Bipolar disorders are highly disabling Reference Rosa, Franco, Martinez-Aran, Sanchez-Moreno, Reinares and Salamero1 and prevalent. Reference Merikangas, Jin, He, Kessler, Lee and Sampson2 More than half of individuals with bipolar disorder experience significant functional impairment in several domains, such as family and social life and work, outside the acute phases of the illness. Reference Sanchez-Moreno, Martinez-Aran and Vieta3 In the past decade, the focus of research in bipolar disorder has changed from clinical remission to functional recovery. Reference Sanchez-Moreno, Martinez-Aran and Vieta3 However, the sources of high variation observed in the psychosocial functioning of individuals with bipolar disorder are still poorly understood. Cognitive impairment is an important determinant of functional impairment in bipolar disorder. Reference Martinez-Aran, Vieta, Torrent, Sanchez-Moreno, Goikolea and Salamero4 Because the strength of association between poor social functioning and cognitive impairment in bipolar disorder is similar to that seen in schizophrenia, Reference Depp, Mausbach, Harmell, savla, Bowie and Harvey5 it seems crucial to characterise the underlying architecture of cognitive performance in bipolar disorder. Previous studies investigating multiple cognitive areas in bipolar disorder have usually focused on domains a priori that were subjectively defined and selected. However, the validity of these domains is questionable, as it relies on the assumption that the latent organisation of human cognition is similar in people with bipolar disorder and healthy controls. This crucial assumption has received little experimental evidence. Reference Schretlen, Pena, Aretouli, Orue, Cascella and Pearlson6 It thus remains possible that the same neuropsychological tests might evaluate different cognitive competencies in these two groups of participants if there is a discrepancy in how cognitive measures relate to one another between individuals with and without bipolar disorder. This lack of equivalence between the constructs could lead to artefacts in the observed differences in cognitive test performances. A few studies have explored the latent cognitive structure in bipolar disorder and found that cognitive functioning was best described by multifactorial models. Reference Schretlen, Pena, Aretouli, Orue, Cascella and Pearlson6–Reference Gallagher, Gray and Watson8 However, some of these studies included patients who were symptomatic. Reference Czobor, Jaeger, Berns, Gonzalez and Loftus7,Reference Gallagher, Gray and Watson8 Here, we have measured performance for a broad range of cognitive domains in euthymic bipolar disorder using a comprehensive battery of neuropsychological tests. We have examined the component structure of bipolar disorder cognitive processes using a principal component analysis (PCA), which allows the data-driven reduction of multiple cognitive measures, avoids arbitrary and a priori categorisation of several tests into domains, and results in a reliable estimate of underlying cognitive constructs in bipolar disorder.
Among clinical factors, residual depressive symptoms have been reported to be the strongest predictor of functional impairment Reference Bonnin, Martinez-Aran, Torrent, Pacchiarotti, Rosa and Franco9 and quality of life Reference Dias, Brissos, Frey and Kapczinski10 in euthymic bipolar disorder. They are also associated with lower adherence to medication in individuals with bipolar disorder. Reference Belzeaux, Correard, Boyer, Etain, Loftus and Bellivier11 In contrast, residual hypomanic symptoms have no impact on functioning in euthymic bipolar disorder. Reference Martinez-Aran, Vieta, Torrent, Sanchez-Moreno, Goikolea and Salamero4,Reference Bonnin, Martinez-Aran, Torrent, Pacchiarotti, Rosa and Franco9,Reference Allen, Bello and Thaler12–Reference Martino, Marengo, Igoa, Scapola, Ais and Perinot14 Whereas residual hypomanic symptoms are not related to cognition, Reference Bourne, Bilderbeck, Drennan, Atkinson, Price and Geddes15 it is less clear whether subthreshold depressive symptoms negatively affect neuropsychological performance in euthymic bipolar disorder or not. The relationship between residual depressive symptoms and cognition is mixed, showing a small impact of subthreshold depression on only a few cognitive components, such as verbal memory, speed, and executive function, but not for others. Reference Bourne, Aydemir, Balanza-Martinez, Bora, Brissos and Cavanagh16 A few studies have explored the role of cognition in mediating the relationship between depressive symptoms and functioning in euthymic bipolar disorder, with inconsistent results. One study showed that verbal memory partially mediated the relationship between subthreshold depressive symptoms and functional outcome in people with bipolar disorder who were euthymic. Reference del Mar Bonnin, González-Pinto, Solé, Reinares, González-Ortega and Alberich17 However, the sample size was not large enough to include other cognitive moderators in the model. In contrast, another study reported that cognition did not mediate the relationship between depressive symptoms and social competencies in bipolar disorder. Reference Bowie, Depp, McGrath, Wolyniec, Mausbach and Thornquist18 Again, only one global neurocognitive composite score was included as the cognitive mediator in the model, because of the limited sample size. There have been no studies, to date, that have investigated simultaneous mediation between subdepressive symptoms and functioning by multiple cognitive components in bipolar disorder. In this study, the cognitive domains obtained with PCA were entered in a path analysis model, as potential mediators between residual depressive symptoms and functioning.
Method
Study design and recruiting network characteristics
This multicentre, cross-sectional study included patients recruited into the FACE-BD (FondaMental Academic Centers of Expertise for Bipolar Disorders) cohort by a French national network of nine bipolar disorder expert centres (Bordeaux, Créteil, Grenoble, Marseille, Monaco, Montpellier, Nancy, Paris and Versailles). This network was set up by the FondaMental Foundation (www.fondation-fondamental.org) and funded by the French Ministry of Research and the French Ministry of Health to build an infrastructure and provide resources to follow clinical cohorts and comparative-effectiveness research on a representative patient population.
Participants
Bipolar disorder was diagnosed based on a structured clinical interview that assessed the DSM-IV-TR criteria. 19 Out-patients of 18 to 65 years of age with type I, II, or not otherwise specified (NOS, including cyclothymia) bipolar disorder were eligible. All patients were euthymic when they were tested according to the DSM-IV-TR criteria, with a Montgomery–Åsberg Depression Rating Scale (MADRS) Reference Montgomery and Asberg20 of less than 10 and a Young Mania Rating Scale (YMRS) Reference Young, Biggs, Ziegler and Meyer21 of less than 10. Exclusion criteria were a history of neurological or sensory disorders, dyslexia, dysorthographia, dyscalculia, dysphasia, dyspraxia, language delay, substance-related disorders in the previous month and electro-convulsive therapy in the past year. The ethics committee (Comité de Protection des Personnes lie de France IX) approved the study protocol on 18 January 2010. Although the committee waived the requirement for written informed consent, the patients received a letter informing them of the study and asking whether they agreed to participate.
Assessment tools
Clinical assessments
The age at onset; number of previous mixed, hypomanic, manic and major depressive episodes; subtype of bipolar disorder; and history of psychotic symptoms were recorded. We used the yes/no format for recording whether the patient was taking lithium carbonate, anticonvulsants, antipsychotics, antidepressants or anxiolytics at the time of the evaluation. Finally, three sociodemo-graphic characteristics were collected: gender, age and educational level.
Psychosocial functioning was measured using the Functioning Assessment Short Test (FAST), Reference Rosa, Sanchez-Moreno, Martinez-Aran, Salamero, Torrent and Reinares22 which covers six functioning domains: autonomy, occupational functioning, cognitive functioning, financial issues, interpersonal relationships and leisure time. The higher the score, the greater the disability. FAST has good internal consistency (Cronbach alpha between 0.87 and 0.96) and test–retest reliability (Pearson correlation coefficient between 0.90 and 0.97; intraclass correlation coefficient between 0.90 and 0.98, see online supplement DS1 for a bibliography of FAST psychometric properties).
Battery of cognitive tests
Experienced psychologists administered the tests in a fixed order. Testing lasted a total of 120 min, including a 5–10 min break. The standardised test battery complied with the recommendations issued by the International Society for Bipolar Disorders. Reference Yatham, Torres, Malhi, Frangou, Glahn and Bearden23 It included 11 tests and evaluated the following six cognitive domains:
-
(a) motor speed with the digit symbol coding and symbol search subtests from the Wechsler Adult Intelligence Scale (WAIS) version III; Reference Wechsler24
-
(b) attention with the Conners' Continuous Performance Test II (CPT-II) Reference Conners and Staff25 and the Trail Making Test (TMT); Reference Reitan26
-
(c) executive functions with the Stroop colour and word test and verbal fluency; Reference Lezak27
-
(d) verbal memory with the California Verbal Learning Test (CVLT); Reference Delis, Kramer, Kaplan and Ober28
-
(e) working memory with the digit span subtest from the WAIS-III and the spatial span subtest from the Wechsler Memory Scale version III; and
-
(f) intellectual functioning with the vocabulary and matrix reasoning subtests from the WAIS-III.
Some of the current cognitive data obtained with this battery have been published previously. Reference Roux, Raust, Cannavo, Aubin, Aouizerate and Azorin29
Statistical analyses
PCA
PCA is a powerful statistical method for identifying the underlying organisation of multiple variables such as cognitive measures and allows data reduction necessary to avoid type I errors from multiple comparisons. The data-set for the PCA included 22 raw cognitive variables: number of correct answers for the digit symbol coding and symbol search tests, percentages of omissions and commission errors for the CPT-II, reaction time (ms) for hits in the CPT, time (s) to complete the TMT-A and -B, number of responses in the colour, word, and colour-word conditions of the Stroop test, number of correct words for phonemic and semantic verbal fluency, number of recalled words in the immediate recall, short and long delay free recall of the CVLT, number of total correct recognised words for the CVLT, span lengths for the forward and backward conditions of the spatial and digit span tests, number of correct answers for matrix reasoning, and total score for vocabulary.
Sampling adequacy was evaluated using the Kaiser-Meyer-Olkin (KMO) measure Reference Dziuban and Shirkey30 for the overall cognitive data-set and Bartlett's test of sphericity. Reference Bartlett31 The number of components to be extracted in the PCA was determined by Horn's parallel analysis. Reference Horn32 This method contrasts eigenvalues produced through a parallel PCA on 1000 random data-sets, with the same number of variables and observations as the observational data-set, to generate eigenvalues for components that are adjusted for sample error-induced inflation. Adjusted eigenvalues > 1 indicate dimensions to retain.
We ran a PCA on cognitive variables followed by an Oblimin rotation. The rotation was performed to simplify the component structure. We used an oblique rotation because the cognitive components were believed to be correlated with each other. In PCA, the usual standard for sample size is a participant-to-variable ratio > 5, Reference Bryant, Yarnold, Grimm and Yarnold33 and therefore required 110 participants for the current study.
Path and mediation analyses
Zero-order correlations between MADRS, cognitive components, FAST scores, age, gender and education were calculated using Pearson correlation coefficients. A path analysis was performed using MADRS, cognitive components and FAST scores to test whether cognitive components mediated the relationship between residual depressive symptoms and functioning. The model tested in the path analysis did not include the YRMS score, as we expected it to correlate with neither cognition nor functioning. The model allowed the residual variances of the cognitive components scores to be correlated. Age, gender and education were used as covariates in the model.
Analyses were performed using the lavaan package of R statistical software version 3.3 with the maximum likelihood estimation method. Linear regression analyses were conducted to evaluate the relationships among the variables and were indexed using standardised path coefficients. Because the FAST total score is usually not normally distributed in euthymic bipolar disorder, Reference Bas, Poyraz, Bas, Poyraz and Tosun34 we used a non-parametric bootstrapping of the standard errors with 2000 iterations for the correlation and path analyses. The fit between the model and the data was assessed using four indices: the chi-square goodness-of-fit statistic (χ2), comparative fit index (CFI), root-mean-squared error of approximation (RMSEA) and standardised root-mean-square residual (SRMR).
Results
Participants
We included 241 patients. Table 1 reports their sociodemographic and clinical characteristics and Table 2 the results of the battery of cognitive tests. No patient had more than 5% missing cognitive data; the missing cognitive data were estimated using a multivariate imputation by chained equations in the mice package of R.
Table 1 Participant sociodemographical and clinical characteristics (n = 241)
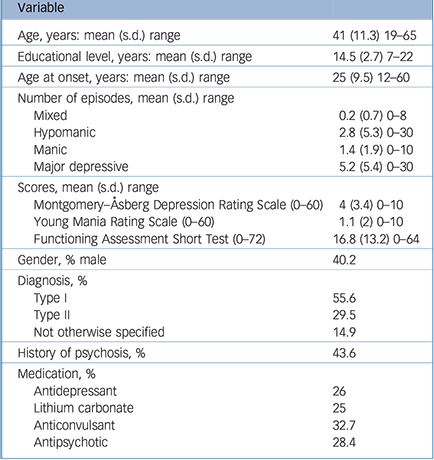
| Variable | |
|---|---|
| Age, years: mean (s.d.) range | 41 (11.3) 19–65 |
| Educational level, years: mean (s.d.) range | 14.5 (2.7) 7–22 |
| Age at onset, years: mean (s.d.) range | 25 (9.5) 12–60 |
| Number of episodes, mean (s.d.) range | |
| Mixed | 0.2 (0.7) 0–8 |
| Hypomanic | 2.8 (5.3) 0–30 |
| Manic | 1.4 (1.9) 0–10 |
| Major depressive | 5.2 (5.4) 0–30 |
| Scores, mean (s.d.) range | |
| Montgomery–Åsberg Depression Rating Scale (0–60) | 4 (3.4) 0–10 |
| Young Mania Rating Scale (0–60) | 1.1 (2) 0–10 |
| Functioning Assessment Short Test (0–72) | 16.8 (13.2) 0–64 |
| Gender, % male | 40.2 |
| Diagnosis, % | |
| Type I | 55.6 |
| Type II | 29.5 |
| Not otherwise specified | 14.9 |
| History of psychosis, % | 43.6 |
| Medication, % | |
| Antidepressant | 26 |
| Lithium carbonate | 25 |
| Anticonvulsant | 32.7 |
| Antipsychotic | 28.4 |
Table 2 Participant neuropsychological performance
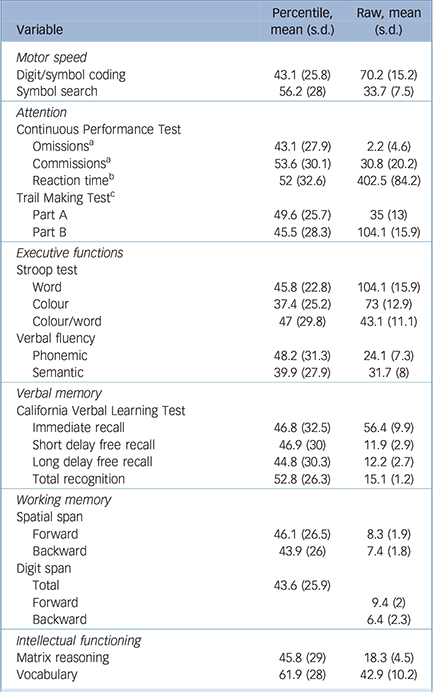
| Variable | Percentile, mean (s.d.) |
Raw, mean (s.d.) |
|---|---|---|
| Motor speed | ||
| Digit/symbol coding | 43.1 (25.8) | 70.2 (15.2) |
| Symbol search | 56.2 (28) | 33.7 (7.5) |
| Attention | ||
| Continuous Performance Test | ||
| Omissions a | 43.1 (27.9) | 2.2 (4.6) |
| Commissions a | 53.6 (30.1) | 30.8 (20.2) |
| Reaction time b | 52 (32.6) | 402.5 (84.2) |
| Trail Making Test c | ||
| Part A | 49.6 (25.7) | 35 (13) |
| Part B | 45.5 (28.3) | 104.1 (15.9) |
| Executive functions | ||
| Stroop test | ||
| Word | 45.8 (22.8) | 104.1 (15.9) |
| Colour | 37.4 (25.2) | 73 (12.9) |
| Colour/word | 47 (29.8) | 43.1 (11.1) |
| Verbal fluency | ||
| Phonemic | 48.2 (31.3) | 24.1 (7.3) |
| Semantic | 39.9 (27.9) | 31.7 (8) |
| Verbal memory | ||
| California verbal Learning Test | ||
| Immediate recall | 46.8 (32.5) | 56.4 (9.9) |
| Short delay free recall | 46.9 (30) | 11.9 (2.9) |
| Long delay free recall | 44.8 (30.3) | 12.2 (2.7) |
| Total recognition | 52.8 (26.3) | 15.1 (1.2) |
| Working memory | ||
| Spatial span | ||
| Forward | 46.1 (26.5) | 8.3 (1.9) |
| Backward | 43.9 (26) | 7.4 (1.8) |
| Digit span | ||
| Total | 43.6 (25.9) | |
| Forward | 9.4 (2) | |
| Backward | 6.4 (2.3) | |
| Intellectual functioning | ||
| Matrix reasoning | 45.8 (29) | 18.3 (4.5) |
| Vocabulary | 61.9 (28) | 42.9 (10.2) |
a. Raw scores are percentages.
b. Raw scores are in ms.
c. Raw scores are in s.
PCA
The KMO measure for the overall cognitive data-set was 0.85 and Bartlett's test of sphericity was significant (χ2(231) = 2295, P < 0.001), both indicating good factorability of the cognitive data. Horn's parallel analysis showed that five components should be retained, as their adjusted eigenvalue was above 1 (online Table DS1). The five-component structure explained 61% of the variance (online Table DS2). All component loadings were greater than 0.4, and all communalities were higher than 0.3 (Table 3). The first component consisted of all measures of the CVLT and was designated ‘verbal memory’. The second component bundled TMT, CPT omissions, symbol search, vocabulary, symbol coding and semantic verbal fluency and was designated ‘speed of processing and verbal knowledge’. The third component included all measures of spatial and digit spans, with matrix reasoning, and was designated ‘working memory and problem-solving’. The fourth component consisted of all measures of the Stroop test, with phonemic verbal fluency, and was designated ‘verbal fluency and inhibition’. The final component included CPT reaction time and commission and was designated ‘visual sustained attention’.
Table 3 Component loadings and communalities for the cognitive variables
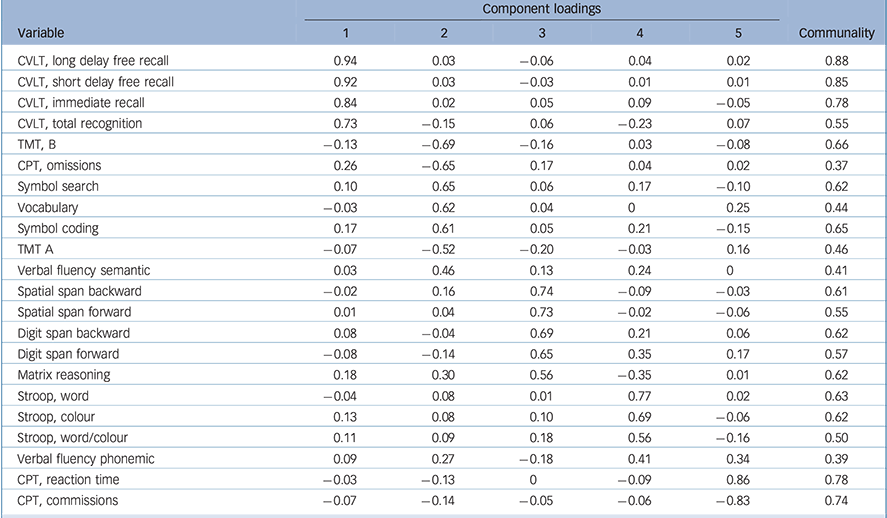
| Component loadings | ||||||
|---|---|---|---|---|---|---|
| Variable | 1 | 2 | 3 | 4 | 5 | Communality |
| CVLT, long delay free recall | 0.94 | 0.03 | −0.06 | 0.04 | 0.02 | 0.88 |
| CVLT, short delay free recall | 0.92 | 0.03 | −0.03 | 0.01 | 0.01 | 0.85 |
| CVLT, immediate recall | 0.84 | 0.02 | 0.05 | 0.09 | −0.05 | 0.78 |
| CVLT, total recognition | 0.73 | −0.15 | 0.06 | −0.23 | 0.07 | 0.55 |
| TMT, B | −0.13 | −0.69 | −0.16 | 0.03 | −0.08 | 0.66 |
| CPT, omissions | 0.26 | −0.65 | 0.17 | 0.04 | 0.02 | 0.37 |
| Symbol search | 0.10 | 0.65 | 0.06 | 0.17 | −0.10 | 0.62 |
| Vocabulary | −0.03 | 0.62 | 0.04 | 0 | 0.25 | 0.44 |
| Symbol coding | 0.17 | 0.61 | 0.05 | 0.21 | −0.15 | 0.65 |
| TMT A | −0.07 | −0.52 | −0.20 | −0.03 | 0.16 | 0.46 |
| Verbal fluency semantic | 0.03 | 0.46 | 0.13 | 0.24 | 0 | 0.41 |
| Spatial span backward | −0.02 | 0.16 | 0.74 | −0.09 | −0.03 | 0.61 |
| Spatial span forward | 0.01 | 0.04 | 0.73 | −0.02 | −0.06 | 0.55 |
| Digit span backward | 0.08 | −0.04 | 0.69 | 0.21 | 0.06 | 0.62 |
| Digit span forward | −0.08 | −0.14 | 0.65 | 0.35 | 0.17 | 0.57 |
| Matrix reasoning | 0.18 | 0.30 | 0.56 | −0.35 | 0.01 | 0.62 |
| Stroop, word | −0.04 | 0.08 | 0.01 | 0.77 | 0.02 | 0.63 |
| Stroop, colour | 0.13 | 0.08 | 0.10 | 0.69 | −0.06 | 0.62 |
| Stroop, word/colour | 0.11 | 0.09 | 0.18 | 0.56 | −0.16 | 0.50 |
| Verbal fluency phonemic | 0.09 | 0.27 | −0.18 | 0.41 | 0.34 | 0.39 |
| CPT, reaction time | −0.03 | −0.13 | 0 | −0.09 | 0.86 | 0.78 |
| CPT, commissions | −0.07 | −0.14 | −0.05 | −0.06 | −0.83 | 0.74 |
CVLT, California Verbal Learning Test; TMT, Trail Making Test; CPT, Conners' Continuous Performance Test II.
Path and mediation analyses
Online Table DS3 reports the zero-order correlations between the variables included in the model. The path analysis model allowed correlations between the residual variances of all cognitive components, except ‘visual sustained attention’, which was not correlated with any other cognitive components.
The path analysis model is shown in Fig. 1. We represent neither covariances between cognitive components, nor the regressions on covariates, to enhance readability. Path analysis requires at least 15 participants for each variable. Reference Bentler and Chou35 We included ten variables in the model and therefore required at least 150 participants. There were 0.8% missing data, which were handled using the full information maximum likelihood estimation. The four patterns of missingness are reported in online Table DS4 and the covariance coverage matrix of missing data in online Table DS5.
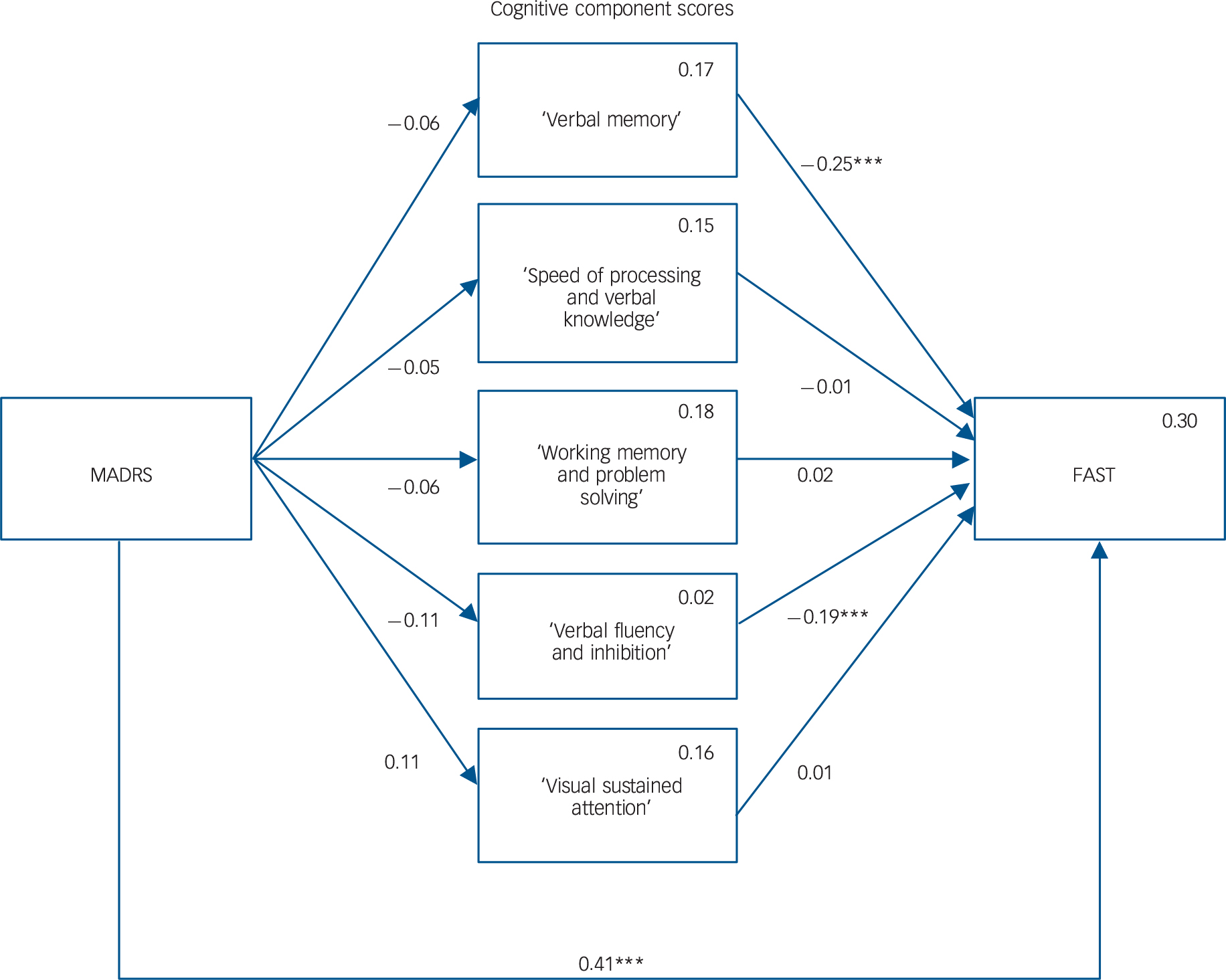
Fig. 1 Mediation model.
Rectangles represent the observed measured variables. Arrows showing the free regression weight are drawn between variables, values are the standardised path coefficients. The squared multiple correlation (R) value for the dependent variable appears in the upper right corner of each rectangle. Covariates and covariation between the cognitive components are not drawn to increase readability but were included in the model. MADRS, Montgomery–Åsberg Depression Rating Scale; FAST, Functioning Assessment Short Test. ***P>0.001.
The model provided a good fit for the data, as suggested by the non-significant chi-square goodness-of-fit statistic (χ2(4) = 7.5, P = 0.113), a CFI of 0.989 (thus greater than 0.95), an RMSEA of 0.06 (which was not significantly greater than 0.05, as the P-value of the one-sided test of the null hypothesis that RMSEA was greater than 0.05 was equal to 0.329), and SRMR of 0.017 (thus lower than 0.08).
The model explained 30% of the variance in functioning. Altogether, the analysis revealed the following relationships between the variables (Fig. 1): a significant positive association between MADRS and FAST, a significant negative association between ‘verbal memory’ cognitive component and FAST, and a significant negative association between the ‘verbal fluency and inhibition’ cognitive component and FAST. We found no other significant associations, and in particular, MADRS was not significantly associated with any cognitive component, thus showing that cognitive component scores did not mediate the relationship between MADRS and FAST. Estimated standardised path coefficients for all variables included in the path analysis model (with the covariates) and residual correlation coefficients between cognitive components are reported in online Table DS6.
Discussion
Main findings and comparison with other studies
Here, we first used a PCA to identify the underlying architecture of cognitive processing in patients with bipolar disorder who were euthymic. We then used a path analysis to evaluate whether cognition mediated the relationship between residual depressive symptoms and functioning. We found five underlying components involved in cognition in euthymic bipolar disorder. Two components were derived from individual variables that were relatively homogeneous and specific regarding modality: the ‘verbal memory’ component was derived only from CVLT measures and the ‘visual sustained attention’ only from CPT measures. The remaining three components were more heterogeneous. The ‘verbal fluency and inhibition’ component consisted of verbal responses sometimes involving inhibition (phonemic verbal fluency and colour–word condition of the Stroop test) and sometimes not (colour and word condition of the Stroop test). The ‘speed of processing and verbal knowledge’ component contained a combination of visuospatial and verbal variables. The ‘working memory and problem-solving’ component bundled non-verbal reasoning and working memory measures. Greater variability within cognitive components has already been reported in bipolar disorder and was interpreted as a decrease in the differentiation of previously discrete cognitive processes through a decline in neural connectivity. Reference Gallagher, Gray and Watson8 The number of extracted cognitive components is similar to that of previous studies that found five Reference Schretlen, Pena, Aretouli, Orue, Cascella and Pearlson6 or six underlying dimensions in cognition for bipolar disorder. Reference Czobor, Jaeger, Berns, Gonzalez and Loftus7 The labels used in these previous studies to describe the cognitive dimensions were similar to those applied in the current study, consisting of verbal memory, speed of processing, working memory, executive functions, verbal knowledge and attention dimensions. This suggests a relative stability and reliability of the method we used to uncover the underlying cognitive components in bipolar disorder.
There was a negative association between residual depressive symptoms and functioning: individuals with more pronounced depressive symptoms had poorer social functioning, which was not explained by age, gender, lower education or poorer cognition. This finding is in accordance with previous cross-sectional Reference Aparicio, Santos, Jimenez-Lopez, Bagney, Rodriguez-Jimenez and Sanchez-Morla13,Reference del Mar Bonnin, González-Pinto, Solé, Reinares, González-Ortega and Alberich17,Reference Bowie, Depp, McGrath, Wolyniec, Mausbach and Thornquist18,Reference Bas, Poyraz, Bas, Poyraz and Tosun34 and longitudinal studies, Reference Bonnin, Martinez-Aran, Torrent, Pacchiarotti, Rosa and Franco9,Reference Martino, Marengo, Igoa, Scapola, Ais and Perinot14 showing that subclinical depressive symptoms in bipolar disorders are the main predictors of poor functional outcome, particularly work functioning. Reference Burdick, Goldberg and Harrow36 The present study included only patients who were euthymic, based on stringent criteria: the mean score for depression was very low but similar to previous studies exploring social functioning in euthymic bipolar disorder. Reference Bonnin, Martinez-Aran, Torrent, Pacchiarotti, Rosa and Franco9,Reference Aparicio, Santos, Jimenez-Lopez, Bagney, Rodriguez-Jimenez and Sanchez-Morla13,Reference del Mar Bonnin, González-Pinto, Solé, Reinares, González-Ortega and Alberich17,Reference Bas, Poyraz, Bas, Poyraz and Tosun34 However, the data show that functional impairment may be associated with even very low residual depressive symptoms, measured with a scale that was not specifically designed to assess subsyndromal depressive symptoms.
Among the five cognitive components found in this study, only two were positively associated with functioning: ‘verbal memory’ and ‘verbal fluency and inhibition’. Patients with better verbal memory, verbal fluency and inhibition also had a better social functioning. These results are consistent with several prior reports indicating that verbal memory Reference Martino, Marengo, Igoa, Scapola, Ais and Perinot14 and inhibitory control (Stroop colour–word test) Reference Reinares, Papachristou, Harvey, Mar Bonnin, Sanchez-Moreno and Torrent37 were more highly associated with functioning than other cognitive functions. That functioning was more highly related to residual depressive symptoms than cognition could be explained by the auto-evaluation method we used to measure everyday functioning. Self-reported measures of social functioning may be influenced less by objective cognitive performance and more by depressive symptomatology, because of pessimistic subjective appraisal of oneself and one's environment. This influence might be particularly important for low FAST scores (better functioning), like in our sample, where the distribution of FAST scores was skewed on the right. In contrast, the reverse may be true for performance-based measures of functioning and real-world functional milestones in bipolar disorder, which may be influenced more by objective cognitive performance and less by depression. Reference Depp, Mausbach, Harmell, savla, Bowie and Harvey5,Reference Allen, Bello and Thaler12
No associations between residual depressive symptoms and cognitive components were significant, showing that cognition does not mediate the relationship between subclinical residual depressive symptoms and functioning. This result is in accordance with a previous study reporting that perceived cognitive impairment and subclinical residual depressive symptoms are two independent sources of variation in the functioning of individuals with bipolar disorder. Reference Samalin, Boyer, Murru, Pacchiarotti, Reinares and Bonnin38 However, this mediation might occur for higher levels of depressive symptoms, as the impact of depressive symptoms on cognition varied according to the clinical response after treatment. Reference Gorwood, Corruble, Falissard and Goodwin39
Our model explained only 30% of the variance in functioning, supporting a role for other factors that were not measured here. Previous studies have suggested that functioning may be impaired when sleep is persistently disrupted, Reference Boland, Stange, Molz Adams, LaBelle, Ong and Hamilton40 when social cognition is impaired, Reference Aparicio, Santos, Jimenez-Lopez, Bagney, Rodriguez-Jimenez and Sanchez-Morla13 and when episode density is high. Reference Reinares, Papachristou, Harvey, Mar Bonnin, Sanchez-Moreno and Torrent37 The mean level of functioning in participants recruited in this study corresponds to moderate functional difficulties. Reference Sanchez-Moreno, Martinez-Aran and Vieta3 It is in the range (from 6 to 29) of those found in studies exploring the relationship between cognition and functioning in euthymic bipolar disorder. Reference Aparicio, Santos, Jimenez-Lopez, Bagney, Rodriguez-Jimenez and Sanchez-Morla13,Reference Bas, Poyraz, Bas, Poyraz and Tosun34,Reference Samalin, Boyer, Murru, Pacchiarotti, Reinares and Bonnin38 This consistency supports the general applicability of our findings to patients with euthymic bipolar disorder, provided the same assessment tools are used.
Limitations
Limitations of our study include the cross-sectional design, which precludes the assessment of causality and the direction of potential causal links. Another limitation is the lack of inclusion of social cognitive tasks in the assessment. We did not compare the cognitive architecture found in participants with bipolar disorder with a control group. Finally, the sample size was not large enough to test a more complex model that includes other variables of the illness (type of bipolar disorder, number of previous episodes, age at onset and history of psychosis) and medication. The time since the last mood episode might also be an important factor lacking in the present study. These variables might have also had an effect on functioning in euthymic bipolar disorder.
Implications
Our findings have important implications for future clinical studies. Individuals with bipolar disorder who respond to treatment may nevertheless continue to experience residual depressive and cognitive symptoms, leading to difficulties in functioning. First, it seems crucial to improving the assessment and the characterisation of residual depressive symptoms, with, for example, specific scales. Treatment approaches should possibly include cognitive performance improvement and full residual depressive symptom remission as important targets to obtain functional recovery in bipolar disorder. The optimal method to treat residual depressive symptoms is not clear, but they may be targeted by the use of mood stabilisers effective in the treatment of depressive polarity. Evidence for the efficacy of pharmacological and psychological interventions that target cognitive deficits in bipolar disorder is still preliminary, despite promising avenues such as functional remediation. Reference Bonnin, Reinares, Martinez-Aran, Balanza-Martinez, Sole and Torrent41 Among cognitive dimensions for cognitive remediation in euthymic bipolar disorder, our data suggest that verbal memory and fluency, and inhibition may be choice targets.
In summary, this study provides further evidence that cognitive impairments in specific dimensions are a core feature of bipolar disorder. This study also suggests that cognition is a separable dimension from depressive symptoms that persist during the inter-episodic period of bipolar disorder. Verbal memory and fluency and Stroop test performance were particularly associated with functioning in our participants with euthymic bipolar disorder and should be assessed in future studies focusing on functional outcome in bipolar disorder.
Funding
This work was supported by the Centre Hospitalier de Versailles, Foundation FondaMental, Créteil, France, and by the Investissements d'Avenir Programs managed by the ANR under references and .
Acknowledgements
We thank the Centre Hospitalier de Versailles and William Hempel of Alex Edelman 8t Associates for editorial assistance.
Appendix
List of FACE-BD collaborators
FACE-BD Clinical Coordinating Center (FondaMental Foundation): B. Etain, C. Henry and M. Leboyer.
FACE-BD Data Coordinating Center (FondaMental Foundation): O. Godin, H. Laouamri and N. Ngo-Nguyen.
FACE-BD clinical sites and principal collaborators in France: AP-HP, Hôpital H. Mondor – A. Chenevier, Pôle de Psychiatrie, Créteil – C. Daban, S. Hotier, S. Lauer, A. Leduc, A. Pelletier and A. Raust; AP-HP, GH Saint-Louis–Lariboisière–Fernand Widal, Pôle Neurosciences, Paris – F. Bellivier, M. Carminati, B. Etain, J. P. Lépine, I Nieto, P. Seguin and S. Sportiche; Hôpital C. Perrens, Centre Expert Trouble Bipolaire, Service de Psychiatrie Adulte, Pôle 3-4-7, Bordeaux – B. Antoniol, A. Desage, S. Gard, A. Jutant, K. Mbailara, I. Minois and L Zanouy; Département d'Urgence et Post Urgence Psychiatrique, CHRU Montpellier, Montpellier – S. Bonnet, F. Coppola, P. Courtet, D. Ducasse, M. Gachet, L. Matos, F. Molière, B. Noisette, E. Olié and G. Tarquini; Département de Psychiatrie, Hôpital Sainte Marguerite, Marseille – J. M. Azorin, R. Belzeaux and N. Corréard; Service de Psychiatrie et Psychologie Clinique, CHU de Nancy, Hôpitaux de Brabois, Vandoeuvre Les Nancy – R. Cohen, J.P. Kahn, P. Kieffer and O. Wajsbrot-Elgrabli; Clinique Universitaire de Psychiatrie, CHU de Grenoble, Grenoble – T. Bougerol, M. A. De Pourtales, B. Fredembach, S. Garçon, Alexandre Perrin and M. Polosan; Centre Hospitalier de Versailles, Service Universitaire de Psychiatrie d'adultes, Le Chesnay – A.M. Galliot, I. Grévin, M. C. Hardy-Bayle, A.S. Cannavo, N. Kayser, C. Passerieux and P. Roux; Service de Psychiatrie, Centre Hospitalier Princesse Grace, Monaco – L. Albertini, V. Aubin, E. Beetz, I. Cussac, J. Loftus and I. Medecin.







eLetters
No eLetters have been published for this article.