Schizophrenia is a diverse disorder. One facet of its diversity is the age at onset. The incidence of schizophrenia peaks between 10 and 25 years for men and between 25 and 35 for women. Reference Buchanan, Carpenter, Sadock and Sadock1 Another peak, particularly among women, occurs in mid-life: about 23% of people with schizophrenia experience their first episode after the age of 40. Reference Harris and Jeste2 In a small group of people, schizophrenia has its onset after age 60, which has been defined as very-late-onset schizophrenia-like psychosis. Reference Howard, Rabins, Seeman and Jeste3 Finally, onset of schizophrenia can occur in childhood or adolescence, typically after the age of 5. Its prevalence is about 1 per 10 000 in children, and 1–2 per 1000 in adolescents. As in early adulthood, higher rates are reported in young males than in young females. Reference McClennan, Sadock and Sadock4
DeLisi has conceptualised age at onset as a surrogate measure of severity of the disease process. Reference Delisi5 An earlier age at onset has been associated with more severe clinical and behavioural symptoms, Reference Hoff, Harris, Faustman, Beal, DeVilliers and Mone6,Reference Johnstone, Owens, Bydder, Colter, Crow and Frith7 more social disability, Reference Eggers and Bunk8 narrower posterior brain segments Reference Crow, Colter, Frith, Johnstone and Owens9 and larger ventricles. Reference Raz and Raz10 Cognitive deficits in schizophrenia are core features of the illness. Reference Heinrichs and Zakzanis11,Reference Rajji and Mulsant12 They are the strongest predictors of function and are considered potential targets for treatment. Reference Green, Kern and Heaton13 They have been well characterised, for example by Heaton et al, Reference Heaton, Paulsen, McAdams, Kuck, Zisook and Braff14 Hoff et al, Reference Hoff, Sakuma, Wieneke, Horon, Kushner and Delisi15 and White et al, Reference White, Ho, Ward, O'Leary and Andreasen16 and are believed to be the manifestation of the disease process affecting different brain systems. Thus, characterising the relationship between age at onset and cognitive deficits will illuminate from a cognitive perspective the unravelling of the disease process at different levels of severity. Although some studies have reported an association of earlier age at onset and more severe cognitive deficits, Reference Hoff, Harris, Faustman, Beal, DeVilliers and Mone6,Reference Jeste, McAdams, Palmer, Braff, Jernigan and Paulsen17–Reference Tuulio-Henriksson, Partonen, Suviusaari, Haukka and Lönnqvist19 others failed to find differences in the cognitive profiles of individuals with early v. late-onset schizophrenia. Reference Heaton, Paulsen, McAdams, Kuck, Zisook and Braff14,Reference Jeste, Harris, Krull, Kuck, McAdams and Heaton20,Reference Sachdev, Brodaty, Rose and Cathcart21 Further, the nature of the cognitive deficits that may be associated with age at onset has varied in different studies. Considering that several of these studies have small sample sizes, and to assess whether such associations exist across the life-span, we present a meta-analysis of the literature, estimating and comparing the severity of cognitive deficits in patients with adult-onset at their first episode of schizophrenia, youth-onset (in childhood or adolescence) schizophrenia and late-onset schizophrenia. We chose to compare individuals with youth-onset or late-onset schizophrenia only to people with adult-onset schizophrenia at their first episode because individuals with youth-onset and late-onset schizophrenia are typically studied close to the onset of the illness. By contrast, in individuals with adult-onset schizophrenia who have been ill for many years, one would expect chronicity and treatment to influence cognitive deficits, thus, confounding the hypothesised specific relationships to age at onset. In this report we use the word ‘schizophrenia’ to refer to schizophrenia and related disorders: schizoaffective, schizophreniform, delusional disorder or very-late-onset schizophrenia-like psychosis.
Method
Literature search
A literature search of 29 databases was performed on 30 September 2008 (see Appendix for details and the search terms used). We searched back to 1980, the year of the introduction of DSM–III. 22
Inclusion and exclusion criteria
Only publications that included a healthy control group were considered for this meta-analysis so that effect sizes could be calculated. Drug trials that presented pre-treatment baseline cognitive data in patients and included a healthy control group were selected. Publications were excluded if individuals with a diagnosis of schizophrenia and those with another diagnosis (e.g. bipolar disorder with psychosis) were analysed together as one sample. Similarly, publications were also excluded if individuals with different age at onset (i.e. childhood, adolescence, adult or late-onset) were not analysed separately or if those with adult-onset schizophrenia included people who were not in their first episode. Publications reporting on treatment trials were excluded if there were no available baseline data to compare between patients and controls. Publications reporting on the same sample were counted as a single study.
Classification of studies
If a publication met the above criteria, individuals were classified into one of three groups: first-episode schizophrenia, youth-onset schizophrenia and late-onset schizophrenia. To be classified into youth-onset schizophrenia, individuals with schizophrenia had to have a maximum age at onset of 19 years. To be classified into late-onset schizophrenia, people with schizophrenia had to have a minimum age at onset of 40 years. For any other age at onset, a selected publication was classified into the first-episode schizophrenia category provided that the study included and analysed separately those individuals with first-episode schizophrenia. Age at onset is defined differently in different studies. It is used to refer to age at first behavioural changes (e.g. DeLisi et al Reference DeLisi, Hoff, Schwartz, Shields, Halthore and Gupta23 ), age at first manifestation of positive symptoms (e.g. White et al Reference White, Ho, Ward, O'Leary and Andreasen16 ) or age at first admission to hospital (e.g. Bellgrove et al Reference Bellgrove, Collinson, Mattingley, Pantelis, Fitzgerald and James24 ). However, these different stages have been shown to be correlated and typically within 6–18 months of each other. Reference Delisi5,Reference DeLisi, Hoff, Schwartz, Shields, Halthore and Gupta23,Reference Delisi, Goldin, Maxwell, Kazuba and Gershon25 Thus, when classifying a study, we accepted the definition of age at onset as described in each individual study.
Extracted variables
After the selection and classification of a publication, the following variables were extracted and recorded: journal name, title, first author and year of publication. In addition, the following variables were recorded for patients and controls separately: number of participants and mean (s.d.) age. Some studies only provided an age threshold (e.g. ‘onset before age 14’); when mean (s.d.) age at onset was provided, it was recorded and mean duration of illness was estimated based on the difference between mean age at onset and mean age. Finally, the cognitive data were recorded as follows.
Cognitive measures
The data from each cognitive test were extracted and classified into one of 22 cognitive measures (see Online supplement). The selection of these 22 measures followed a seminal quantitative review of cognitive deficits in schizophrenia. Reference Heinrichs and Zakzanis11 As noted in that review, ‘organizing the myriad of neurocognitive test variables reported in the literature into a coherent classification [is] a major challenge’. We adopted the strategy employed in that review because it accounts for two opposing yet complementary approaches. The first approach is to avoid aggregating cognitive tests and to report results of individual tests. This approach is supported by the fact that most individual tests are mediated by multiple cognitive processes and tap into more than one cognitive function. Thus, aggregation into a single cognitive measure could result in misleading effect sizes that do not correspond to a specific cognitive function. This is also relevant to our review considering that in different groups (first-episode, youth-onset and late-onset schizophrenia), different cognitive tests have been used to assess particular cognitive measures. Consequently, contrasting effect sizes that correspond to these cognitive measures could be problematic. Thus, whenever possible, results of individual tests were reported separately. The second approach is to aggregate cognitive tests into a single measure based on theoretical or factor-analytical underpinnings. This approach facilitates the management of data based on various tests that tap into the same cognitive function (e.g. both California Verbal Learning Test and Rey Auditory Verbal Learning Test assess verbal memory). It also adds to the weight of effect sizes by increasing the number of individual scores contributing to a mean effect size. This is particularly relevant to our review considering the relatively small number of participants in some studies.
Calculation and comparison of effect sizes
Individual Cohen's effect sizes (d i) Reference Cohen26 were calculated for each cognitive test as the difference between the means of the schizophrenia and control groups divided by the pooled standard deviation. In some studies the groups' means and standard deviations were not available and d i-values were calculated based on the reported inferential statistics. Reference Wolf27 Effect size raw means (d-values) and their 95% confidence intervals (CIs) were generated for each cognitive measure based on the individual d i-values aggregated in this measure. Finally, effect size weighted means (weighted d-values) and their standard errors (s.e.) were generated for each cognitive measure by calculating the mean of individual d i-values weighted by the inverse of their individual variance. Weighted means were also generated for age, age at onset and duration of illness. We used the Q between test to compare the three groups' weighted d-values statistically. Reference Lipsey and Wilson28 When Q between was significant, we compared each of the weighted d-values of each pair separately using Q between with Bonferroni's adjustment. We also used two difference cut-offs to compare effect sizes qualitatively: 0.3 and 0.6. Following well-established conventions, an effect size <0.2 is considered small, an effect size of 0.2–0.8 is considered medium and an effect size above 0.8 is considered large. Reference Cohen26 Thus, a difference of 0.3 separates a small effect size (0.2) and a typical medium effect size (0.5) or a typical medium effect size (0.5) and a large effect size (0.8). A difference of 0.6 separates a small effect size and a large effect size.
Results
The 913 retrieved publications and their relevant cited and citing references were reviewed by one of the authors (T.K.R.). A total of 78 first-episode schizophrenia, 23 youth-onset schizophrenia and 9 late-onset schizophrenia publications were included in the analysis (Fig. 1; see also the Online supplement for a full list of publications included in the analysis). Age at onset was operationally defined in all of the late-onset schizophrenia and 12 of the youth-onset schizophrenia publications. The remaining 11 youth-onset schizophrenia publications did not define age at onset operationally. However, in all these publications, individuals met DSM–III or later diagnostic manual criteria for schizophrenia before the age of 19. In first-episode schizophrenia publications, the first episode was always operationally defined: 61 did not define age at onset operationally.
Descriptive data of participants are reported in Table 1. The mean effect sizes (d-values) of cognitive deficits, the 95% CIs and the contributing number of studies are described in Fig. 2. Adults with first-episode schizophrenia demonstrate large deficits (d≥0.8) on most cognitive tests and measures. Deficits are most pronounced on performance IQ, followed by digit symbol coding, Tower of London and similar tests, visual and verbal memory, vocabulary, arithmetic and fluency. Deficits on most of these functions also rank high among individuals with youth-onset schizophrenia. However, these people demonstrate comparably larger deficits on visuospatial construction, Stroop test and Trail Making Test B. This pattern is in contrast to that observed in individuals with late-onset schizophrenia where auditory and visual attention, fluency and visuospatial construction are relatively more impaired than arithmetic, digit symbol coding, verbal memory and vocabulary.
Table 1 Descriptive data

| Group | Total, n a | Age, years: weighted means (s.d.) | Age at onset, years: weighted means (s.d.) | Duration of illness, b years: weighted means (s.d.) |
|---|---|---|---|---|
| First-episode schizophrenia | 4057 | 24.0 (0.6) | 23.7 (1.5) | 0.3 (0.4) |
| Youth-onset schizophrenia | 692 | 16.0 (0.3) | 13.6 (0.7) | 2.3 (0.2) |
| Late-onset schizophrenia | 261 | 68.4 (2.6) | 60.7 (4.2) | 7.7 (2.3) |
a. For first-episode, youth-onset and late-onset schizophrenia the total number of individuals for whom age at onset was reported was 850, 187 and 74 respectively.
b. Duration of illness was calculated by subtracting age at onset from age at time of assessment.
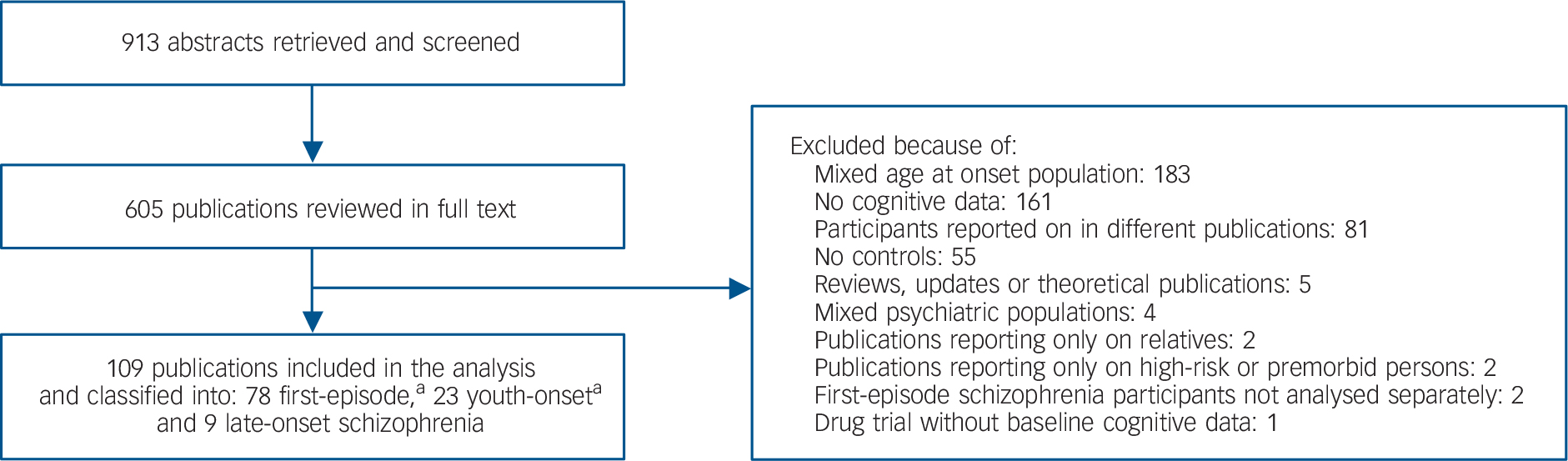
Fig. 1 Flow chart of literature review. a. One publication contributed to both first-episode schizophrenia and youth-onset schizophrenia groups.
The Q between test revealed statistical differences in the weighted mean effect sizes (weighted d-values) of the three groups for 19 of the 22 measures (there was no statistical difference for verbal general memory and verbal IQ in the three groups, and for continuous performance test between first-episode schizophrenia and youth-onset schizophrenia, Table 2). On these 19 measures, post hoc Q between tests with Bonferroni's adjustment showed that:
-
(a) individuals with youth-onset schizophrenia are more impaired than individuals with first-episode schizophrenia on full-scale IQ, psychomotor speed of processing, Trail Making Tests A and B, verbal special memory, and Wisconsin card sorting and similar tests;
-
(b) individuals with youth-onset schizophrenia are more impaired than those with late-onset schizophrenia on arithmetic, digit symbol coding, vocabulary, and Wisconsin card sorting and similar tests, but less impaired on auditory and visual attention; and
-
(c) individuals with first-episode schizophrenia are more impaired than those with late-onset schizophrenia on digit symbol coding, but less impaired on auditory attention, fluency, full-scale IQ, global measure of cognition and visual attention.
Table 2 Cognitive deficits effect sizes: weighted mean (s.e)
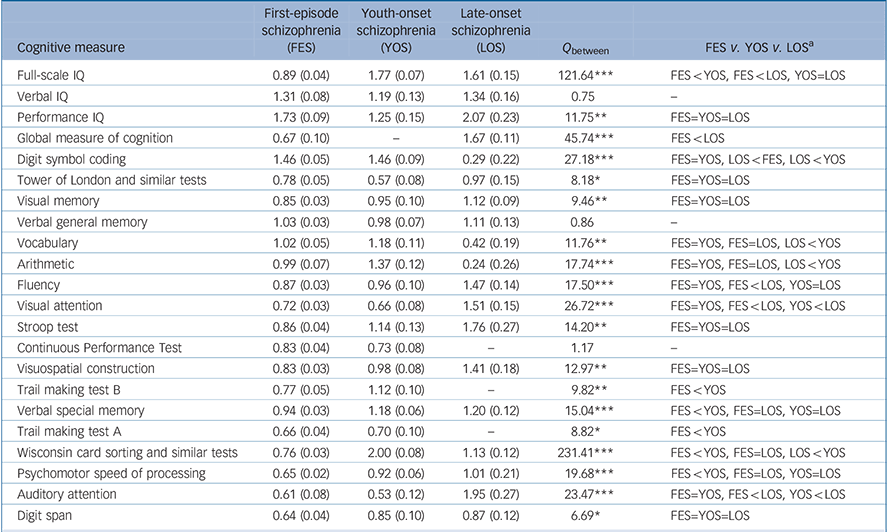
| Cognitive measure | First-episode schizophrenia (FES) | Youth-onset schizophrenia (YOS) | Late-onset schizophrenia (LOS) | Q between | FES v. YOS v. LOS a |
|---|---|---|---|---|---|
| Full-scale IQ | 0.89 (0.04) | 1.77 (0.07) | 1.61 (0.15) | 121.64*** | FES<YOS, FES<LOS, YOS=LOS |
| Verbal IQ | 1.31 (0.08) | 1.19 (0.13) | 1.34 (0.16) | 0.75 | — |
| Performance IQ | 1.73 (0.09) | 1.25 (0.15) | 2.07 (0.23) | 11.75** | FES=YOS=LOS |
| Global measure of cognition | 0.67 (0.10) | — | 1.67 (0.11) | 45.74*** | FES<LOS |
| Digit symbol coding | 1.46 (0.05) | 1.46 (0.09) | 0.29 (0.22) | 27.18*** | FES=YOS, LOS<FES, LOS<YOS |
| Tower of London and similar tests | 0.78 (0.05) | 0.57 (0.08) | 0.97 (0.15) | 8.18* | FES=YOS=LOS |
| Visual memory | 0.85 (0.03) | 0.95 (0.10) | 1.12 (0.09) | 9.46** | FES=YOS=LOS |
| Verbal general memory | 1.03 (0.03) | 0.98 (0.07) | 1.11 (0.13) | 0.86 | — |
| Vocabulary | 1.02 (0.05) | 1.18 (0.11) | 0.42 (0.19) | 11.76** | FES=YOS, FES=LOS, LOS<YOS |
| Arithmetic | 0.99 (0.07) | 1.37 (0.12) | 0.24 (0.26) | 17.74*** | FES=YOS, FES=LOS, LOS<YOS |
| Fluency | 0.87 (0.03) | 0.96 (0.10) | 1.47 (0.14) | 17.50*** | FES=YOS, FES<LOS, YOS=LOS |
| Visual attention | 0.72 (0.03) | 0.66 (0.08) | 1.51 (0.15) | 26.72*** | FES=YOS, FES<LOS, YOS<LOS |
| Stroop test | 0.86 (0.04) | 1.14 (0.13) | 1.76 (0.27) | 14.20** | FES=YOS=LOS |
| Continuous Performance Test | 0.83 (0.04) | 0.73 (0.08) | — | 1.17 | — |
| Visuospatial construction | 0.83 (0.03) | 0.98 (0.08) | 1.41 (0.18) | 12.97** | FES=YOS=LOS |
| Trail making test B | 0.77 (0.05) | 1.12 (0.10) | — | 9.82** | FES<YOS |
| Verbal special memory | 0.94 (0.03) | 1.18 (0.06) | 1.20 (0.12) | 15.04*** | FES<YOS, FES=LOS, YOS=LOS |
| Trail making test A | 0.66 (0.04) | 0.70 (0.10) | — | 8.82* | FES<YOS |
| Wisconsin card sorting and similar tests | 0.76 (0.03) | 2.00 (0.08) | 1.13 (0.12) | 231.41*** | FES<YOS, FES=LOS, LOS<YOS |
| Psychomotor speed of processing | 0.65 (0.02) | 0.92 (0.06) | 1.01 (0.21) | 19.68*** | FES<YOS, FES=LOS, YOS=LOS |
| Auditory attention | 0.61 (0.08) | 0.53 (0.12) | 1.95 (0.27) | 23.47*** | FES=YOS, FES<LOS, YOS<LOS |
| Digit span | 0.64 (0.04) | 0.85 (0.10) | 0.87 (0.12) | 6.69* | FES=YOS=LOS |
a. Q between comparison when there are only two groups; post hoc pair-wise comparison using Bonferroni adjustments when there are three groups and Q between is statistically significant. =, no significant difference between the weighted-mean effect sizes, X<Y, X weighted mean effect size is smaller than Y weighted mean effect size.
* P <0.05, **P <0.01, ***P <0.001.
This differential pattern of cognitive deficits is also demonstrated when comparing the magnitudes of the corresponding weighted d-values in first-episode, youth-onset and late-onset schizophrenia (Fig. 3 and Table 2). When comparing youth-onset schizophrenia with first-episode schizophrenia, the weighted d-values are within 0.3 points for 16 of the 22 cognitive measures. The weighted d-values are larger in the youth-onset schizophrenia than in the first-episode schizophrenia group by 0.3 points or more for four measures: arithmetic, full-scale IQ, Trail Making Test B, and Wisconsin card sorting and similar tests, and smaller for performance IQ. When comparing youth-onset schizophrenia with late-onset schizophrenia, the weighted d-values are within 0.3 points for 7 of the 22 measures and they differ by more than 0.3 for 11 measures. Furthermore, weighted d-values are larger in the late-onset schizophrenia than in the youth-onset schizophrenia group by 0.6 points or more for four measures: auditory attention, performance IQ, Stroop test and visual attention. Conversely, weighted d-values are smaller in the late-onset schizophrenia than in the youth-onset schizophrenia group by 0.6 points or more for four other measures: arithmetic, digit symbol coding, vocabulary and Wisconsin card sorting and similar tests. When comparing first-episode schizophrenia with late-onset schizophrenia, the weighted d-values are within 0.3 points for only 5 of the 22 measures and they differ by more than 0.3 for 13 measures. Furthermore, weighted d-values are larger in the late-onset schizophrenia than in the first-episode schizophrenia group by 0.6 points or more for six of these measures: auditory attention, fluency, full-scale IQ, global measure of cognition, Stroop test and visual attention. Conversely, weighted d-values are smaller in the late-onset schizophrenia than in the first-episode schizophrenia group by 0.6 points or more for three measures: arithmetic, digit symbol coding and vocabulary.
Discussion
We meta-analysed the results of 109 published studies that have compared cognitive functions of 5010 people with schizophrenia with that of healthy controls. To our knowledge, this is the first meta-analysis that addresses the relationship between age at onset and cognition in schizophrenia. When individuals were classified based on their age at onset into those with adult-onset at their first episode of schizophrenia, youth-onset schizophrenia and late-onset schizophrenia, the three groups demonstrated strikingly different patterns of cognitive deficits.

Fig. 2 The mean effect sizes of cognitive deficits of participants with first-episode schizophrenia (a), youth-onset schizophrenia (b) and late-onset schizophrenia (c). y-axis: various cognitive measures. d, mean effect size; n, number of studies contributing to the mean effect size. Vertical line corresponds to effect size of 0.8, the typical threshold for large effect sizes. No studies contributed to global measure of cognition in youth-onset schizophrenia or to Continuous Performance Test, Trail Making Tests A or B in late-onset schizophrenia. FSIQ, full-scale IQ; VIQ, verbal IQ; PIQ, performance IQ; GLOBAL, global measure of cognition; DIG SY, digit symbol coding; TOWER, Tower of London and similar tests; VISM, visual memory; VER GM, verbal general memory; VOCAB, vocabulary; ARTH, arithmetic; FLUEN, fluency; VIS ATT, visual attention; STROOP, Stroop test; CPT, continuous performance test; VS, visuospatial construction; TMB, trail making test B; VER SM, verbal special memory; TMA, trail making test A; WISC, Wisconsin card sorting and similar tests; PSY MOT, psychomotor speed of processing; AUD ATT, auditory attention; DIG SP, digit span.
This meta-analysis has a number of limitations. First, although the classification of different age at onset is based on current expert opinion, it is to a large extent arbitrary. For example, some consider childhood-onset schizophrenia to be merely a more severe form of, and on a clinical and neurobiological continuum with, adult-onset schizophrenia. Reference Nicolson and Rapoport29,Reference Rapoport, Addington, Frangou and Psych30 The results of our analysis are consistent with this view. In contrast, the inclusion of very-late-onset schizophrenia-like psychosis under the broader category of late-onset schizophrenia is controversial because very-late-onset schizophrenia-like psychosis appears to have less genetic underpinnings, to be associated with more sensory deficits, and to have different phenomenology. Reference Howard, Rabins, Seeman and Jeste3
Second, as stated above, we based our classification of age at onset on the definition used in each individual study even though these definitions differed. We believe this approach is sound because these different definitions have been shown to yield age at onsets that are highly correlated and within a few months of each other. Reference Delisi5,Reference DeLisi, Hoff, Schwartz, Shields, Halthore and Gupta23,Reference Delisi, Goldin, Maxwell, Kazuba and Gershon25 Furthermore, it would not be possible to apply retrospectively a single definition of age at onset to a large number of samples characterised with different variables.
Third, the number of studies of late-onset schizophrenia compared with those of first-episode schizophrenia or youth-onset schizophrenia is small. This limitation impedes the consideration in the analysis of factors such as duration of illness, education level, premorbid intellectual abilities or comorbid diseases.
Fourth, some cognitive tests that may not be equivalent were aggregated into a single cognitive measure. However, such aggregation was based on theoretical or factor-analytical grounds. It also enabled the capture of a large quantity of data generated by related cognitive tests and the reduction of the number of comparisons.
Finally, we used control groups of the different studies to generate the effect sizes. The cognitive profiles of these various control groups were naturally different. However, the normal controls were typically matched to the participants with schizophrenia, supporting the validity of comparing effect sizes.
Notwithstanding these limitations, our results extend the large literature on cognitive deficits in schizophrenia, in particular five recent meta-analyses Reference Heinrichs and Zakzanis11,Reference Aleman, Hijman, de Haan and Kahn31–Reference Laws34 and they need to be considered in this context. The diversity of individuals with schizophrenia is likely to be a reflection of multiple pathological processes. Understanding this diversity is critical to the development of personalised treatment interventions. Age at onset and cognition of individuals with schizophrenia are two aspects of the illness where diversity manifests itself. Age at onset can range from childhood to very late in life. Cognitive function can vary from normality Reference Palmer, Heaton, Paulsen, Kuck, Braff and Harris35 to severe impairment.
Our analysis reports large cognitive deficits in most cognitive measures in first-episode schizophrenia. The magnitudes of these deficits are consistent with the magnitudes reported in other meta-analyses. However, the deficits we report in verbal memory, and to a lesser extent executive function and attention, are smaller than those reported in other meta-analyses. Reference Heinrichs and Zakzanis11,Reference Aleman, Hijman, de Haan and Kahn31,Reference Laws34,Reference Lee and Park36 In these other analyses, participants were not restricted to those presenting during their first episode. Thus, the larger deficits reported in these previous analyses could reflect the harmful impact of chronicity of psychosis on cognition, especially on verbal memory. Psychosis in another neuropsychiatric disorder (Alzheimer's disease) has also been associated with acceleration of cognitive decline. Reference Drevets and Rubin37,Reference Lopez, Wisniewski, Becker, Boller and DeKosky38 The potential impact of chronicity on cognition emphasises the importance of age at onset and its corollary, duration of illness.
Individuals with youth-onset schizophrenia have cognitive deficits that are larger than those with first-episode schizophrenia in arithmetic, executive function, full-scale IQ, psychomotor speed of processing and verbal memory. In contrast, they have deficits that are comparable in magnitudes with those in first-episode schizophrenia in the other measures. These findings are in agreement with Tuulio-Henriksson et al Reference Tuulio-Henriksson, Partonen, Suviusaari, Haukka and Lönnqvist19 who reported an association between younger age at onset (range 13–44 years) and larger deficits in verbal learning and memory, and semantic clustering, an executive function, but an absence of such an association in attention (verbal and visual), visuospatial function and vocabulary. Basso et al Reference Basso, Nasrallah, Olson and Bornstein39 also reported more severe deficits in executive function and memory in individuals with adolescent-onset schizophrenia (age at onset <21) compared with those with adult-onset schizophrenia (age at onset >24). However, our findings are in conflict with the absence of an association between age at onset and deficit in psychomotor speed of processing reported by Tuulio-Henriksson et al. Reference Tuulio-Henriksson, Partonen, Suviusaari, Haukka and Lönnqvist19 This discrepancy could be because they included participants with chronic schizophrenia and excluded those with age at onset less than 13 years. White et al Reference White, Ho, Ward, O'Leary and Andreasen16 did report a more severe impairment in psychomotor speed in participants with adolescent-onset schizophrenia (range 12–19 years) when compared with adults with first-episode schizophrenia. Further, Hoff et al's Reference Hoff, Harris, Faustman, Beal, DeVilliers and Mone6 study that included participants with chronic schizophrenia but with age at onset as young as 7 (range 7–29 years) also found an association between younger age at onset and larger deficits on Trail Making Test B and psychomotor speed of processing, in agreement with our findings. However, in contrast with our findings, Hoff et al Reference Hoff, Harris, Faustman, Beal, DeVilliers and Mone6 did not find such an association in verbal memory or Wisconsin card sorting test. This is probably because of their small sample size, hence the advantage of a meta-analysis. Overall, our comparison of individuals with youth-onset schizophrenia or first-episode schizophrenia is consistent with the conceptualisation of age at onset as a surrogate measure of severity of disease process: an earlier onset reflects a more severe illness. In our analysis, this is the case even though individuals with youth-onset schizophrenia had a brief duration of illness (2.3 years at time of testing on average). These larger deficits suggest a high disease burden consistent with the high rate of chromosomal abnormalities, higher familial rates of schizophrenia-spectrum disorders, Reference Rapoport, Addington, Frangou and Psych30,Reference Nicolson, Brookner, Lenane, Gochman, Ingraham and Egan40 and early and progressive cortical grey matter loss observed in the frontal lobes of individuals with childhood-onset schizophrenia (e.g. Vidal et al Reference Vidal, Rapoport, Hayashi, Geaga, Sui and McLemore41 ).
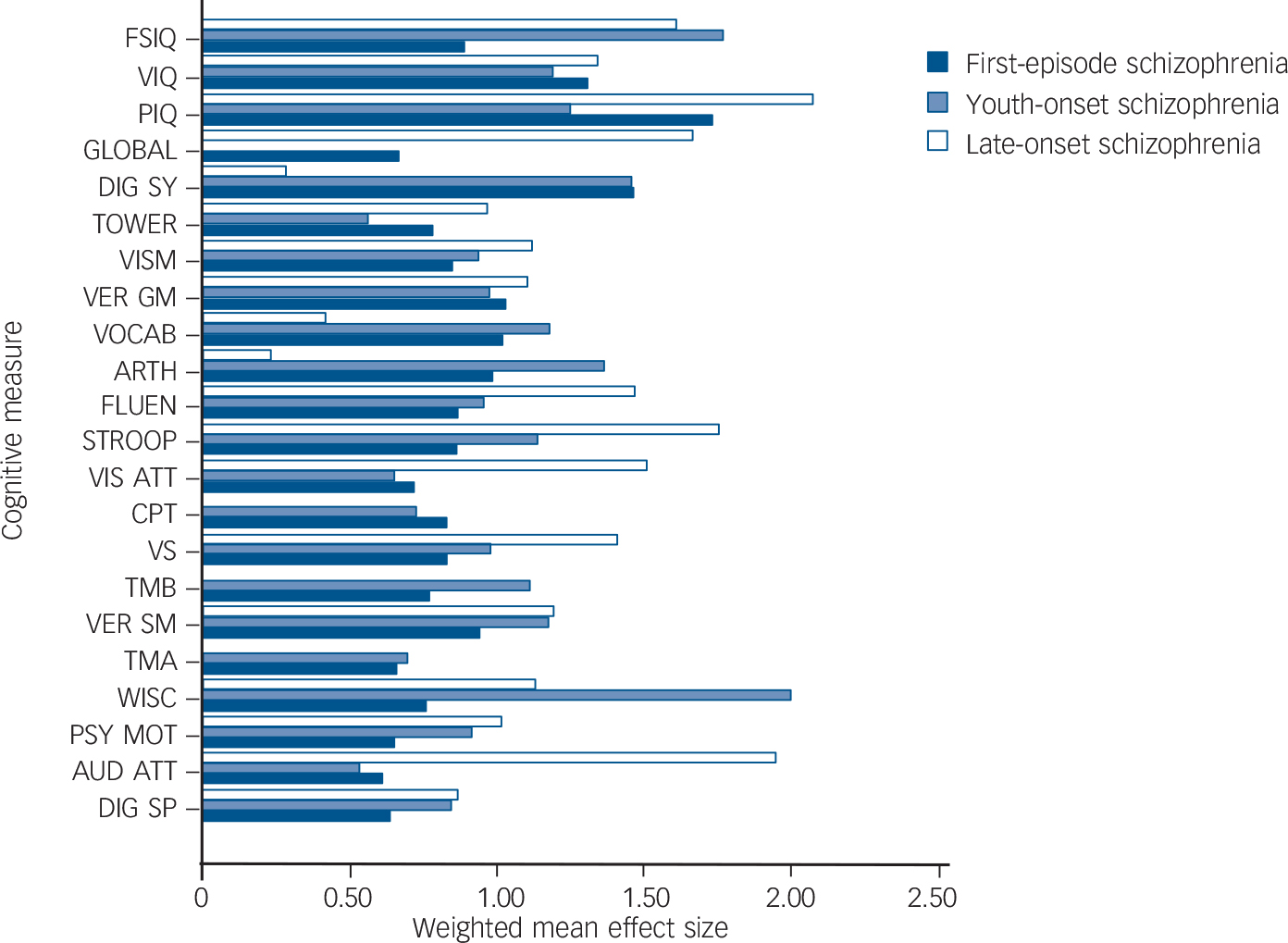
Fig. 3 Qualitative comparison of weighted mean effect sizes (weighted d-values) of cognitive deficits of participants with first-episode, youth-onset schizophrenia and late-onset schizophrenia compared with healthy controls. FSIQ, full-scale IQ; VIQ, verbal IQ; PIQ, performance IQ; GLOBAL, global measure of cognition; DIG SY, digit symbol coding; TOWER, Tower of London and similar tests; VISM, visual memory; VER GM, verbal general memory; VOCAB, vocabulary; ARTH, arithmetic; FLUEN, fluency; VIS ATT, visual attention; STROOP, Stroop test; CPT, continuous performance test; VS, visuospatial construction; TMB, trail making test B; VER SM, verbal special memory; TMA, trail making test A; WISC, Wisconsin card sorting and similar tests; PSY MOT, psychomotor speed of processing; AUD ATT, auditory attention; DIG SP, digit span.
Also consistent with this conceptualisation, individuals with late-onset schizophrenia seem to have some relatively preserved cognitive functions such as arithmetic, digit symbol coding and vocabulary. This is consistent with a less severe disease process, even though the duration of their illness is longer than the duration of those with youth-onset schizophrenia (7.7 v. 2.3 years on average at the time of testing). Assessing semantic organisation, Paulsen et al Reference Paulsen, Romero, Chan, Davis, Heaton and Jeste18 reported that individuals with late-onset schizophrenia (age at onset >45 years) were preserved compared with people with an early onset. In contrast, our analysis reveals that participants with late-onset schizophrenia are severely impaired on measures of auditory and visual attention, fluency, global measure of cognition, IQ and visuospatial construction, and more so than individuals with youth-onset schizophrenia or first-episode schizophrenia on most of these measures. These findings are in conflict with those by the University of California, San Diego group Reference Heaton, Paulsen, McAdams, Kuck, Zisook and Braff14,Reference Jeste, Harris, Krull, Kuck, McAdams and Heaton20 and Sachdev et al Reference Sachdev, Brodaty, Rose and Cathcart21 who showed no difference between participants with late-onset schizophrenia (age at onset >45); Reference Heaton, Paulsen, McAdams, Kuck, Zisook and Braff14,Reference Jeste, Harris, Krull, Kuck, McAdams and Heaton20 age at onset >49 Reference Sachdev, Brodaty, Rose and Cathcart21 and early-onset (age at onset ≤45; Reference Heaton, Paulsen, McAdams, Kuck, Zisook and Braff14,Reference Jeste, Harris, Krull, Kuck, McAdams and Heaton20 age at onset <35) Reference Sachdev, Brodaty, Rose and Cathcart21 schizophrenia on any cognitive measure. A number of factors could have resulted in these discrepancies. First and foremost, the absence of any detected differences may be a result of the small sample sizes, a limitation of any single study of late-onset schizophrenia. This limitation highlights the contribution of a meta-analysis. Second, in the University of California, San Diego studies, participants with early-onset schizophrenia included some people with age at onset as late as 45. Such a high range may have prevented the detection of differences between early-onset schizophrenia and late-onset schizophrenia that we observed in our analysis. In fact, when the University of California, San Diego group assessed the relationship between cognition and age at onset (range 6–64 years) as a continuous variable in the same participants, Reference Jeste, McAdams, Palmer, Braff, Jernigan and Paulsen17 younger age at onset was associated with a more severe deficit in executive function in agreement with our findings. Third, in the University of California, San Diego and Sachdev et al Reference Sachdev, Brodaty, Rose and Cathcart21 studies, individuals with early-onset schizophrenia had significantly longer duration of illness than those with late-onset schizophrenia (e.g. about 30 years for early-onset v. 6 years for late-onset schizophrenia in the study by Lee & Park). Reference Lee and Park36 Thus, the effect of chronicity on individuals with an early onset could have contributed to the absence of differences between the two groups. In the same vein, in our analysis, the relatively longer duration of illness in individuals with late-onset schizophrenia as compared with those with youth-onset or first-episode schizophrenia may be contributing to the observed larger deficits on some measures.
The large impairment in attention, fluency and visuospatial construction function in participants with late-onset schizophrenia compared with age-matched healthy controls suggests specific deficits in these cognitive functions in the context of minimal impairment in arithmetic, digit symbol coding and vocabulary. These large deficits could be underlying the similarly observed large impairment in global cognition and IQ. The small deficit on digit symbol coding is of particular interest. Performance on this task is thought to depend on non-specific and ubiquitous neurological processes Reference Dickinson, Ramsey and Gold42 and correlates with broad prefrontal and temporal grey matter volumes. Reference Sanfilipo, Lafargue, Rusinek, Arena, Loneragan and Lautin43 Thus, a lesser impairment on this task suggests specific rather than generalised cognitive deficits in late-onset schizophrenia. However, the number of studies contributing to these effect sizes is small (e.g. only one study contributed to arithmetic, auditory attention and digit symbol coding in late-onset schizophrenia). Further, performance on a specific task is likely to involve multiple cognitive mechanisms. Reference Keefe44 Thus, a more impaired performance in visuospatial construction than in digit symbol coding, for example, does not necessarily imply that the disease process is specific to a single cognitive mechanism underlying visuospatial construction. It could merely suggest that some of several cognitive mechanisms underlying digit symbol coding are less affected by the disease process than some of those underlying visuospatial construction. Such a less pervasive disease process results in the relative preservation of a particular cognitive function compared with another.
Another possible model is that the disease-related cognitive deficits observed in late-onset schizophrenia could be as a result of an interaction between the disease process and ageing: these deficits could have been present but less prominent prior to the onset of the clinical syndrome. Then, the ageing process and associated allostatic factors such as cerebrovascular disease, diabetes and hypertension, could amplify these deficits resulting in larger deficits compared with younger people with first-episode schizophrenia, even after correcting for the pure effect of ageing observed in older controls.
Longitudinal and controlled studies will be necessary to address these questions of specific deficits v. preserved cognitive functions, and to advance our understanding of the relationship between the disease process underlying schizophrenia, cognition, age at onset, duration of illness, ageing and associated allostatic factors. Such studies would need to target individuals at their first episode or in their prodromal phase, or high-risk people across the life cycle. A more feasible study that would focus on older individuals and controls and follow them longitudinally could disentangle the cognitive deficits or changes that are a result of the illness from those resulting from normal ageing or certain allostatic factors. Characterising such pathological relationships will help towards the development of personalised interventions to enhance cognition or prevent its decline.
Funding
T.K.R. is supported by the Centre for Addiction and Mental Health (CAMH) and the Canadian Institutes of Health Research (CIHR180087).
Appendix
Literature search: databases and search terms
A literature search of 29 databases was performed using CINAHL, EMBASE, and the multi-database search engine, Scholars Portal that includes MEDLINE, PsycARTICLES, PsycINFO, Psychology: A SAGE Full-Text Collection, and the following: AGRICOLA, Aqualine, ASFA: Aquatic Sciences and Fisheries Abstracts, Biological and Agricultural Index, Biological Sciences, Biology Digest, Biotechnology and Bioengineering Abstracts, Compendex, Conference Papers Index, Digital Dissertations, E-Journals, EIS: Digests of Environmental Impact Statements, Environmental Sciences and Pollution Mgmt, General Science Abstracts, Geobase, GeoRef, GeoRef In Process, Meteorological & Geoastrophysical Abstracts Oceanic Abstracts, Pharmaceutical News Index, Physical Education Index, Plant Science, Science Citation Index Expanded, Scopus Natural Sciences, TOXLINE and Zoological Record Plus.
The following search terms were used: schizophrenia, schizoaffective, psychotic disorder, age of onset, age at onset, childhood onset, adolescent onset, late onset, first episode, cognition, neuropsychology, memory, learning, Wechsler, complex figure, face recognition, spatial perception, line orientation, Purdue, motor dexterity, finger tapping, motor disorder, Stroop test, digit span, continuous performance test, backward masking, trails, trail making, Wisconsin card sorting test, IQ, vocabulary, block design, word fluency, token, comprehension, affect recognition, dichotic listening.
Acknowledgements
We thank Dr Dilip V. Jeste for his encouragement to conduct this review.








eLetters
No eLetters have been published for this article.