 0.55 | 1.10 | 0.149 |
| Nausea, vomiting | 1.01 | 0.74 | 1.37 | 0.962 | 0.42 | 0.31 | 0.58 | <0.001 | 0.74 | 0.54 | 1.03 | 0.072 |
| Urination problems | 1.01 | 0.61 | 1.66 | 0.977 | 0.89 | 0.56 | 1.43 | 0.636 | 1.71 | 1.02 | 2.86 | 0.042 |
| Sexual problems | 0.61 | 0.50 | 0.74 | <0.001 | 0.46 | 0.36 | 0.60 | <0.001 | 0.78 | 0.57 | 1.07 | 0.123 |
| Palpitations | 0.37 | 0.29 | 0.48 | <0.001 | 0.47 | 0.35 | 0.62 | <0.001 | 1.79 | 1.31 | 2.43 | <0.001 |
| Orthostatic dizziness | 0.55 | 0.43 | 0.71 | <0.001 | 1.01 | 0.78 | 1.32 | 0.921 | 2.28 | 1.67 | 3.11 | <0.001 |
| Vertigo | 0.62 | 0.42 | 0.93 | 0.020 | 1.01 | 0.67 | 1.52 | 0.970 | 2.70 | 1.83 | 3.99 | <0.001 |
| Sweating | 1.09 | 0.84 | 1.41 | 0.523 | 0.96 | 0.71 | 1.31 | 0.809 | 1.02 | 0.76 | 1.38 | 0.888 |
| Increased temperature | 1.09 | 0.66 | 1.79 | 0.747 | 0.75 | 0.45 | 1.26 | 0.277 | 1.05 | 0.65 | 1.69 | 0.846 |
| Tremor | 0.83 | 0.62 | 1.12 | 0.228 | 0.95 | 0.69 | 1.31 | 0.753 | 2.00 | 1.41 | 2.84 | <0.001 |
| Disorientation | 0.40 | 0.27 | 0.59 | <0.001 | 0.59 | 0.39 | 0.88 | 0.010 | 2.12 | 1.25 | 3.58 | 0.005 |
| Yawning | 1.13 | 0.84 | 1.52 | 0.434 | 0.51 | 0.36 | 0.72 | <0.001 | 0.61 | 0.43 | 0.87 | 0.006 |
| Weight gain | 0.72 | 0.49 | 1.06 | 0.096 | 1.72 | 1.08 | 2.75 | 0.022 | 1.75 | 1.27 | 2.41 | 0.001 |
To address the question of whether the two antidepressants differed in their adverse effect profile, we compared the frequency of each complaint in individuals randomly allocated to receive escitalopram (n = 233) and those randomly allocated to receive nortriptyline (n = 235). The last column of Table 3 shows the odds ratios resulting from a logistic regression comparison between escitalopram and nortriptyline in randomly allocated individuals. Diarrhoea, yawning and insomnia were more commonly reported during treatment with escitalopram than with nortriptyline. Dryness of mouth, constipation, blurred vision, orthostatic dizziness, vertigo, palpitations, disorientation, tremor, increased appetite and weight gain were more frequent during treatment with nortriptyline.
Are adverse reactions dose-related?
The relationships between individual complaints and antidepressant dose were generally weak and non-significant. The exception was dry mouth, which showed a positive relationship to the dose of both escitalopram (OR = 1.43 per 10 mg dose increase, 95% CI 1.13–1.82, P = 0.003) and nortriptyline (OR = 2.18 per 50 mg dose increase, 95% CI 1.62–2.92, P<0.001). The summed total ASEC score showed a weak negative relationship with dose of escitalopram (β = −0.12, 95% CI −0.16 to −0.08, P<0.001) and nortriptyline (β = −0.08, 95% CI −0.13 to −0.03, P = 0.003). This relationship reflected the fact that the dose was progressively increased and adverse reactions tended to decrease as the study progressed. This negative relationship between adverse reactions and dose disappeared when time in the study was entered as a covariate.
Time course: do adverse reactions wear off?
Most complaints decreased progressively over the 12-week follow-up (Fig. 1). For example, complaints of drowsiness, headache, nausea and orthostatic dizziness were half as common towards the end of the trial than during the first weeks (10-week OR = 0.31–0.51, all P<0.001). On the other hand, complaints of constipation and urination problems remained elevated across the 12 weeks, especially in participants treated with nortriptyline (OR = 1.02 and 0.93 respectively, not significant). Dryness of mouth also tended to persist in the majority of individuals treated with nortriptyline (OR = 0.75, 95% CI 0.56–1.00, P = 0.053). Sexual problems remained similarly common over the 12 weeks of treatment, but were consistently reduced in comparison with the pre-treatment baseline. Reports of weight gain grew slightly over the 12 weeks, and this increase was significant in the escitalopram-treated individuals (OR = 1.77, 95% CI 1.23–2.55, P = 0.002). The summed total ASEC score decreased as the study progressed in both escitalopram-treated (β = −0.34, 95% CI −0.39 to −0.28, P<0.001) and nortriptyline-treated (β = −0.36, 95% CI −0.43 to −0.29, P<0.001) individuals. The association between time and adverse effect score remained significant after controlling for dose and severity of depressed mood.

Fig. 1 Time-course of adverse effects. The bars show the proportion of participants reporting each complaint at weeks 0–12.
Adverse reactions and depressed mood
Most complaints of potential adverse reactions were positively related to the severity of depressed mood, with odds ratios between 1.2 and 1.9 for one standard deviation increase in observed depressed mood. Urination problems, increased temperature and yawning were not related to the severity of depressed mood in escitalopram-treated individuals. Dry mouth, constipation, sweating and increased temperature were unrelated to the severity of depressed mood in nortriptyline-treated individuals. Weight gain and appetite increase were the only items that were negatively related to depressed mood with odds ratios between 0.80 and 1.00. For 14 of the 21 items, the association with mood remained significant when only complaints attributed to the antidepressants were considered.
The total ASEC score was strongly positively related to the severity of depressed mood in escitalopram-treated individuals (β = 0.28, 95% CI 0.26–0.30, P<0.001), in nortriptyline-treated individuals (β = 0.25, 95% CI 0.22–0.28, P<0.001) and overall (β = 0.28, 95% CI 0.26–0.30, P<0.001). The associations between adverse effects score and severity of depressive symptoms was similar when only linked adverse effects were considered, and remained significant after controlling for time in the study and dose of antidepressants.
Do adverse reactions lead to discontinuation?
We used Cox proportional hazard models to test whether adverse reactions predicted discontinuation of antidepressants. As severity of depressed mood predicted discontinuation and was positively associated with most physical complaints, the models were repeated with depressed mood as a covariate. Table 4 shows the hazard ratios (HRs) for each adverse effect predicting discontinuation. Complaints of decreased appetite, diarrhoea, orthostatic dizziness and dry mouth predicted discontinuation in escitalopram-treated individuals independently of the effect of mood. Urinary problems and drowsiness predicted discontinuation of nortriptyline, independently of the effect of depressed mood. Urinary problems, dry mouth, blurred vision and orthostatic dizziness predicted discontinuation across the whole sample and remained significant after controlling for the severity of depressed mood.
Table 4 Predictive validity of adverse reactions for antidepressant discontinuationa

| Overall | Escitalopram | Nortriptyline | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | Lower | Upper | P | HR | Lower | Upper | P | HR | Lower | Upper | P | |
| Dry mouth | 1.67 b | 1.31 | 2.14 | <0.001 * | 1.72 | 1.20 | 2.46 | 0.003 * | 1.12 | 0.76 | 1.66 | 0.565 |
| Drowsiness | 1.30 | 0.99 | 1.69 | 0.055 | 0.94 | 0.62 | 1.42 | 0.764 | 1.68 | 1.18 | 2.39 | 0.004 * |
| Insomnia | 1.36 | 1.06 | 1.73 | 0.014 | 1.47 | 1.03 | 2.09 | 0.032 | 1.31 | 0.94 | 1.84 | 0.115 |
| Blurred vision | 1.60 | 1.19 | 2.15 | 0.002 * | 1.55 | 0.97 | 2.48 | 0.067 | 1.50 | 1.02 | 2.22 | 0.040 |
| Headache | 1.27 | 0.98 | 1.66 | 0.070 | 1.34 | 0.92 | 1.95 | 0.128 | 1.25 | 0.86 | 1.81 | 0.237 |
| Constipation | 1.55 | 1.17 | 2.06 | 0.002 * | 1.42 | 0.85 | 2.38 | 0.177 | 1.32 | 0.93 | 1.88 | 0.124 |
| Diarrhoea | 1.52 | 1.03 | 2.25 | 0.037 | 2.10 | 1.32 | 3.33 | 0.002 * | 1.00 | 0.44 | 2.27 | 0.994 |
| Appetite increase | 0.86 | 0.57 | 1.31 | 0.484 | 0.59 | 0.26 | 1.34 | 0.206 | 0.90 | 0.55 | 1.48 | 0.692 |
| Appetite decrease | 1.59 | 1.21 | 2.10 | 0.001 | 2.20 | 1.52 | 3.20 | <0.001 * | 1.15 | 0.75 | 1.75 | 0.524 |
| Nausea, vomiting | 1.44 | 1.04 | 1.99 | 0.027 | 1.62 | 1.04 | 2.53 | 0.033 | 1.34 | 0.83 | 2.16 | 0.226 |
| Urination problems | 1.90 | 1.32 | 2.74 | 0.001 * | 1.41 | 0.72 | 2.78 | 0.320 | 1.97 | 1.27 | 3.05 | 0.003 * |
| Sexual problems | 1.32 | 1.03 | 1.69 | 0.031 | 1.35 | 0.94 | 1.94 | 0.103 | 1.33 | 0.94 | 1.88 | 0.110 |
| Palpitations | 1.32 | 0.99 | 1.75 | 0.060 | 1.42 | 0.91 | 2.20 | 0.122 | 1.13 | 0.77 | 1.65 | 0.526 |
| Orthostatic dizziness | 1.45 | 1.11 | 1.89 | 0.007 * | 2.05 | 1.38 | 3.03 | <0.001 * | 0.95 | 0.66 | 1.37 | 0.773 |
| Vertigo | 1.56 | 1.07 | 2.28 | 0.022 | 2.27 | 1.27 | 4.05 | 0.006 | 1.04 | 0.63 | 1.73 | 0.880 |
| Sweating | 1.29 | 1.00 | 1.67 | 0.048 | 1.57 | 1.09 | 2.26 | 0.015 | 1.04 | 0.73 | 1.49 | 0.829 |
| Increased temperature | 1.50 | 1.03 | 2.20 | 0.036 | 1.19 | 0.64 | 2.22 | 0.574 | 1.71 | 1.05 | 2.78 | 0.030 |
| Tremor | 1.41 | 1.07 | 1.87 | 0.016 | 1.64 | 1.09 | 2.48 | 0.018 | 1.15 | 0.78 | 1.68 | 0.486 |
| Disorientation | 1.23 | 0.79 | 1.90 | 0.354 | 1.38 | 0.70 | 2.72 | 0.354 | 1.02 | 0.58 | 1.81 | 0.941 |
| Yawning | 1.08 | 0.77 | 1.51 | 0.652 | 1.00 | 0.62 | 1.62 | 0.998 | 1.24 | 0.78 | 1.97 | 0.373 |
| Weight gain | 1.21 | 0.85 | 1.71 | 0.291 | 0.76 | 0.39 | 1.51 | 0.437 | 1.35 | 0.89 | 2.04 | 0.160 |
The total ASEC score predicted discontinuation of escitalopram (HR = 1.41 per ASEC standard deviation (5.6 points), 95% CI 1.22–1.63, P<0.001), nortriptyline (HR = 1.28, 95% CI 1.11–1.48, P = 0.001) and overall (HR = 1.37, 95% CI 1.24–1.51, P<0.001). After correction for the severity of depressed mood, the effect of total ASEC score on discontinuation remained significant for escitalopram (HR = 1.25, 95% CI 1.07–1.45, P = 0.005) and overall (HR = 1.22, 95% CI 1.10–1.35, P<0.001), but not for nortriptyline (HR = 1.15, 95% CI 0.99–1.33, P = 0.060). The addition of the UKU score to the model did not improve the prediction of discontinuation provided by the ASEC.
Discussion
Measurement of adverse reactions
The GENDEP study demonstrates that adverse reactions to antidepressants can be usefully measured with a brief self-report instrument that does not require medical input. There was good agreement between self-report and psychiatrists' ratings and the much longer UKU did not add predictive value above the ASEC self-report. The various adverse reactions tended to be only weakly correlated. As a consequence, summed total scores on scales measuring adverse reactions do not represent sufficient statistics and specific complaints merit separate evaluation. Individual adverse reactions differ in their time course during antidepressant treatment and have significance in predicting discontinuation of antidepressants that is not captured by the sum of weakly correlated items. For example, the urinary complaints are a stronger predictor of discontinuation than the sum of all potential adverse reactions.
Adverse reactions and severity of depression
Many physical complaints that are listed among adverse reactions were more common in participants who were not taking medication compared with the same individuals during treatment with antidepressants, were related to the severity of depression rather than antidepressant dose and decreased over time with continued treatment. The blurred boundary between symptoms of depression and adverse reactions to antidepressants may confound the assessment of efficacy and tolerability. Reference Uher, Maier, Hauser, Marušič, Schmael and Mors9,Reference Moller31 It has been previously reported that adverse reactions are more frequently experienced by individuals with more severe depression. 11 In GENDEP, we have extended this finding by testing whether the association between adverse reactions and severity of depressed mood is as a result of content overlap between instruments used to assess depression and adverse reactions. We found evidence that this is not the case. First, most adverse reactions were positively associated with scores on the observed mood dimension, Reference Uher, Farmer, Maier, Rietschel, Hauser and Marusic22 which captures the core symptoms of depression and has no content overlap with the adverse effects checklist. Second, even linked adverse effects that were attributed to the antidepressants were strongly positively correlated with the severity of depressed mood at the time of assessment. We conclude that more severely depressed individuals are more likely to experience physical adverse reactions to antidepressants. This may be because of the increased sensitivity and attention to physical discomfort that accompanies depressed mood. Reference Tang, Salkovskis, Hodges, Wright, Hanna and Hester32,Reference Klauenberg, Maier, Assion, Hoffmann, Krumova and Magerl33
Antidepressant-specific profiles of adverse reactions
Although there may be an overlap between adverse reactions to antidepressants and symptoms of depression, it is clear that the two antidepressants used in GENDEP have distinct adverse effect profiles. We have confirmed that people treated with nortriptyline, a tricyclic antidepressant, experience anticholinergic adverse effects including dry mouth, constipation, orthostatic dizziness and blurred vision more commonly than those taking escitalopram, an SSRI. On the other hand, escitalopram was associated with more complaints of diarrhoea and yawning. These adverse effect profiles are in agreement with previous reports. Reference Remick16–Reference Gutierrez-Alvarez19,Reference Wilson and Mottram34 The increased appetite and weight gain on nortriptyline and decreased appetite and insomnia on escitalopram can be interpreted either in terms of adverse reactions or as differential efficacy of the two drugs on neurovegetative symptoms of depression. Reference Uher, Maier, Hauser, Marušič, Schmael and Mors9 The fact that decreased appetite and insomnia are more frequently reported in the medication-free depressed state than during treatment with escitalopram suggests that at least part of this difference can be attributed to differential efficacy.
Dry mouth was the most commonly reported adverse effect. This adverse reaction was more common during treatment with either nortriptyline or escitalopram than in the medication-free state, and showed a positive relationship with the dose of both antidepressants. Dry mouth was reported by three-quarters of participants taking nortriptyline. This is a much higher proportion than the 27% found by a meta-analysis of studies with various, often non-standard, methods of eliciting information about adverse reactions. Reference Trindade, Menon, Topfer and Coloma18 This suggests that common adverse reactions are underreported when specific questions are not asked.
Time course of adverse reactions
The weekly assessments allowed us to explore the time course of adverse effects. Most complaints decreased over the 12 weeks. For example, orthostatic dizziness was more common in the first 4 weeks of treatment with nortriptyline but then decreased to the pre-treatment level. On the other hand, dry mouth and urinary problems tended to persist throughout the study period. It may be important to give realistic information as to which adverse effects are likely to habituate and to manage those effects that have a tendency to persist: persistent adverse effects may be an important determinant of long-term non-adherence. Reference Frank and Judge2,Reference Lingam and Scott3
Impact of adverse reactions on adherence
Most of the commonly reported adverse effects were not associated with discontinuation of antidepressants. However, several adverse reactions were strong predictors of discontinuation. For example, urinary problems, although relatively uncommon, doubled the rate of discontinuation of treatment with nortriptyline within the 12 weeks. Urinary hesitancy and retention are known adverse reactions to drugs with anticholinergic properties, including tricyclic antidepressants. Reference Remick16,Reference Degner, Grohmann, Kropp, Ruther, Bender and Engel17,Reference Verhamme, Sturkenboom, Stricker and Bosch35 As urinary retention is treatable, Reference Demyttenaere and Huygens36 specific attention to this problem may be needed to enable collaborative decision-making and maximise the chance of an individual receiving effective medication with a minimal burden of adverse effects. Reference Vinberg37
Limitations and future direction
The GENDEP study compared two active antidepressants in an open-label design and did not include a placebo arm. This made GENDEP more acceptable to treatment-seeking individuals with depression and their general practitioners and enabled us to recruit a more representative sample. On the other hand, the lack of masking may have introduced a source of bias as the participants knew which drug they were taking and the lack of placebo precluded the differentiation of effects that are common to both study medications from placebo effects. We were able to compare complaints during medication-free status with those during antidepressant treatment. However, because of the lack of placebo condition, we were unable to make such comparison specifically for linked adverse effects that were subjectively attributed to medication. We hope that the pragmatic ASEC measure will facilitate a systematic exploration of adverse reactions in future placebo-controlled studies of antidepressants and the resulting data will enable a more accurate interpretation of the present results.
Appendix: The Antidepressant Side-Effect Checklist (ASEC)
Please score the following list of symptoms 0 = absent, 1 = mild, 2 = moderate, 3 = severe.
Please indicate if the symptom is likely to be a side-effect of antidepressant medication (Y = YES, N = NO). Write a comment to provide relevant information if the item is not a side-effect.
Acknowledgements
We thank the following collaborators for their contribution: Richard J. Williamson, Abraham Sterne, Helen Dean, Bhanu Gupta, Joanna Gray, Cerisse Gunasinghe, Amanda Elkin, Desmond Campbell, Julien Mendlewicz, Thomas Schulze, Christine Schmael, Susanne Höfels, Anna Schuhmacher, Ute Pfeiffer, Sandra Weber, Lisbeth Jorgensen, Anne Schinkel Stamp, Alenka Tancic, Jerneja Sveticic, Zrnka Kovacic, Pawel Kapelski, Maria Skibiñska, Piotr M Czerski, Aleksandra Rajewska, Monika Dmitrzak-Weglarz, Aleksandra Szczepankiewicz and Elzbieta Cegielska. We specially acknowledge the contribution of Jorge Perez, who was the lead investigator at Brescia, Italy, and who died in October 2007, and the important contribution made by Dr Andrej Marušič, the principal investigator at Ljubljana, Slovenia, who died in June 2008.








eLetters
No eLetters have been published for this article.