Studies strongly suggest that genetic transmission accounts for most of the familial aggregation of schizophrenia (Reference GottesmanGottesman, 1991; Reference Kendler and DiehlKendler & Diehl, 1993). Environmental risk factors have also been implicated: these include pregnancy and delivery complications (Reference Parnas, Schulsinger and TeasdaleParnas et al, 1982; Reference Geddes and LawrieGeddes & Lawrie, 1995) and influenza during the mother's pregnancy (Reference Mednick, Machon and HuttunenMednick et al, 1988; Reference O'Callaghan, Sham and TakeiO’Callaghan et al, 1991; Reference Kunugi, Nanko and TakeiKunugi et al, 1992; Reference McGrath, Pemberton and WelhamMcGrath et al, 1994). Some studies have been unable to confirm an association between prenatal exposure to influenza and later schizophrenia (Reference Crow, Done and JohnstoneCrow et al, 1991; Reference Selten and SlaetsSelten & Slaets, 1994; Reference Susser, Lin and BrownSusser et al, 1994), and it is conceivable that maternal intake of medication during pregnancy to alleviate symptoms associated with influenza and similar diseases might influence the risk of schizophrenia in the offspring. Thus, maternal intake of analgesics might be related to the offspring's risk of schizophrenia. To our knowledge, no study linking psychiatric morbidity in the offspring with maternal intake of analgesics during pregnancy has been reported; we therefore analysed data from the Copenhagen Perinatal Cohort to examine the associations between schizophrenia in adult life and prenatal exposure to analgesics.
METHOD
The Copenhagen Perinatal Cohort
The Copenhagen Perinatal Cohort consists of 9125 individuals delivered by 8949 pregnant women between October 1959 and December 1961 at the maternity department of the Copenhagen University Hospital, Rigshospitalet. A total of 8400 infants survived the first month after birth. Information on exposure to analgesics and psychiatric hospitalisation was available for 7999 individuals (4098 males and 3941 females) from the Perinatal Cohort.
Consumption of medication during pregnancy
The mothers’ reported use of medication during pregnancy (information obtained in prenatal and postnatal interviews on any medication taken for at least 5 days) was computerised, using binary indicator variables without dosage information. The following types of drugs were included in this self-report: antihistamines, barbiturates, psychoactive medications (this category included meprobamate, chlordiazepoxide, glutethimide, chlorpromazine or perphenazine, imipramine and reserpine), analgesics, chemotherapeutics (sulphonamides, nitrofurantoin or phenyl salicylate preparations for disinfection of the urinary tract), hormones (this category included oestrogens, progestogens and corticosteroids) and diuretics.
The category of analgesics was pharmacologically heterogeneous. It included both prescribed analgesics and analgesics bought over the counter, and it was not possible to separate analgesics used for treating fever from those used for treating pain. Aspirin and other antipyretics were commonly used; other analgesics included phenacetin, aspirin in combination with codeine and, more rarely, stronger analgesics such as morphine or synthetic forms of morphine. The category of analgesics also included mixed-formula drugs containing aspirin-like analgesics in combination with a smaller amount of opioid analgesic. Other combinations included two types of anti-inflammatory drugs (for instance antipyrine plus phenacetin) and analgesics in combination with caffeine.
The mothers were asked about their intake of analgesics in the first, second and third months and in the second and third trimesters of pregnancy. For the study population of 7999 cohort members, the missing data rate was less than 1% for the first and the third months of pregnancy, and 4.4% for the second month. Information on use of analgesics was complete for the second trimester, while the missing data rate was 1.7% for the third trimester. The prevalence of exposure to analgesics at any time during the first trimester was 0.9% (71/7997), during the second trimester 1.8% (144/7999) and during the third trimester 2.0% (154/7867). Nineteen individuals were exposed exclusively during the first trimester, 58 were exposed exclusively during the second trimester and 82 were exposed exclusively during the third trimester, while 41 were exposed in all three trimesters.
Demographic factors
Maternal age (in years) at the time of delivery was obtained from the records at Rigshospitalet. Information on social status was obtained from an interview with the mother when the child was 1 year old. The socio-economic classification was based on information about breadwinner's occupation, breadwinner's education, type of income (wage or salary) and quality of housing. Sufficient data for classification of social status were available for 6333 individuals (79.2% of the study population). The overall social status mean was substituted for missing data, and a dummy variable was included to indicate missing data on social status in the logistic regression analyses.
Psychiatric hospitalisation
Written approval to conduct a registry-based psychiatric follow-up was obtained from the regional scientific ethics committee. The Danish Psychiatric Central Register has been computerised since 1 April 1969 (Reference Munk-Jørgensen and MortensenMunk-Jørgensen & Mortensen, 1997). It contains data on all admissions to Danish psychiatric in-patient facilities. The diagnostic system in use when the Danish Psychiatric Central Register was computerised was the ICD–8 (World Health Organization, 1967). The cohort members and their parents were followed in the Danish Psychiatric Central Register to identify all hospital admissions with a diagnosis of schizophrenia (ICD–8 code 295 or ICD–10 code F20) until December 1999. In ICD–8, schizophrenia is defined by prototypic descriptions of symptoms, such as bizarre delusions, delusions of control, abnormal affect, autism, hallucinations and disorganised thinking. In 1994, the more operational ICD–10 criteria were implemented (World Health Organization, 1992). The cohort and their parents were categorised as having a history of schizophrenia (ICD–8 code 295 or ICD–10 code F20) if they had been admitted with one of these diagnoses.
Other variables
Weighted pregnancy and delivery complications scales were constructed for use with the Copenhagen Perinatal Cohort by a team of American and Danish obstetricians and paediatric neurologists (Reference Zachau-Christiansen and RossZachau-Christiansen & Ross, 1975). To control for pregnancy complications, we used the pregnancy complications scale, which included items such as bleeding or illness during pregnancy, use of radiation during pregnancy and pre-eclampsia. Data on viral infections in the second trimester were available for 7970 pregnancies (99.6% of the study population). A few days after delivery, the mothers were asked about infections during the different periods of pregnancy, and the vast majority of viral infections recorded were minor respiratory illnesses or influenza (Reference VillumsenVillumsen, 1970).
We have recently reported that the combination of maternal hypertension and third-trimester (but not second-trimester) intake of diuretics during pregnancy was associated with an elevated risk in the offspring of schizophrenia according to the ICD–8 classification (Reference Sorensen, Mortensen and ReinischSørensen et al, 2003). Both second-trimester and third-trimester exposures to diuretics were included as potential confounders.
Statistical analysis
For each recorded period of gestation (first, second and third months and second and third trimesters), we estimated the risk of developing schizophrenia in cohort members exposed to analgesics (exposure group) and in those who were not exposed to these drugs (non-exposure group). Sub-periods showing an odds ratio significant at the 5% level were included in the subsequent multivariate analyses.
The distributions of potential confounders, including parental demographic and psychiatric characteristics, were compared between the two groups. One-way analysis of variance was used to compare the distributions of the continuous variables: parental age, parental social status and Pregnancy Complications score. Chisquared tests were used to compare the proportions of exposures to viral infections (second trimester), classes of medication in the second trimester, and the proportions of maternal and paternal schizophrenia in the exposure and non-exposure groups.
The risk of developing schizophrenia associated with prenatal exposure to analgesics was estimated in multivariate logistic regression models. The multivariate analyses included exposure to analgesics in the first and second trimesters and the following binary variables: second-trimester exposure to viral infections, other medication in the second trimester (anti-histamines, psychoactive medications, barbiturates, chemotherapeutics, hormones and diuretics) and third-trimester exposure to diuretics. In addition, maternal and paternal schizophrenia were included as covariates, and the following parameters were included as continuous covariates: maternal and paternal age, parental social class and Pregnancy Complications score. The first model adjusted for parental age, social status and Pregnancy Complications score, whereas the second model (full model) studied the joint effects of all the variables listed.
RESULTS
A total of 116 cases of schizophrenia (cumulative incidence 1.5%) were identified. Among 4058 male cohort members, 76 (1.9%) had developed schizophrenia, and among 3941 female cohort members, 40 (1.0%) had developed schizophrenia.
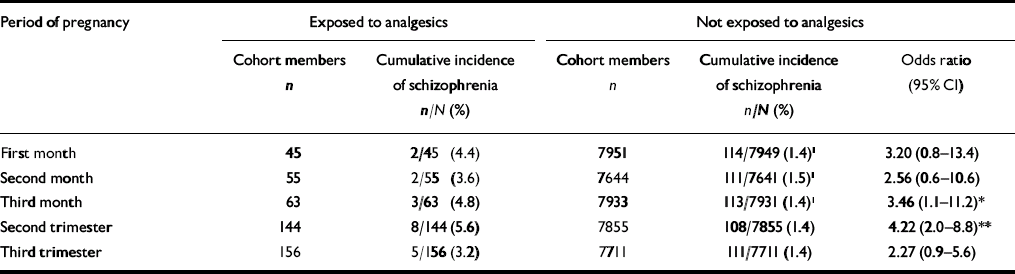
Table 1 shows that exposure to analgesics in the registered sub-periods of gestation was associated with an elevated risk of schizophrenia. For the first trimester the odds ratio was only significant for the third month. The odds ratio was also not significant for the third trimester, whereas the cumulative incidence of schizophrenia in the exposure group in the second trimester was significantly higher than in the non-exposure group in that period (5.6% v. 1.4%; P < 0.001). Thus, the odds ratio for the second trimester was 4.22 (95% CI 2.0–8.8).
Table 1 Risks of developing schizophrenia in members of the Copenhagen Perinatal Cohort associated with exposure to analgesics during gestation
| Period of pregnancy | Exposed to analgesics | Not exposed to analgesics | |||
|---|---|---|---|---|---|
| Cohort members n | Cumulative incidence of schizophrenia n/N (%) | Cohort members n | Cumulative incidence of schizophrenia n/N (%) | Odds ratio (95% CI) | |
| First month | 45 | 2/45 (4.4) | 7951 | 114/7949 (1.4)1 | 3.20 (0.8–13.4) |
| Second month | 55 | 2/55 (3.6) | 7644 | 111/7641 (1.5)1 | 2.56 (0.6–10.6) |
| Third month | 63 | 3/63 (4.8) | 7933 | 113/7931 (1.4)1 | 3.46 (1.1–11.2)* |
| Second trimester | 144 | 8/144 (5.6) | 7855 | 108/7855 (1.4) | 4.22 (2.0–8.8)** |
| Third trimester | 156 | 5/156 (3.2) | 7711 | 111/7711 (1.4) | 2.27 (0.9–5.6) |
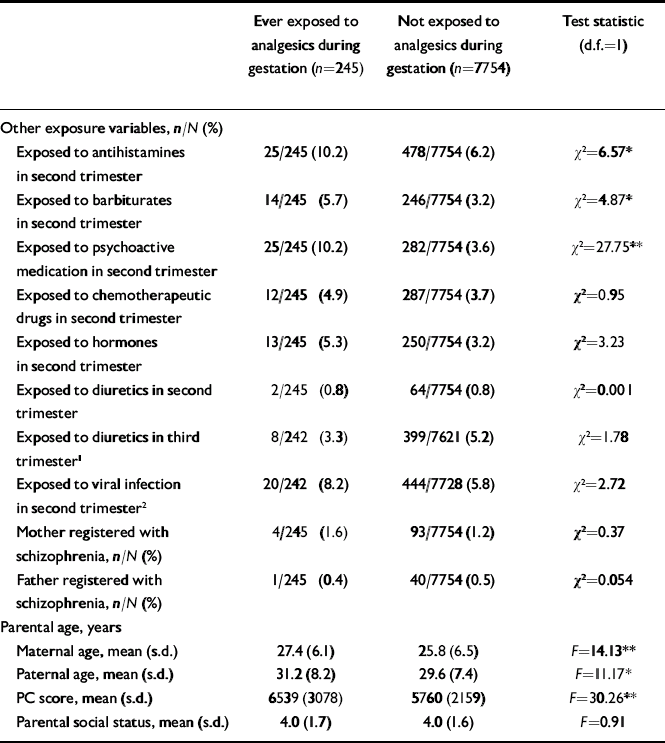
Some cohort members were exposed to analgesics in more than one period of gestation. The total number of cohort members who had been exposed at any time during gestation was 245 (ever exposed), whereas 7754 had never been exposed. The unadjusted odds ratio associated with exposure to analgesics at any time during gestation was 2.39 (95% CI 1.2–5.0), whereas it was 2.71 (95% CI 1.3–5.4) when adjusted for social status, mother's and father's age, and Pregnancy Complications score. Table 2 shows the distribution of demographic and other characteristics of individuals who had ever been exposed to analgesics during gestation v. the non-exposed comparison group. Exposure correlated with viral infections, antihistamine intake, barbiturate intake and use of psychoactive medication. Moreover, the mean parental age and the mean Pregnancy Complications score were higher. The two groups did not differ with respect to parental social status or proportions of mothers and fathers with schizophrenia.
Table 2 Characteristics of the exposure and non-exposure groups of the Copenhagen Perinatal Cohort
| Ever exposed to analgesics during gestation (n=245) | Not exposed to analgesics during gestation (n=7754) | Test statistic (d.f.=1) | |
|---|---|---|---|
| Other exposure variables, n/N (%) | |||
| Exposed to antihistamines in second trimester | 25/245 (10.2) | 478/7754 (6.2) | χ2=6.57* |
| Exposed to barbiturates in second trimester | 14/245 (5.7) | 246/7754 (3.2) | χ2=4.87* |
| Exposed to psychoactive medication in second trimester | 25/245 (10.2) | 282/7754 (3.6) | χ2=27.75** |
| Exposed to chemotherapeutic drugs in second trimester | 12/245 (4.9) | 287/7754 (3.7) | χ2=0.95 |
| Exposed to hormones in second trimester | 13/245 (5.3) | 250/7754 (3.2) | χ2=3.23 |
| Exposed to diuretics in second trimester | 2/245 (0.8) | 64/7754 (0.8) | χ2=0.001 |
| Exposed to diuretics in third trimester1 | 8/242 (3.3) | 399/7621 (5.2) | χ2=1.78 |
| Exposed to viral infection in second trimester2 | 20/242 (8.2) | 444/7728 (5.8) | χ2=2.72 |
| Mother registered with schizophrenia, n/N (%) | 4/245 (1.6) | 93/7754 (1.2) | χ2=0.37 |
| Father registered with schizophrenia, n/N (%) | 1/245 (0.4) | 40/7754 (0.5) | χ2=0.054 |
| Parental age, years | |||
| Maternal age, mean (s.d.) | 27.4 (6.1) | 25.8 (6.5) | F=14.13** |
| Paternal age, mean (s.d.) | 31.2 (8.2) | 29.6 (7.4) | F=11.17* |
| PC score, mean (s.d.) | 6539 (3078) | 5760 (2159) | F=30.26** |
| Parental social status, mean (s.d.) | 4.0 (1.7) | 4.0 (1.6) | F=0.91 |
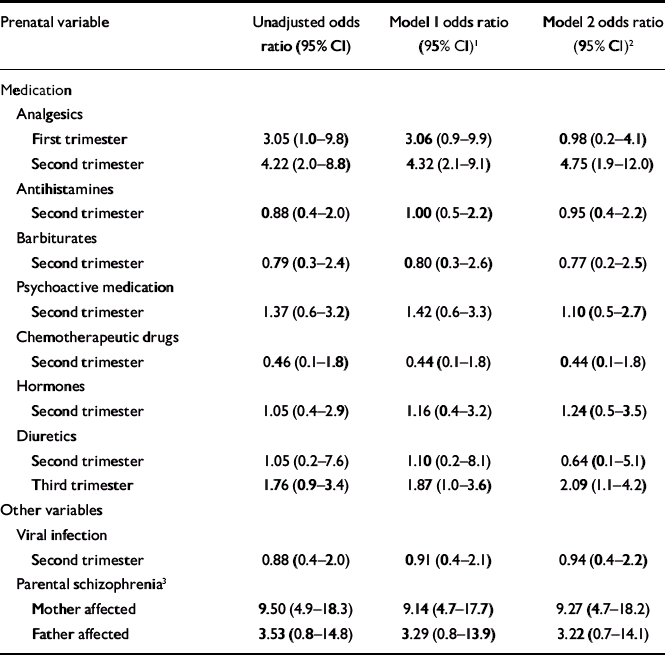
The relative risks (odds ratios) associated with exposure to analgesics are shown in Table 3. This table also shows the relative risk associated with exposure to viral infection or other medication in the second trimester and the risk associated with maternal or paternal hospitalisation with schizophrenia (Table 3, first column). Maternal schizophrenia emerged as the strongest risk factor (adjusted OR=9.27, 95% CI 4.7–18.2). The second strongest effect was noted for second-trimester exposure to analgesics (adjusted OR=4.75, 95% CI 1.9–12.0). The risk of schizophrenia associated with paternal schizophrenia was lower than expected (adjusted OR= 3.22, 95% CI 0.7–14.1). Similarly, the risk of schizophrenia associated with second-trimester viral infection was lower than expected (adjusted OR=0.94, 95% CI 0.4–2.2).
Table 3 Multiple logistic regression analysis of exposures during pregnancy and of parental psychiatric diagnoses predicting schizophrenia
| Prenatal variable | Unadjusted odds ratio (95% CI) | Model 1 odds ratio (95% CI)1 | Model 2 odds ratio (95% CI)2 |
|---|---|---|---|
| Medication | |||
| Analgesics | |||
| First trimester | 3.05 (1.0–9.8) | 3.06 (0.9–9.9) | 0.98 (0.2–4.1) |
| Second trimester | 4.22 (2.0–8.8) | 4.32 (2.1–9.1) | 4.75 (1.9–12.0) |
| Antihistamines | |||
| Second trimester | 0.88 (0.4–2.0) | 1.00 (0.5–2.2) | 0.95 (0.4–2.2) |
| Barbiturates | |||
| Second trimester | 0.79 (0.3–2.4) | 0.80 (0.3–2.6) | 0.77 (0.2–2.5) |
| Psychoactive medication | |||
| Second trimester | 1.37 (0.6–3.2) | 1.42 (0.6–3.3) | 1.10 (0.5–2.7) |
| Chemotherapeutic drugs | |||
| Second trimester | 0.46 (0.1–1.8) | 0.44 (0.1–1.8) | 0.44 (0.1–1.8) |
| Hormones | |||
| Second trimester | 1.05 (0.4–2.9) | 1.16 (0.4–3.2) | 1.24 (0.5–3.5) |
| Diuretics | |||
| Second trimester | 1.05 (0.2–7.6) | 1.10 (0.2–8.1) | 0.64 (0.1–5.1) |
| Third trimester | 1.76 (0.9–3.4) | 1.87 (1.0–3.6) | 2.09 (1.1–4.2) |
| Other variables | |||
| Viral infection | |||
| Second trimester | 0.88 (0.4–2.0) | 0.91 (0.4–2.1) | 0.94 (0.4–2.2) |
| Parental schizophrenia3 | |||
| Mother affected | 9.50 (4.9–18.3) | 9.14 (4.7–17.7) | 9.27 (4.7–18.2) |
| Father affected | 3.53 (0.8–14.8) | 3.29 (0.8–13.9) | 3.22 (0.7–14.1) |
Assuming a causal connection between prenatal exposure to analgesics and increased risk of schizophrenia, the attributable risk – i.e. the proportion of individuals in the total population with the disease attributed to exposure to analgesics in the second trimester – was calculated (Reference Altman, Machin and BryantAltman et al, 2000) and found to be 5.2% (95% CI 1.8–11.4).
The effect of analgesics exposure in the second trimester was statistically significant in both genders. The unadjusted odds ratio was 3.77 (95% CI 1.5–9.6) in males and 4.94 (95% CI 1.6–16.4) in females.
Second-trimester analgesics exposure and other putative risk factors
Out of 7970 cohort members, 464 (5.8%) had been exposed to a viral infection during the second trimester. Six cases of schizophrenia (1.3%) emerged among those exposed. There was no interaction between second-trimester exposure to analgesics and second-trimester viral exposure in relation to outcome. Only 15 cohort members had both these exposures during the second trimester of gestation, none of whom had a subsequent diagnosis of schizophrenia.
Second-trimester exposure to medication other than analgesics did not confer an elevated risk of schizophrenia, but third-trimester exposure to diuretics was significantly associated with risk of the disease in the multivariate analysis (adjusted OR=2.09, 95% CI 1.1–4.2). The association between exposure to psychoactive medication in the second trimester and outcome was in the expected direction but was not statistically significant. Mothers who took many analgesics might be at higher risk of developing an affective disorder or a substance dependence disorder later in life. Assuming that many with such diagnoses would have been in contact with a psychiatric in-patient facility in Denmark after 1969, we decided to carry out additional analyses in a subsample of 6109 cohort members whose mother and father did not appear in the Danish Psychiatric Central Register. Those excluded were all offspring of mothers or fathers with a diagnosis of schizophrenia or any other psychiatric disorder. In the subsample the unadjusted odds ratio of developing schizophrenia associated with prenatal exposure to analgesics in the second trimester was 4.51 (95% CI 1.6–12.7).
Out of 144 members of the subsample who had been exposed to analgesics at any time during gestation, 18 people had been exposed to opium or morphine, and 2 cases of schizophrenia (11.1%) arose in the latter subgroup. A preliminary review (data not shown) of some of the original data forms indicated that some of the opioid-treated mothers had undergone abdominal surgery a short time before. The Pregnancy Complications score for these women was higher than the scores for the cohort as a whole (data not shown). We found no indication in the data forms that any of these women had ever misused opioids.
A subgroup of the cohort (n=45) had been exposed to mixed-formula analgesic drugs that contained, among other things, older pyrazolone derivatives (antipyrine, isopropylantipyrine, salipyrine, phenazone, propyphenazone). Four cases of schizophrenia (8.9%) arose in that subgroup. The unadjusted odds of developing schizophrenia was 6.74 (95% CI 2.4–19.1) in those who had been exposed to mixed-formula analgesics of this sort and the estimate remained significant when we adjusted for maternal and paternal age, social status and Pregnancy Complications score.
DISCUSSION
Independent of a wide range of potential confounders, an association between prenatal exposure to analgesics and risk of schizophrenia was observed. The estimate of the risk of schizophrenia was more than four times greater in individuals who were exposed to analgesics in the second trimester (the association was slightly stronger in females than in males). The association was independent of maternal schizophrenia, which was the strongest risk factor for schizophrenia in this study.
From a methodological viewpoint, our prospective study is well suited to examine putative associations between prenatal exposure to medication and adverse outcomes many years later. Exposure to drug treatment was recorded shortly after delivery, thus minimising the risk of recall bias. The fact that only hospitalised cases of schizophrenia were identified should not be important, as most individuals meeting ICD–8 or ICD–10 criteria for schizophrenia are admitted to hospital before the age of 40 years. Up to 1994, the (ICD–8) concept of schizophrenia in Denmark reflected diagnostic caution rather than overinclusiveness. By extending the period of follow-up beyond 1994, some new cases of schizophrenia were identified using the more operational ICD–10 criteria.
We are not aware of any previous study suggesting a link between prenatal exposure to analgesic drugs and increased risk of severe mental illness. Tentatively, three types of explanations may be considered:
-
(a) chemical substances might have caused a subtle disruption of foetal neurodevelopment (or interacted with yet unknown genetic liability factors forthe disorder) to increase the risk for later development of schizophrenia;
-
(b) maternal somatic conditions for which the mothers took analgesics during pregnancy might underlie the observed association;
-
(c) the association could be explained by unidentified factors (which could not be fully controlled in the analysis) that correlate with both maternal intake of analgesics during pregnancy and outcome (residual confounding).
The unusually high risks of schizophrenia (11.1% and 8.9%, respectively) in the small subgroup of offspring of mothers who were treated with morphine or opioid analgesics and the subgroup with prenatal exposure to mixed-formula analgesics could be interpreted as a possible indication of a drug-related effect. Some of the mixed-formula analgesics contained antipyrine and related substances. The derivatives of these substances undergo extensive metabolisation in humans. Little toxicological information on their embryotoxic and teratogenic properties exists for these substances (Reference BurdanBurdan, 2002). However, to establish a drug-associated risk of a specific adult outcome in a non-experimental setting requires comparability of the exposed and non-exposed groups. The above-mentioned small subgroups may not be comparable with the pool of non-exposed cohort controls on all the characteristics thought relevant to the development of schizophrenia.
It is attractive to speculate that in utero exposure to one or several types of analgesics could be implicated in disruption of foetal neurodevelopment. During the second trimester of pregnancy, the cortical subplate reaches its peak of development (Reference Akbarian, Kim and PotkinAkbarian et al, 1995). This may be a period when the immature brain is particularly sensitive to a range of intrauterine environmental influences.
Based on data from the Copenhagen Perinatal Cohort, Villumsen noted that the incidence of malformed offspring of mothers who did not take medication during pregnancy was 3.6%, whereas the incidence of malformed children in offspring of mothers who had taken analgesics was 7.4% (Reference VillumsenVillumsen, 1970). This is of potential relevance to theories on aetiological factors in schizophrenia as well. A large number of minor physical anomalies (high-steepled palate, large or small distance between tear ducts, adherent earlobes) have been linked to schizophrenia-spectrum schizophrenia-spectrum disorders in a subsample of the Copenhagen Perinatal Cohort (Reference Schiffman, Ekstrom and LaBrieSchiffman et al, 2002). It has even been suggested that common genetic or environmental factors might be associated with schizophrenia and congenital malformations (Reference GoodmanGoodman, 1996; Reference Ismail, Cantor-Graae and McNeilIsmail et al, 1998).
We observed no difference in the proportions of maternal and paternal schizophrenia in analgesic-exposed and non-exposed cohort members. The mothers of the two groups did, however, differ in other important aspects. Maternal intake of psychotropic drugs during the second trimester correlated with intake of analgesics in the same period. Intake of these medications might be associated with higher psychiatric morbidity and/or a tendency to consult a physician more often. When the cohort was established, few Danish women consumed alcohol and the use of illicit drugs was extremely rare. Consequently, data were not collected on maternal alcohol or illicit drug use during pregnancy.
Maternal viral infection in the second trimester was not significantly associated with schizophrenia, but in this study statistical power might not have been sufficient to detect such a relationship. As mentioned previously, only 15 cohort members had been exposed to both viral infection and maternal analgesics, and consequently statistical power is also a concern when interpreting the non-significance of the interaction between these two factors.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Children born to mothers who took analgesics for periods that extended into the second trimester of their pregnancy may have an increased risk of developing schizophrenia.
-
▪ The association between prenatal exposure to analgesics and increased risk of schizophrenia was independent of other known risk factors for the disorder.
-
▪ It would have required a larger cohort study and the collection of additional prenatal and perinatal data to disentangle effects of prenatal exposure to analgesic drugs from the effects of the somatic and psychosomatic conditions prompting their use.
LIMITATIONS
-
▪ The study lacked adequate statistical power to test whether differential effects of particular analgesic substances were present.
-
▪ The data on prenatal exposure to medication dates back to the early 1960s and some of the drugs used at that time are now obsolete in most countries.
-
▪ No information on the dosage of the drugs taken during the pregnancy was available.
In conclusion, prenatal exposure to analgesics in the second trimester of pregnancy conferred a more than four-fold greater risk of schizophrenia. However, only 6.9% of all the cohort members with schizophrenia had been exposed to analgesics in the second trimester. Replication of this research by other investigators is clearly needed before prenatal exposure to analgesics can be added to the list of demonstrated risk factors for schizophrenia.
Acknowledgements
The study was supported by a Sygekassernes Helsefond (Health Insurance Foundation) grant to H.J.S., grants HD-17655 and HD-20263 from the National Institute of Child Health and Human Development to J.M.R., grant DA-05056 from the National Institute on Drug Abuse to J.M.R., grant 9700093 from the Danish Research Council to E.L.M., and grant 1400/2-4-1997 from the Danish National Board of Health to E.L.M. The authors thank Vibeke Munk, BA, for help with the manuscript and critical comments.






eLetters
No eLetters have been published for this article.