Cardiovascular disease (CVD) and major depression are common diseases and constitute a major proportion of the total burden of disease.1 There is a long history linking CVD and depression. As early as 1905 a German psychiatrist, Robert Gaupp, described depression secondary to arteriosclerosis in elderly patientsReference Post2 and in 1937 Maltzberg et al showed that patients with depression had higher mortality than the general population and that this was mainly attributed to CVD.Reference Maltzberg3 Since then, a large number of studies, reviews and meta-analyses have reported associations between CVD (defined as ischaemic heart disease (IHD), stroke or combined) and higher risk of subsequent depression and/or the reverseReference Ayerbe, Ayis, Wolfe and Rudd4–Reference Huffman, Celano, Beach, Motiwala and Januzzi11 and these findings have led researchers to argue that the association between CVD and depression is bidirectional.Reference Alexopoulos12,Reference de Jonge and Roest13 The mechanisms underlying these associations are, however, less well-described and several mechanisms have been put forward to explain the link.Reference Stapelberg, Neumann, Shum, McConnell and Hamilton-Craig10,Reference Huffman, Celano, Beach, Motiwala and Januzzi11 The associations may be causal, with one disease mechanism (e.g. metabolic factors, inflammation and autonomic dysregulation) leading to the other. Other potential mechanisms include shared behavioural risk factors or genetic selection. In addition, shared symptomatology and study bias may affect the risk estimates. It can arise from detection or information bias (such as that cohort members diagnosed in the healthcare system may be more likely to get additional diagnoses) or misclassification of exposure or outcome (especially when including only physicians’ diagnoses from hospitals). Finally, selection bias will occur if some individuals are more likely to participate than others. This may undermine the external validity of the results.
In our study, we included data from ten population-based cohorts to examine the following research questions: (a) if a bidirectional association between IHD/stroke and depression could be explained by shared common risk factors such as age, gender, calendar time, education, marital status, alcohol use, smoking, physical activity, body mass index (BMI), systolic blood pressure, total cholesterol, statin use, and stroke/IHD, and (b) if the associations were present in both men and women; and (c) to what extent study bias such as detection bias (for example because of systematic difference in depression diagnostic between those admitted to hospital and those not) or non-response explained any association.
Method
Study populations
We reviewed the Danish data archive and cohort profiles for Danish cohorts with a sufficient sample size, identification of individuals for register linkage and relevant covariates measured at health examinations. It was possible to obtain data for all the relevant studies, except for one, and include them in the study. This selection procedure meant that we did not have any information on whether the studies would agree with our hypotheses before inclusion. We ended up with ten Danish population-based cohorts studied between 1981 and 2015. For the first analyses we pooled the seven cohorts from the Center for Clinical Research and Prevention, the Diet, Cancer and Health Study and the Copenhagen City Heart Study 2nd examination to study if a bidirectional association between IHD, stroke and depression could be explained by common risk factors and if they were present in both men and women. This pooled cohort included 93 076 individuals and information on each study is listed in supplementary Table 1 (available at https://doi.org/10.1192/bjp.2019.130). The studies have been described in detail elsewhere.Reference Nordahl, Hvidtfeldt and Diderichsen14,Reference Osler, Linneberg, Glumer and Jorgensen15 For the analyses of study bias we used data from the Metropolit cohort that includes men born in 1953 in the Copenhagen area.Reference Osler, Lund, Kriegbaum, Christensen and Andersen16 Of these 10 510 were alive in September 2004 (at age 51) and 6292 men (59.8%) responded to a mailed health questionnaire that included self-reported measures of chest pain and depression. All study participants in the population-based studies gave written informed consent and the study protocol was approved by the Danish Data Protection Agency (CSU-FCFS-2017-002 with I-Suite no. 05295).
The pooled nine cohorts were used to examine the first two research questions whereas the Metropolit study was used to examine the third. We used these cohorts instead of register-based cohorts because they included a range of measures that could not be obtained from the registers such as self-reported lifestyle, objectively measured height and weight, blood pressure and blood tests. The Metropolit study was used to explore the associations using both self-reported and register-based disease that were not available in the other cohorts.
Exposures
In the pooled cohorts, exposure was an incident hospital diagnosis (including both in-patients and out-patients) of either IHD, stroke or depression in the Danish National Patient Registry or the Danish Psychiatric Central Registry that holds data from 1977 and 1969, respectively.
IHD was defined according to the International Classification of Diseases 10 (ICD-10)17 codes I20–25 or ICD-8 codes 410–414. Stroke was defined as ICD-10 codes I60–69 or G45 or ICD-818 codes 430–438 and included both cerebral infarction, haemorrhage and transient ischaemic attack. Depression was defined as ICD-10 codes F32–33 or ICD-8 codes 296.09, 296.29, 298.09 or 300.49.
In the Metropolit cohort self-reported measures of IHD and depression were based on positive answers to the questions: ‘Have you ever had chest pain?’, ‘Has a doctor ever told you that you suffer from depression?’. Participants with a positive response on depression were also asked ‘How old were you when the disease occurred for the first time?’ and ‘Do you or have you ever taken medicine for depression?’ with four categories: present use, use within the past 3 years, more than 3 years ago or never. Furthermore, participants were evaluated on the Major Depression Inventory (MDI),Reference Olsen, Jensen, Noerholm, Martiny and Bech19 which is a frequently used and well-validated self-report of 12 items assessing the frequency of depressive symptoms during the past 2 weeks. Each item was rated on a six-point scale ranging from zero (not at all) to five (all the time). Ratings were summed to calculate a total score that can range from 0 to 50. Based on a previous study of diagnostic validity, a cut-off of 26 points or more was defined as major depression.Reference Bech, Rasmussen, Olsen, Noerholm and Abildgaard20
Outcomes
CVD and depression outcomes were followed from study entry (baseline) or change in disease exposure status and until end of follow-up (11 July 2017 for the pooled cohorts and January 2018 for the Metropolit cohort) in the Danish National Patient Registry and the Psychiatric Central Register using the same ICD-8 and ICD-10 codes as outlined above. All analyses were done separately for IHD and stroke as they were assumed to be linked to depression through different aetiological mechanisms.
Explanatory covariables
Information on education (basic education (7–9 grade of obligatory schooling), medium education (high school degree/vocational), higher education (more than high school degree) or missing) was obtained from the Integrated Database for Labor Market Research. Marital status was based on data from the Danish Civil Registration System and categorised as married, unmarried, divorced or widow/widower. We had self-reported information on alcohol use (number of alcoholic drinks per day), smoking status (never, former or current smoker) and physical activity (sedentary, light, moderate and hard exercise). Furthermore, study participants had a physical examination including height and weight (from which BMI was calculated), systolic blood pressure (in mmHg, included in quintiles) and total plasma cholesterol level (included in quintiles). Plasma cholesterol was determined with colorimetric assays (Boehringer Mannheim) in the Copenhagen City Heart Study 2, 1936 cohort, Health 2006 and DanFund. In the MONICA cohorts and Inter99 enzymatic techniques were used. In the Diet, Cancer and Health study, cholesterol was determined using a Lipotrend®C reflectance photometer (Boehringer Mannheim). In a subset of individuals (the MONICA cohorts and Inter99), we had measurements on C-reactive protein (CRP) measured by high-sensitivity latex-enhanced immunoturbidimetric assays (Abbott Architect) in the MONICA cohorts and using an ultra-sensitive single molecule counting technology in Inter99 (Singulex). Information on statin use at the time of study participation was obtained from the Danish Prescription Register using the Anatomical Therapeutic Classification (ATC) code C10AA. In the Metropolit cohort we used self-reported information on education, alcohol use, smoking status and physical activity.
Statistical analysis
Stata (version 15, StataCorp, College Station, TX, USA) was used for all statistical analyses. Missing values in the variables from the pooled cohorts were imputed based on age, gender and marital status if the variables were assumed to be missing completely at random (supplementary Table 2). For education, missing information was included as a fixed number.
We studied the first two study questions in the pooled cohorts using Cox proportional hazards regressions to calculate hazard ratios (HR) with 95% CIs. For the analyses of IHD and depression, individuals with IHD (n = 3098) or depression (n = 1467) before study entry were excluded, and similarly, for the analyses of stroke and depression, individuals with stroke (n = 1574) or depression (n = 1467) before study entry were excluded. The exposure was defined as a time-dependent variable, indicating that upon diagnosis of the exposure disease, the individual's status changed from unexposed to exposed. Individuals were followed from study entry (the date of study participation) until first outcome, death or emigration or end of follow-up, whichever came first. We examined both directions in the same cohorts: first, we studied if individuals who developed CVD during follow-up had a higher risk of subsequent depression and next we repeated analyses to see if individuals who developed depression during follow-up had a higher subsequent risk of CVD. Age was the underlying timescale in analyses examining CVD as outcome and follow-up time the underlying timescale in analysis using depression as outcome as the proportional hazards assumption were not met when using age in these analyses. The proportional hazards assumption was tested graphically by plotting –log (−log(survival)) versus log (follow-up time) and no important violations were found.
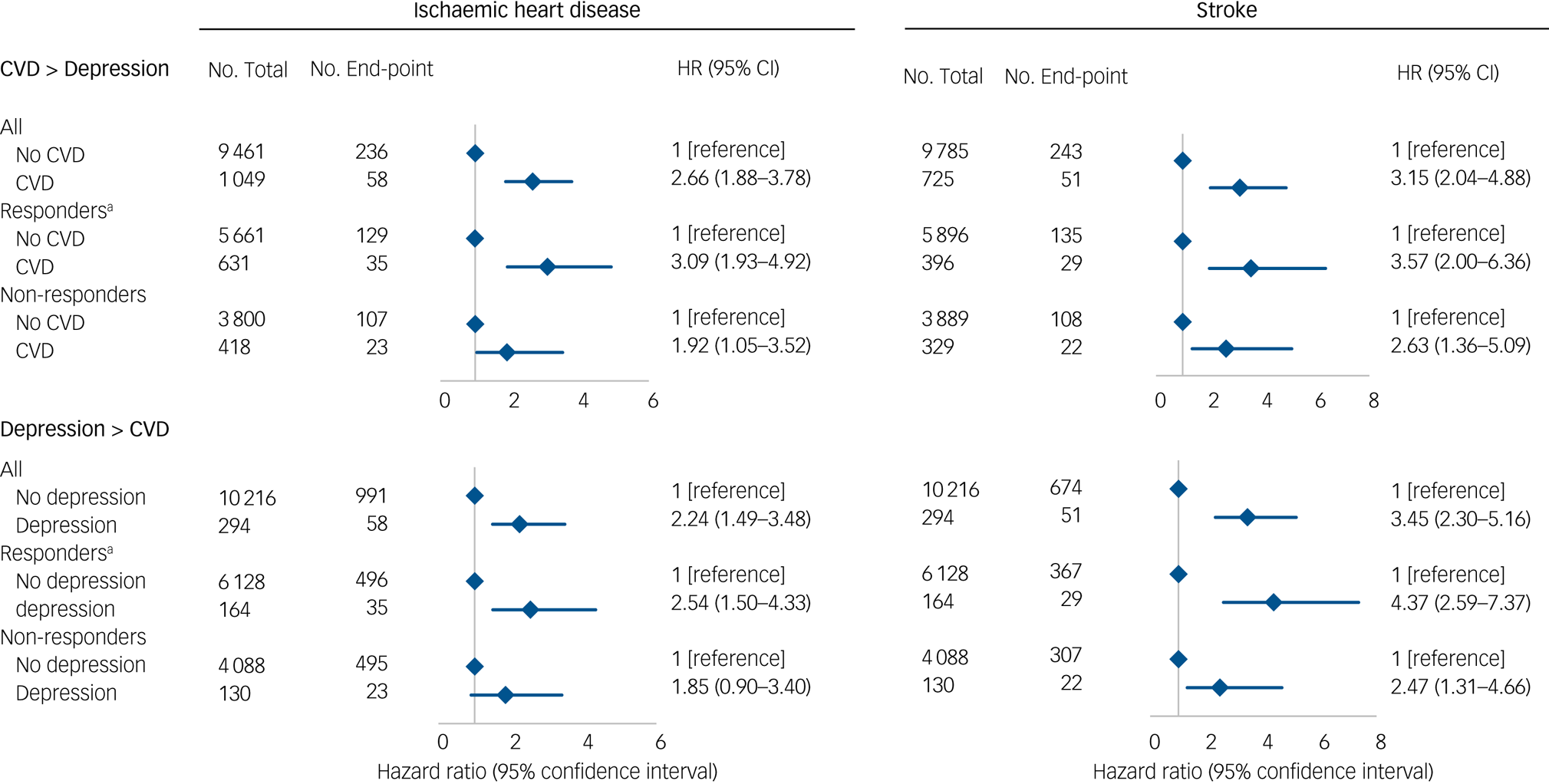
The influence of potentially explanatory covariables was analysed using six different levels of adjustment as shown in Fig. 1. To maximise power, we combined data from all cohorts in one analysis with adjustment for cohort as a stratum variable. We also explored if the associations were present in both men and women in gender-specific analyses (supplementary Fig. 1). We next examined the role of study bias in the Metropolit cohort first by using both register-based hospital diagnoses and self-reported measures of CVD and depression (Fig. 2). The register-based exposures were defined as having a register-based diagnosis at baseline in order to compare results with the self-reported exposures at baseline. However, we also included exposures as time-dependent variables similar to in the combined cohort (supplementary Table 3). Finally, we examined the association in both responders and non-responders followed through the registers (Fig. 3) with exposure as a time-dependent variable.

Fig. 1 Bidirectional associations between cardiovascular disease (CVD) and depression in the combined cohorts.

Fig. 2 Bidirectional associations between depression and cardiovascular disease (CVD) in the Metropolit cohort. (a) Associations between hospital-diagnosed and self-reported measures of cardiovascular disease and subsequent risk of depression. (b) Associations between hospital diagnosed and self-reported measures of depression and subsequent risk of ischaemic heart disease and stroke.

Fig. 3 Bidirectional associations between cardiovascular disease (CVD) and depression in the Metropolit cohort.
In sensitivity analyses of the pooled cohorts, we examined the associations from the three main cohorts separately and combined the estimates in meta-analyses assuming a fixed-effects model in case of low heterogeneity (I 2 < 25%) or a random-effects model in case of moderate to high heterogeneity (I 2≥25%). In the subset of individuals (MONICA cohorts and Inter99) where high-sensitivity CRP, triglyceride and high-density lipoprotein (HDL) cholesterol were available we included an analysis further adjusted for these variables.
Results
A total of 93 076 individuals were included in the pooled cohorts. The mean age was 54 years (range: 18–98 years) and 53% were women. In the Metropolit study of men born in 1953, 6292 responded to the questionnaire in September 2004 at mean age 51.2 (range 50.6–51.6 years). The distribution of baseline characteristics in each cohort are shown in Table 1.
Table 1 Baseline characteristics of the cohortsa

IQR, Interquartile range; NA, not available.
a. The cohorts at Center for Clinical Research and Prevention, the Diet, Cancer and Health Study and the Copenhagen City Heart Study was used to study our first and second research questions and the Metropolit cohort was used to study the third research question. In the main analyses, the cohorts at Center for Clinical Research and Prevention, the Diet, Cancer and Health Study and the Copenhagen City Heart Study was combined. Only data on questionnaire responders in the Metropolit study is included. In the Metropolit study, 5 individuals had missing data on smoke status while 66 had missing data on physical activity.
The bidirectional association between CVD and depression in the pooled cohorts
Participants were followed for a median of 20.6 years (range: 0–36 years, interquartile range (IQR) 15–22 years). After exclusion of individuals who developed IHD or depression before study entry, 15 844 were diagnosed with IHD during follow-up and 2237 developed depression. After exclusion of individuals who developed stroke or depression before study entry, 11 787 developed stroke during follow-up and 2276 developed depression.
IHD was associated with higher risk of developing depression with a HR of 1.70 (95% CI 1.37–2.12, P<0.001) after adjustment for age, cohort and calendar year (Fig. 1). Median time between being diagnosed with IHD and subsequent depression was 4.7 years (IQR 1.5–9.1 years). After further adjustments the association was still present after stepwise inclusion of variables into the model. After adjustment for all explanatory covariables, the HR was 1.79 (95% CI 1.43–2.23, P<0.001). The analysis of the reverse association showed that a diagnosis of depression was associated with higher risk of subsequent IHD (HR 1.64, 95% CI 1.37–1.96, P<0.001) in the first model). After adjustment for all covariables the HR was 1.63 (95% CI 1.36–1.95, P<0.001). Median time between being diagnosed with depression and subsequent IHD was 5.9 years (IQR 2.8–10.2 years).
For stroke, similar patterns of associations were seen. Being diagnosed with stroke was associated with higher risk of subsequent depression with a HR of 2.62 (95% CI 2.09–3.29, P<0.001) after adjustment for all explanatory covariables, whereas the HR for the reverse association was 1.94 (95% CI 1.63–2.30, P<0.001). Median time between being diagnosed with stroke and subsequent depression was 3.2 years (IQR 1.1–6.7 years) whereas for the opposite direction it was 4.4 years (IQR 1.9–8.1 years). Thus, we did not find that the common shared risk factors explained much of the association. When the analyses were stratified by gender the associations were present in both men and women (supplementary Fig. 1).
Analyses of detection bias
Misclassification related to using only hospital diagnoses of CVD and depression were explored in the Metropolit cohort by examining self-reported disease measures instead. In the middle-aged men from the Metropolit cohort, self-reported chest pain was associated with higher risk of depression with a HR of 2.10 (95% CI 1.24–3.54, P < 0.001) (Fig. 2(a)). For the opposite direction, self-reported depression as well as MDI score at baseline was similarly associated with higher risk of both IHD (HR 1.48 (95% CI 1.17–1.87, P<0.001) and 1.58 (95% CI 1.03–2.42, P = 0.04)) and stroke (HR 1.47 (95% CI 1.09–1.98, P = 0.01) and 1.71 (95% CI 1.04–2.82, P = 0.03)) (Fig. 2(b)).
In both populations, the risk estimates from the analyses based on hospital diagnoses pointed in the same direction but were imprecisely estimated as hospital diagnoses of CVD and depression were less frequent than the self-reported measures. The analyses also showed that only 12% (n = 88) of those with self-reported depression had been registered with this diagnosis in the hospital registers. Among the 88% (n = 644) men with self-reported depression but no hospital diagnosis, the risk of developing CVD was the same as for the 12% also diagnosed at hospital (data not shown).
Analyses of non-response
A total of 6292 men in the Metropolit cohort responded to the questionnaire in 2004 and 4218 did not respond. When we examined whether non-response modified the association between register-based IHD or stroke diagnosis and subsequent depression by comparing the risk estimates for responders and non-responders we found similar results in both groups with similar risk estimates for the reverse associations (Fig. 3).
Sensitivity analysis
When we examined the estimates from the meta-analyses of the individual cohorts, risk estimates were similar (supplementary Fig. 2 and 3). Finally, in the subset of individuals with CRP, HDL and triglycerides, further adjustment for these covariables only changed the association minimally (supplementary Fig. 4).
Discussion
In this study based on ten population-based cohorts we found that IHD and stroke were associated with depression in a bidirectional manner in both men and women. The associations were still present after adjustment for several shared risk factors including age, gender, calendar time, education, marital status, alcohol use, smoking, physical activity, BMI, systolic blood pressure, total cholesterol, statin use and IHD/stroke. The associations were replicated even in further analyses using both self-reported and register-based disease measures to account for detection bias and including non-responders.
Comparison with findings from other studies
Previous studies have suggested that 20–28% of individuals with IHD will get a subsequent depression within 2 years,Reference Meijer, Conradi, Bos, Thombs, van Melle and de Jonge21 and around 30% of individuals with stroke will get a depression within 5–10 years years.Reference Ayerbe, Ayis, Wolfe and Rudd4,Reference Hackett and Pickles22 In our study, the incidence was lower, but this could be the result of a different definition of depression but also perhaps other risk factors. However, whereas most previous studies only examined the prevalence of depression in patients after CVD without comparison with the background population, our result are similar to those studies that made reference to the fact that the background population patients with IHD and stroke have a higher risk of depression.Reference Osler, Martensson, Wium-Andersen, Prescott, Andersen and Jorgensen23,Reference Jorgensen, Wium-Andersen, Wium-Andersen, Jorgensen, Prescott and Maartensson24 Previous studies have suggested that risk factors for development of depression after CVD are younger age, female gender, diabetesReference Huffman, Celano, Beach, Motiwala and Januzzi11 and for stroke also cognitive impairment and stroke severity.Reference Ayerbe, Ayis, Wolfe and Rudd4 A recent review suggested that women in particular had a higher risk of depressive symptoms following a hospital admission with IHD.Reference Buckland, Pozehl and Yates25 In our study we found that the associations between CVD and depression were present in both men and women.
For the opposite direction, several meta-analyses of cohort studies have reported that depression increases risk of both IHD and stroke.Reference Dong, Zhang, Tong and Qin5–Reference Wu and Kling9 A few address various explanatory factors and discuss potential mechanism. As ours, these studies show an association between depression and IHD,Reference Gan, Gong, Tong, Sun, Cong and Dong6,Reference Nicholson, Kuper and Hemingway7,Reference Wu and Kling9,Reference Rugulies26 and the two latest examined only prospective studies and included several sensitivity analyses.Reference Gan, Gong, Tong, Sun, Cong and Dong6,Reference Wu and Kling9 They both reported a risk of IHD between 1.249 and 1.306 and these estimates were relatively consistent across studies adjusting for various covariables including socioeconomic status, smoking, hypertension, diabetes and BMI. Fewer studies have adjusted for physical activity, alcohol use, cholesterol levels or inflammation but Gan et al reported that studies that did control for physical activity and cholesterol levels still found a significant association.Reference Gan, Gong, Tong, Sun, Cong and Dong6 Inflammation, however, could also be on the causal pathway between depression and IHD and adjustment might therefore be questioned. Finally, in both meta-analyses the risk estimates for IHD were lower among women than in men, which is similar to what we found in our subanalyses (supplementary Fig. 1).
For stroke, our results are comparable with meta-analyses that have reported increased risk of stroke among individuals with depression. In the largest meta-analysis to date including 28 prospective cohort studies and 8478 stroke cases, Pan et al found that depression was associated with stroke with a HR of 1.45 and the increased risk was evident in studies that adjusted for smoking and BMI and across several subgroups.Reference Pan, Sun, Okereke, Rexrode and Hu8 For both IHD and stroke, no significant difference between the association in men and women have been reported.Reference Dong, Zhang, Tong and Qin5,Reference Wu and Kling9
Interpretation of our findings
Except for reverse causation of impaired mood being a part of symptomatic but undiagnosed depression,Reference Nicholson, Kuper and Hemingway7 no studies have previously explored the effect of other potential sources of bias. In the present study, misclassification or selective participation did not seem to explain the associations that were consistent when we compared responders and non-responders and when we used self-reported exposure measures. However, the results indicated that hospital diagnoses only include a small number of all the individuals with depression in the population who also seem to have higher risk of CVD and this could lead to underestimation of the risk in studies based solely on hospital diagnoses.
Thus, the present study suggests that shared risk factors or bias did not account for the associations. One possibility could be that CVD and depression are associated because the risk factors for both diseases are associated. Another possibility could be that the association is explained by other factors that we did not account for, such as genetic factors or treatment of depression or IHD or stroke. Although, it is known that genetic factors are aetiologically important in CVD and depression, it is unclear to what extent their co-occurrence is explained by shared genetic or early environmental factors. A significant genetic correlation between CVD and depression would provide evidence in support of a shared genetic pathway. However, the association could also be causal mediated through inflammation, endothelium dysfunction, alterations of the hypothalamic–pituitary–adrenocortical axis or cardiac autonomic dysfunction, which have all been proposed to explain the link between depression and CVD.Reference Nemeroff and Goldschmidt-Clermont27,Reference Chrysohoou, Kollia and Tousoulis28 In our study, we only had measurements of CRP in a small group of participants, and adjustment in this group did not affect the association. Furthermore, because of its anti-inflammatory effects and its ability to improve endothelium dysfunction, statins have been suggested to reduce the risk of depression,Reference Kohler-Forsberg, Gasse, Berk and Ostergaard29 however, further adjustment for statins (model 6 in Fig. 1) did not explain the association. Nevertheless, treatment for depression and stroke might be important to consider. For example, antidepressant medications have been shown to promote CVDReference Stapelberg, Neumann, Shum, McConnell and Hamilton-Craig10 and beta blockers might cause depressionReference Steffensmeier, Ernst, Kelly and Hartz30 and could thus explain a part of the association.
Strengths and limitations
An important strength of our study is the large number of individuals as a result of the pooling of several population-based cohorts and complete follow-up for register-based outcomes. Furthermore, IHD and stroke were measured simultaneously, and we adjusted for common risk factors including both socioeconomic status, lifestyle factors and objectively measured BMI, blood pressure and cholesterol levels. The register-based hospital diagnosis allowed us to examine the associations between developing CVD and the subsequent risk of depression and the reverse in the same population as well as to study the associations in non-participants. This attempt to study the temporal combinations of the two diseases could contribute to a better understanding of their interactive course.
However, our study also has some limitations in that all outcomes were based on hospital diagnoses. This may not be as important for IHD or stroke outcomes as these patients are often admitted to hospital but may be so for depression as patients treated in general practice or psychiatry clinics will not be included in the hospital-based patient registries. Our comparison of self-reported and hospital-diagnosed depression indicated that nearly 90% of those with depression are not recorded in the registers. This is in accordance with previous validation studiesReference Thielen, Nygaard, Andersen, Rugulies, Heinesen and Bech31,Reference Osler, Kriegbaum, Christensen, Lund and Nybo Andersen32 and suggests that studies based on hospital diagnoses might underestimate the association between CVD and depression. This was also one of the reasons for including the Metropolit cohort, which included measures on self-reported depression and MDI scores, but unfortunately, these could not be used as an outcome.
Furthermore, we only had information on the potential shared risk factors at baseline in midlife. With a long follow-up time these risk factors could have changed especially after receiving a diagnosis of for example depression and consequently not reflect the status at the time of disease development or the effects operating early in life. Finally, we did not have information on potential important mediators such as heart rate variability (a measure of cardiac autonomic dysfunction) or markers of endothelium dysfunction.
Implications
In conclusion, our study supports that IHD and stroke are associated with depression in a bidirectional manner in both men and women. These associations could not be explained by adjustment for several shared risk factors, misclassification of disease measures or selective non-response. This might reflect that several mechanisms over the life course contribute to the association and consequently the effect of risk factors linked to specific casual chains becomes modest. However, lifestyle medication could still improve the risks for development of CVD and depression. This is valuable information for future studies, which could focus on potential shared genetic and early-life environmental risk factors as potential explanations for the association.
Acknowledgements
The authors would like to thank the participants in the study cohorts and the team behind the cohorts for their great work in collecting and assuring data of high quality.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2019.130.
Funding
This work was supported by the Lundbeck Foundation, the Danish Heart Foundation, Eva and Henry Frænkels Mindelegat and the Independent Research Fund Denmark. This study was a part of the Danish study of Functional Disorders (DanFunD) funded by The Lundbeck foundation (grant number R155-2013-14070) and TrygFonden (grant number 7-11-0213). The DanFunD scientific management group consists of Professor MD DMSC Torben Jørgensen (PI), Professor MD DMSc Per Fink and Senior Consultant MD PhD Lene Falgaard Eplov.







eLetters
No eLetters have been published for this article.