Few randomised controlled trials (RCTs) have been targeted at reducing substance use among people with psychotic disorders. Two large RCTs have reported encouraging but short-term effects of single-session motivational interventions among psychiatric hospital in-patients with mixed diagnoses and coexisting alcohol and/or other drug use problems (Reference Baker, Lewin and ReichlerBaker et al, 2002; Reference Hulse and TaitHulse & Tait, 2003). In a pilot study of 25 in-patients with early psychosis, Kavanagh et al (Reference Kavanagh, Waghorn and Jenner2004) reported that a total of 3 hours’ motivational interviewing resulted in significantly better outcomes. Cognitive–behavioural therapy (CBT) has been shown to be effective for problems associated with alcohol (Reference Shand, Gates and FawcettShand et al, 2003), cannabis (Reference Copeland, Swift and RoffmanCopeland et al, 2001) and amphetamine use (Reference Baker, Lee and ClaireBaker et al, 2005a ), for improving psychotic symptomatology (Reference Haddock, Barrowclough and TarrierHaddock et al, 2003) and in related service contexts (Reference Graham, Copello and BirchwoodGraham et al, 2004). In the first RCT to investigate the efficacy of CBT among people with coexisting schizophrenia and substance use disorder, Barrowclough et al (Reference Barrowclough, Haddock and Tarrier2001) reported modest yet promising findings. Eighteen months after study entry, the treatment group had superior general functioning and negative symptom scores, but there was no differential effect on percentage of days of abstinence from substances (Reference Haddock, Barrowclough and TarrierHaddock et al, 2003). The authors suggested that larger studies are required that examine the efficacy of the different components of CBT interventions. The aim of the present study was to investigate whether a 10-session motivational interviewing/CBT intervention administered to a relatively large sample of people with psychosis and substance use disorders was more efficacious than routine treatment in reducing substance use and improving symptomatology and general functioning.
METHOD
Design
In the current RCT, all of the participants provided written informed consent and were assessed at baseline (pre-treatment), 15 weeks (post-treatment), 6 months and 12 months after the initial assessment. Participants were randomly allocated to one of the following two groups: the treatment group, which received 10 1-hour sessions of motivational interviewing and CBT (in addition to an assessment schedule, treatment as usual and provision of self-help material for substance use); or the control group, which received self-help material for substance use, treatment as usual, and the same assessment schedule as that for the treatment group. After the initial assessment, participants drew a card from an envelope, which allocated them to either the treatment group or the control group.
Participants
The study participants were 130 regular users of alcohol, cannabis and/or amphetamines who had a non-acute psychotic disorder, and who were recruited from the Hunter region, 150 kilometres north of Sydney, New South Wales, Australia. Substance use intervention thresholds included alcohol consumption exceeding National Health and Medical Research Council (NHMRC) recommended levels (an average of four standard drinks per day for men and two standard drinks per day for women) (Reference Pols and HawksPols & Hawks, 1992) or at least weekly use of cannabis or amphetamines as recorded on the Opiate Treatment Index (OTI; Reference Darke, Ward and HallDarke et al, 1991) for the month before the initial assessment. Other inclusion criteria were as follows: age at least 15 years; ability to speak English; and having a confirmed ICD–10 psychotic disorder (World Health Organization, 1992). Exclusion criteria were: failure to meet at least one of the specified substance use thresholds; having an organic brain impairment; and intending to move from the geographical area within the subsequent 12 months. Referrals to the present study were received from community health agencies (33.8%), in-patient psychiatric hospital units (33.1%), an early psychosis service (27.7%), media advertisements (3.1%) and the Neuroscience Institute of Schizophrenia and Allied Disorders (NISAD; (Reference Loughland, Carr and LewinLoughland et al, 2001) Schizophrenia Research Register (2.3%). Participants who were initially approached via in-patient units were recontacted 2 months after discharge and invited to participate in the study.
Procedure
All of the participants read an information sheet before giving their written consent to participate in the study. Parental/guardian consent was sought for individuals under 18 years of age. Participants were informed that they would be randomly assigned to one of two conditions. Each participant was reimbursed with a Aus$20 fee for their time, travel and participation at each assessment (but not for treatment sessions). This amount was considered small enough not to influence participants’ responses unduly, but sufficient to reduce non-adherence caused by the inconvenience of attending assessment sessions. If possible, treatment sessions were conducted at the research centre or a community clinic. However, if participants were unable to attend these centres, sessions were conducted in the participant's home. Any participant who missed three consecutive treatment sessions was considered to have dropped out of treatment. Follow-up assessments were conducted by clinical interviewers who were masked to intervention status.
Measures
Key demographic and clinical characteristics and outcome measures are reported in this paper. The assessment instruments that were used have been reported previously (Reference Baker, Bucci and LewinBaker et al, 2005b ), and are described only briefly here. Data were collected on various demographic characteristics, treatment history (mental health and alcohol and/or other drug use) and current substance use. Diagnosis in accordance with the ICD–10 was achieved by administering the Diagnostic Interview for Psychosis (DIP; Reference Jablensky, McGrath and HerrmanJablensky et al, 2000) and applying the Operational Criteria for Psychosis (OPCRIT; Reference McGuffin, Farmer and HarveyMcGuffin et al, 1991). The diagnosis obtained from this interview were later collapsed to match the psychosis categories reported in the Low Prevalence Disorders Study (LPDS) of the National Survey of Mental Health and Wellbeing (NSMHWB) (Reference Jablensky, McGrath and HerrmanJablensky et al, 2000), which are as follows: severe depression with psychosis (F32.3); bipolar, mania (F30, F31); schizophrenia (F20); schizoaffective disorder (F25); and other psychosis (F22, F28, F29).
The Drug Use Scale of the OTI (Darke et al, Reference Darke, Ward and Hall1991, Reference Darke, Hall and Wodak1992), which was the primary measure of alcohol and/or other drug use, was administered at each assessment. The OTI yields an average daily consumption score for 11 classes of drug during the month (28 days) before interview, with weekly use of a single dose of cannabis or amphetamines being equivalent to an OTI score of 0.14 (4/28). The OTI also provides a poly-drug use score which identifies the number of drug classes used that month. In addition, an aggregate substance use index score was used as a global measure to describe the number of ‘day equivalents’ of hazardous use. This was necessary because the substance use measures varied with regard to the units recorded (e.g. number of standard drinks v. number of occasions of cannabis use). For each illicit substance the estimated number of days of consumption during the past 28 days was determined, and for alcohol the number of days on which consumption exceeded NHMRC recommended levels was calculated. Ten substances (excluding nicotine) were included in the aggregate index. Thus it was theoretically possible to have a score ranging from 0 day equivalents to 280 day equivalents. The sections on alcohol use disorders and nonalcohol psychoactive substance use disorders in the Structured Clinical Interview for DSM–IV Axis I Disorders – Research Version (SCID–I–RV; Reference First, Gibbon and SpitzerFirst et al, 2003) were also used at the baseline assessment and at the 6- and 12-month follow-ups to determine current and lifetime substance misuse or dependence, as well as that during the past 12 months. A modified version of the Readiness to Change Questionnaire (RCQ; Reference Heather and RollnickHeather & Rollnick, 1993) was used to assess stage of change with regard to alcohol, cannabis and amphetamines.
Psychiatric symptomatology was assessed using the Brief Psychiatric Rating Scale (BPRS; Reference Ventura, Lukoff and NuechterleinVentura et al, 1993), which was also administered at each assessment time point. Thomas et al (Reference Thomas, Donnell and Young2004) have recently reviewed the published factor analyses of the 24-item BPRS and undertaken a two-tiered analysis (exploratory and confirmatory factor analyses) of BPRS data from 640 psychiatric in-patients. Unfortunately, their four-factor solution effectively discarded over a third of the items (9/24), many of which have reasonably consistent loadings in earlier studies and also according to Ventura et al (Reference Ventura, Nuechterlein and Subotnik2000). In the interests of finding a more parsimonious solution, we factor-analysed the 1531 sets of BPRS ratings that were collected as part of the present study and a concurrent treatment study of smokers with a psychotic disorder (Reference Baker, Bucci and LewinBaker et al, 2005b ), giving a total of 427 participants who were assessed at baseline and on up to three follow-up occasions. The solution that was extracted, based on a principal-components analysis with an oblique rotation, resulted in the assignment of five items to each of four factors (with scores in the range 5–35 for each factor) as follows: factor 1, mania (motor hyperactivity, excitement, tension, distractibility, elevated mood); factor 2, dysphoria (depression, guilt, anxiety, suicidality, somatic concern); factor 3, negative symptoms (blunted affect, emotional withdrawal, motor retardation, disorientation, self-neglect); and factor 4, positive symptoms (unusual thought content, grandiosity, hallucinations, bizarre behaviour, suspiciousness). These factors are generally consistent with those reported previously (Reference Ventura, Nuechterlein and SubotnikVentura et al, 2000; Reference Thomas, Donnell and YoungThomas et al, 2004) and have acceptable reliability estimates (alpha coefficients of 0.73, 0.75, 0.70 and 0.70 respectively), with an overall reliability estimate of 0.82 for the BPRS total score (range 24–168).
At each assessment time point, the Beck Depression Inventory–II (BDI–II; Beck et al, Reference Beck, Steer and Garbin1988, Reference Beck, Steer and Brown1996) was also employed to measure severity of depression during the past 2 weeks, and the Global Assessment of Functioning (GAF; American Psychiatric Association, 1994) was used to measure overall functioning. On all scales that measure alcohol and/or other drug use and psychiatric symptomatology, higher scores indicate poorer functioning, except for the GAF, in which higher scores indicate better functioning.
Components of the intervention
The treatment was manualised (Reference Baker, Bucci and Kay-LambkinBaker et al, 2004) and consisted of 10 weekly, 1-hour sessions (motivational interviewing in sessions 1 to 4 and CBT in sessions 5 to 10), with the last two sessions concentrating on relapse prevention for substance use and mental health problems. A treatment contract was established early in the intervention, and this outlined both therapist and participant expectations. A therapist checklist, adapted from the National Institute on Drug Abuse (Reference SchusterSchuster, 1989), was completed at the end of each treatment session to monitor therapist adherence to core treatment components. The three therapists were state-registered psychologists with a minimum of 2 years’ postgraduate clinical training, who received training and weekly clinical supervision from A.B.
Motivational interviewing
Treatment sessions commenced with motivational interviewing the week after the baseline assessment. The therapists followed the four general principles outlined by Miller & Rollnick (Reference Miller and Rollnick2002), namely expressing empathy, developing discrepancy, rolling with resistance and supporting self-efficacy. Feedback was given with regard to current levels of alcohol and/or other drug use and the possible interaction with symptoms. Information was delivered interactively with regard to current substance use and safer consumption levels, covering each problematic substance used (except for nicotine). Participants were asked to complete self-monitoring records (Reference Jarvis, Tebbutt and MattickJarvis et al, 1995) of their symptoms and alcohol and/or other drug use to prepare them for the subsequent transition to CBT. Therapists also completed a case formulation sheet in collaboration with the participant. When a participant had demonstrated that they had arrived at the ‘determination’ or ‘action’ stage of change (Reference Prochaska, DiClemente, Miller and HeatherProchaska & DiClemente, 1986), the cognitive–behavioural component of the intervention commenced.
CBT
An agenda was set at the beginning of each session, and homework from the previous week's session was reviewed before continuing with the CBT goals for that session. The material that was covered during sessions was applied flexibly according to the needs of each individual, and included the following: presenting the rationale for CBT and the process of therapy; the cognitive model of problematic substance use and psychotic symptoms (Reference Graham, Copello and BirchwoodGraham et al, 2004); specific techniques for managing alcohol and/or other drug use and symptoms more effectively; and identification of situational triggers and beliefs that could lead to substance use and exacerbation of psychotic symptoms (Reference Jarvis, Tebbutt and MattickJarvis et al, 1995; Reference Graham, Copello and BirchwoodGraham et al, 2004). Finally, the identification and avoidance of high-risk situations (Reference Monti, Abrams and KaddenMonti et al, 1989) that could lead to maintenance of substance use were explored, and various coping strategies were practised in the form of role-plays. Other topics included the following: discussion of seemingly irrelevant decisions (Reference Monti, Abrams and KaddenMonti et al, 1989); problem-solving strategies (Reference Jarvis, Tebbutt and MattickJarvis et al, 1995); identification and management of ‘unhelpful’ patterns of thinking (Reference Graham, Copello and BirchwoodGraham et al, 2004); management of cravings, the abstinence/rule violation effect and drink/drug refusal skills (Reference Monti, Abrams and KaddenMonti et al, 1989); and lifestyle issues. The final two sessions focused on strategies for relapse prevention (Reference Marlatt and GordonMarlatt & Gordon, 1998).
Treatment as usual
Participants were informed that they were using substances at above the recommended levels. They received a self-help booklet on substance use (Centre for Education and Information on Drugs and Alcohol, 2000), and were encouraged to maintain or increase their contact with local health services.
Statistical analysis
Data were analysed using SPSS for Windows (version 12.0). For the continuous outcome variables (e.g. alcohol, cannabis, amphetamine use), analysis of variance (ANOVA)-based planned comparisons were used to examine differences between groups and patterns of change across assessment time points. Categorical variables were analysed using chi-squared tests. As a partial control for the number of statistical tests, the threshold for significance was set at P<0.01.
RESULTS
Baseline characteristics of participants
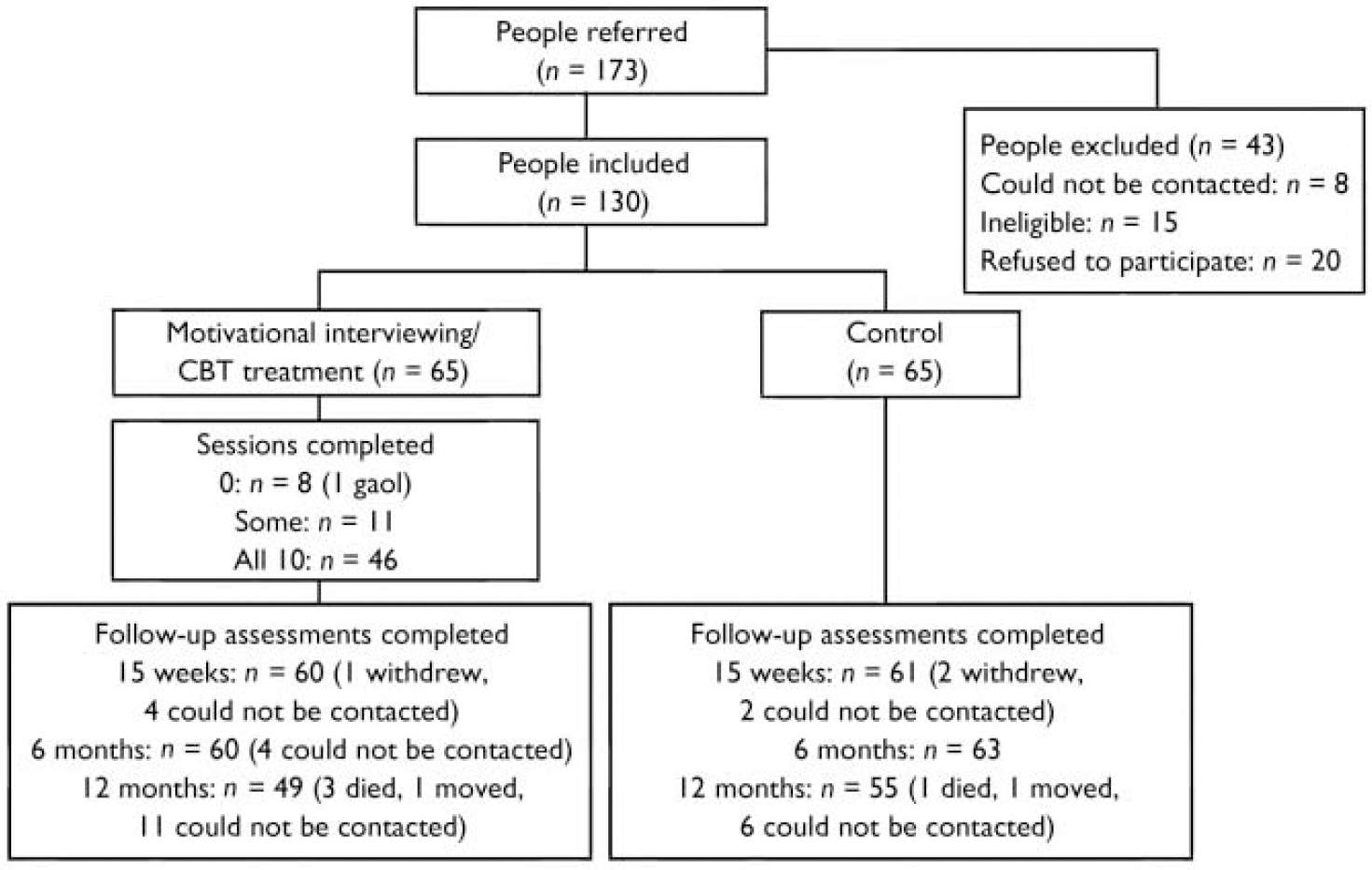
Overall recruitment and attrition profiles are presented in Fig. 1. The recruited sample consisted of 130 individuals with an ICD–10 psychotic disorder and coexisting problems with alcohol, cannabis and/or amphetamine use (hazardous levels). The baseline (pre-treatment) sample characteristics and patterns of substance use have been reported elsewhere (Reference Baker, Bucci and LewinBaker et al, 2005b ). Of those individuals who met the intervention threshold criteria for alcohol use at baseline, 37.7% were at the precontemplation stage of change and 26.4% were at the contemplation stage, based on responses to the RCQ (Reference Heather and RollnickHeather & Rollnick, 1993). The corresponding baseline rates for the other substances indicated somewhat higher levels of motivation to change (cannabis: pre-contemplation, 25.0%; contemplation, 48.8%; amphetamine: pre-contemplation, 13.6%; contemplation, 50.0%).
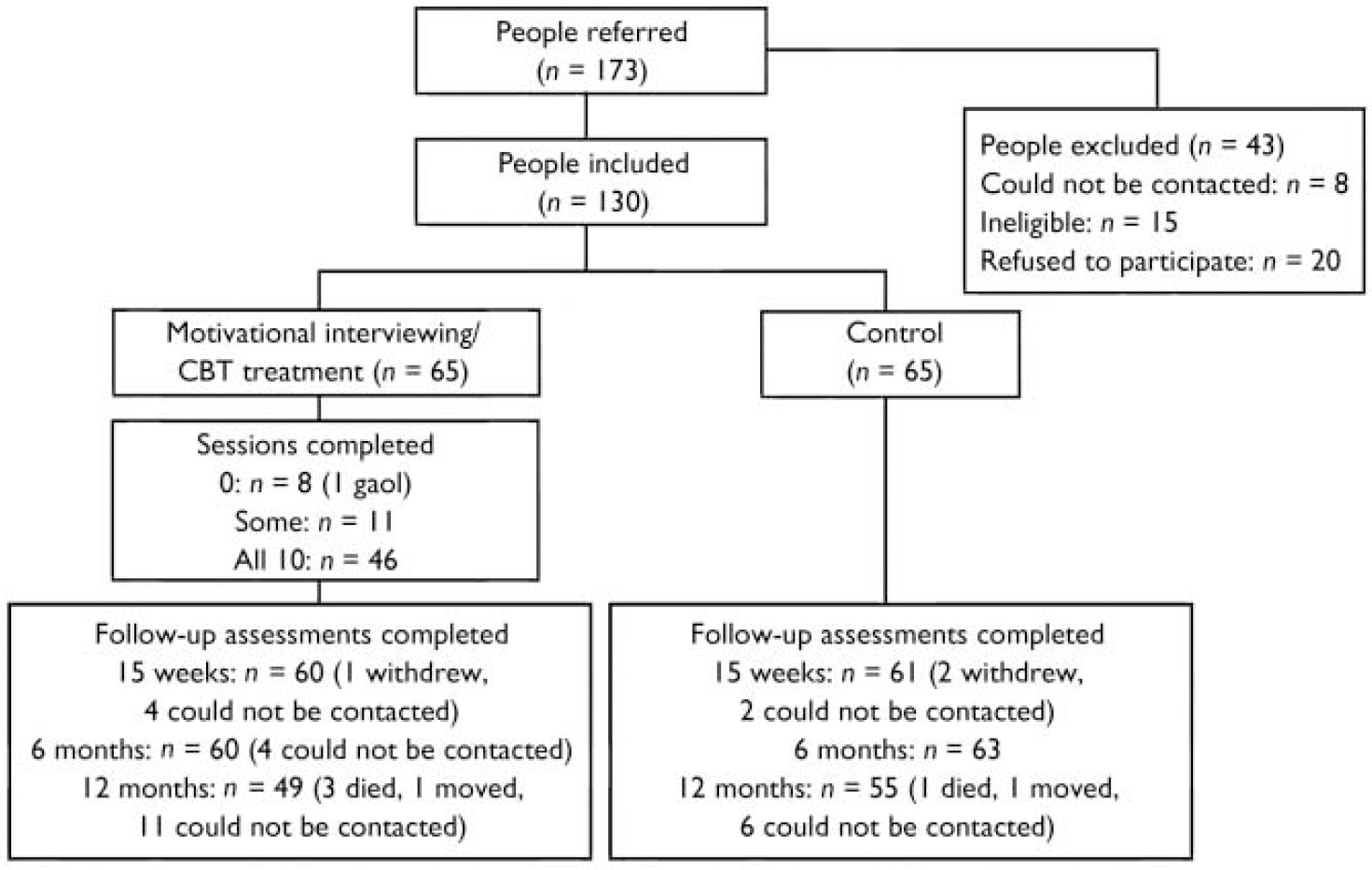
Fig. 1 Recruitment and attrition profiles. CBT, cognitive–behavioural therapy.
The demographic and clinical characteristics of the participants who completed the first three assessments (n=119) are shown in Table 1 (58 treatment group and 61 control group participants). The mean age was 28.83 years and the majority of the participants in the sample were male (78.2%), born in Australia (90.8%), single (78.2%) and receiving welfare support (88.2%). Schizophrenia was the primary diagnosis (62.2%), and the majority of the sample met the criteria for lifetime or past 12 months’ alcohol and cannabis misuse or dependence, whereas 42.0% of the sample reported amphetamine misuse or dependence in the past 12 months. The intervention thresholds for current substance use were met by 43.7% for alcohol (treatment group, 21/58; control group 31/61), 61.3% for cannabis (treatment group, 39/58; control group, 34/61) and 16.8% for amphetamine (treatment group, 11/58; control group, 9/61). More than half of the sample had experienced a psychosocial stressor before the onset of their disorder. The majority of the participants (67.7%) used antipsychotic medication, which most of them (82.9%) reported to be helpful. Approximately two-thirds of the participants had had at least one hospital admission within the past 12 months.
Table 1 Characteristics of participants who completed the baseline, post-treatment and 6-month follow-up phases of the study (n=119)
| Participant characteristics | |
|---|---|
| Demographic factors | |
| Age (years): mean (s.d., range) | 28.83 (10.27, 15-61) |
| Male (%) | 78.2 |
| Born in Australia (%) | 90.8 |
| Single, never married | 78.2 |
| Age on leaving school (years): mean (s.d., range)1 | 16.01 (1.50, 10-20) |
| Post-school qualifications obtained (%) | 65.5 |
| Receiving welfare support (%) | 88.2 |
| ICD—10 primary diagnosis | |
| Severe depression with psychosis (%) | 4.2 |
| Bipolar, mania (%) | 9.2 |
| Schizophrenia (%) | 62.2 |
| Schizoaffective disorder (%) | 12.6 |
| Other psychosis (%) | 11.8 |
| SCID—I diagnosis (%): abuse (only)/dependence | |
| Alcohol | |
| Past 12 months (%) | 11.8/55.5 |
| Lifetime (%) | 13.4/72.3 |
| Cannabis | |
| Past 12 months (%) | 7.6/65.5 |
| Lifetime (%) | 6.7/82.4 |
| Amphetamine | |
| Past 12 months (%) | 10.1/31.9 |
| Lifetime (%) | 10.1/43.7 |
| Patterns of substance use (OTI for past month) | |
| Alcohol status: ≥hazardous use (NHMRC) (%) | 43.7 |
| Cannabis status: ≥weekly use (%) | 61.3 |
| Amphetamine status: ≥weekly use (%) | 16.8 |
| Illness factors | |
| Family history of schizophrenia (%) | 36.1 |
| Psychosocial stressor before onset of illness (%) | 60.5 |
| Course of psychotic disorder | |
| Single episode, good or unknown recovery (%) | 19.3 |
| Multiple episodes, good recovery (%) | 41.2 |
| Multiple episodes, minimal recovery or deterioration (%) | 30.3 |
| Chronic, clear deterioration (%) | 9.2 |
| Medication | |
| Use of antipsychotic medication (%) | 67.7 |
| Antipsychotic medication helpful (%) | 82.9 |
| Age at onset of illness (years): mean (s.d., range) | 19.32 (6.70, 5-38) |
| Service utilisation (past 12 months) | |
| Hospital admissions (past 12 months) | |
| At least one admission (%) | 62.3 |
| Number of admissions: mean (s.d., range)2 | 1.03 (1.22, 0-6) |
| Length of admission (days): mean (s.d., range)3 | 28.91 (22.95, 7-105) |
Treatment attendance and completion of follow-up
In the treatment group 8 out of 65 participants (12.3%) did not attend any sessions, 11 (16.9%) attended some sessions and 46 (70.8%) attended all 10 sessions. Approximately a fifth of those who completed more than half the treatment sessions (9 out of 50 participants; 18.0%) required six to eight motivational interviewing sessions before making the transition to CBT. Overall, 28.3% of treatment sessions and 12.6% of assessments involved home visits, and 30.5% of follow-up assessments were conducted by telephone. There were similar patterns of attendance at the 15-week (93.1%) and 6-month (94.6%) follow-up, with the lowest participation rate occurring at the 12-month follow-up (80.0%), although attendance levels still remained high. Two separate data-sets were established to take into account these different patterns of follow-up, namely participants who completed the baseline, 15-week and 6-month assessments (n=119, 91.5%), and participants who completed all four assessments (n=97, 74.6%). There were no significant differences between groups in the pattern of completion of follow-up. In the analyses which follow, planned comparisons between the first three assessments were based on the first block (n=119), whereas comparisons between the final assessment and each of the earlier assessments were based on the second block (n=97).
Changes in substance use
Mean baseline, 15-week, 6-month and 12-month follow-up scores for the key substances are shown in Table 2 for participants who were above the relevant substance use thresholds at baseline, together with standardised differences (in effect size units) between baseline and the 12 month follow-up. It can be seen that there were significant time effects for alcohol, poly-drug use and the aggregate hazardous use index, but there were no group main effects or group×time interactions. Alcohol consumption decreased significantly for the sample as a whole, with the 15-week, 6-month and 12-month follow-up assessments all having lower OTI scores than at baseline. The reduction in alcohol consumption between baseline and the 12-month follow-up was equivalent to an overall effect size change of 0.80 units. This difference tended to be more marked for the control group (0.97) than for the treated group (0.54).
Table 2 Substance use patterns across study phases

| Group/phase | Estimated daily consumption during past month (OTI score)1 | Aggregate substance use index score (day equivalents) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohol | Cannabis | Amphetamine | Poly-drug use | ||||||||||||
| n | Mean | (s.d.) | n | Mean | (s.d.) | n | Mean | (s.d.) | n | Mean | (s.d.) | n | Mean | (s.d.) | |
| Treatment | |||||||||||||||
| Baseline | 21 | 6.15 | (4.94) | 39 | 8.18 | (7.28) | 11 | 2.39 | (3.71) | 58 | 3.17 | (1.05) | 58 | 32.03 | (17.73) |
| 15 weeks | 21 | 4.92 | (4.69) | 39 | 5.09 | (7.21) | 11 | 0.34 | (0.53) | 58 | 2.81 | (1.08) | 58 | 23.78 | (20.47) |
| 6 months | 21 | 3.73 | (4.07) | 39 | 5.37 | (11.75) | 11 | 0.19 | (0.41) | 58 | 2.60 | (1.14) | 58 | 21.34 | (18.34) |
| 12 months | 18 | 3.58 | (4.80) | 29 | 8.53 | (14.59) | 9 | 0.14 | (0.26) | 44 | 2.84 | (1.18) | 44 | 23.68 | (21.72) |
| Control | |||||||||||||||
| Baseline | 31 | 6.30 | (4.49) | 34 | 4.80 | (4.83) | 9 | 0.56 | (0.60) | 61 | 2.64 | (0.90) | 61 | 27.89 | (12.85) |
| 15 weeks | 31 | 3.35 | (4.10) | 34 | 5.66 | (8.72) | 9 | 0.25 | (0.57) | 61 | 2.44 | (1.05) | 61 | 20.28 | (17.56) |
| 6 months | 31 | 2.52 | (4.20) | 34 | 4.67 | (8.68) | 9 | 1.47 | (2.28) | 61 | 2.41 | (1.15) | 61 | 18.28 | (19.06) |
| 12 months | 28 | 2.19 | (3.04) | 29 | 4.12 | (6.51) | 8 | 0.01 | (0.25) | 53 | 2.19 | (1.12) | 53 | 16.74 | (17.00) |
| Standardised change between baseline and 12 months (effect size units)2 | |||||||||||||||
| Treatment | 18 | 0.54 | [0.52] | 29 | 0.04 | [0.14] | 9 | 1.28 | [1.00] | 44 | 0.21 | [0.31] | 44 | 0.48 | [0.43] |
| Control | 28 | 0.97 | [0.93] | 29 | 0.06 | [0.01] | 8 | 0.33 | [0.13] | 53 | 0.40 | [0.28] | 53 | 0.58 | [0.51] |
| Overall | 46 | 0.80 | [0.77] | 58 | 0.01 | [0.08] | 17 | 0.83 | [0.62] | 97 | 0.31 | [0.30] | 97 | 0.53 | [0.47] |
| Pattern of significant differences3 | Time: | Time: | Time: | ||||||||||||
| Baseline v. 15 weeks: F(1,50)=7.34* | Baseline v. 15 weeks: F(1,117)=12.48** | Baseline v. 15 weeks: F(1,117)=23.52** | |||||||||||||
| Baseline v. 6 months: F(1,50)=14.95** | Baseline v. 6 months: F(1,117)=16.19** | Baseline v. 6 months: F(1,117)=36.03** | |||||||||||||
| Baseline v. 12 months: F(1,44)=16.57** | Baseline v. 12 months: F(1,95)=9.91* | Baseline v. 12 months: F(1,95)=23.14** | |||||||||||||
There were no significant time effects for either cannabis or amphetamine use. For cannabis, there tended to be higher consumption in the treatment group than in the control group initially (8.18 v. 4.80), and there was a non-significant trend for a differential reduction in cannabis consumption between the baseline and 15-week assessments for the treatment group compared with the control group (F (1,71)=6.25, P=0.02). For this period, mean daily cannabis consumption decreased by 0.36 standardised units for the treatment group compared with -0.02 standardised units for the control group. This amounts to a differential change of 0.38 standardised units (a moderate effect size), which was not maintained at the subsequent assessments (Table 2).
For amphetamine, there was a nonsignificant trend towards a differential (baseline v. 6 months) reduction in amphetamine use in the treatment group compared with the control group (F (1,18)=4.70, P=0.04). The mean daily number of occasions of amphetamine use fell by 1.33 standardised units for the treatment group compared with -0.40 for the control group, which represents a differential change of 1.73 standardised units (a large effect size). As is shown in Table 2, this differential was less marked (0.95) for the 12-month follow-up, but was still strong. Reflecting the significant reduction in alcohol use among the whole sample, and the trends towards a change in the level of amphetamine use, there was a significant overall reduction in poly-drug use scores over time, with significant differences between baseline and each of the follow-up assessments (Table 2). A similar pattern emerged for the aggregate substance use index.
Table 3 shows the percentage of participants who remained above the alcohol, cannabis and amphetamine thresholds at each follow-up assessment, and the corresponding abstinence rates. There were no significant group differences in threshold rates or abstinence rates for any substance at any of the follow-up assessments.
Table 3 Threshold and abstinence rates at follow-up for alcohol, cannabis and amphetamine1
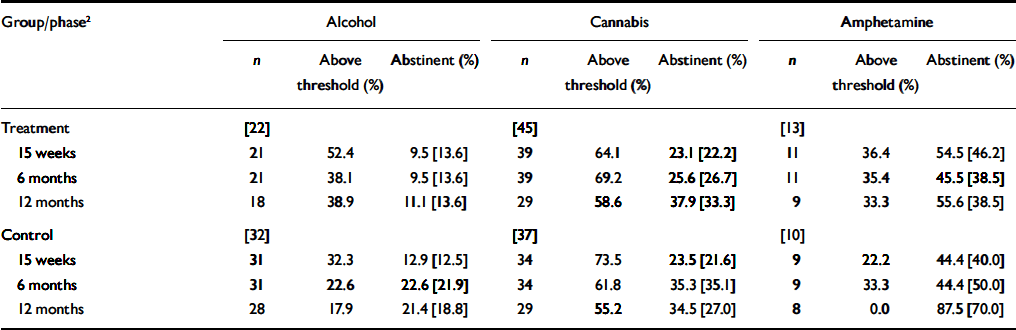
| Group/phase2 | Alcohol | Cannabis | Amphetamine | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Above threshold (%) | Abstinent (%) | n | Above threshold (%) | Abstinent (%) | n | Above threshold (%) | Abstinent (%) | |
| Treatment | [22] | [45] | [13] | ||||||
| 15 weeks | 21 | 52.4 | 9.5 [13.6] | 39 | 64.1 | 23.1 [22.2] | 11 | 36.4 | 54.5 [46.2] |
| 6 months | 21 | 38.1 | 9.5 [13.6] | 39 | 69.2 | 25.6 [26.7] | 11 | 35.4 | 45.5 [38.5] |
| 12 months | 18 | 38.9 | 11.1 [13.6] | 29 | 58.6 | 37.9 [33.3] | 9 | 33.3 | 55.6 [38.5] |
| Control | [32] | [37] | [10] | ||||||
| 15 weeks | 31 | 32.3 | 12.9 [12.5] | 34 | 73.5 | 23.5 [21.6] | 9 | 22.2 | 44.4 [40.0] |
| 6 months | 31 | 22.6 | 22.6 [21.9] | 34 | 61.8 | 35.3 [35.1] | 9 | 33.3 | 44.4 [50.0] |
| 12 months | 28 | 17.9 | 21.4 [18.8] | 29 | 55.2 | 34.5 [27.0] | 8 | 0.0 | 87.5 [70.0] |
Changes in symptomatology
Table 4 shows the symptom profiles for the intervention and control groups, together with standardised change scores between baseline and the 12 months follow-up. There was a significant improvement between baseline and the 12-month assessment on the BPRS mania factor, and between baseline and each of the follow-up assessments on the BPRS negative symptoms factor. The overall standardised change in BPRS negative symptoms between baseline and the 12-month assessment was around half a standard deviation. There were no other significant effects for the BPRS scales (i.e. for dysphoria, positive symptoms or BPRS total scores). BDI–II depression scores were also significantly lower at each of the follow-up assessments than at baseline, with a more marked reduction between baseline and the 6-month assessment for the intervention group than for the control group (0.78 v. 0.28 standardised units, or a half a standard deviation of differential impact). Although there were no main effects in the GAF analyses, there was a significant group×time interaction, with a deterioration in global functioning between baseline and the 12-month assessment for the control group, and a small improvement in the treatment group. This is reflected by the fact that the standardised change scores for this variable were negative for the treatment group, indicating an improvement in functioning. Thus the decrease of -0.15 units in the treatment group compared with 0.43 in the control group represents a differential impact of over half a standard deviation (0.58) (a moderate effect size).
Table 4 Symptom scores across study phases1
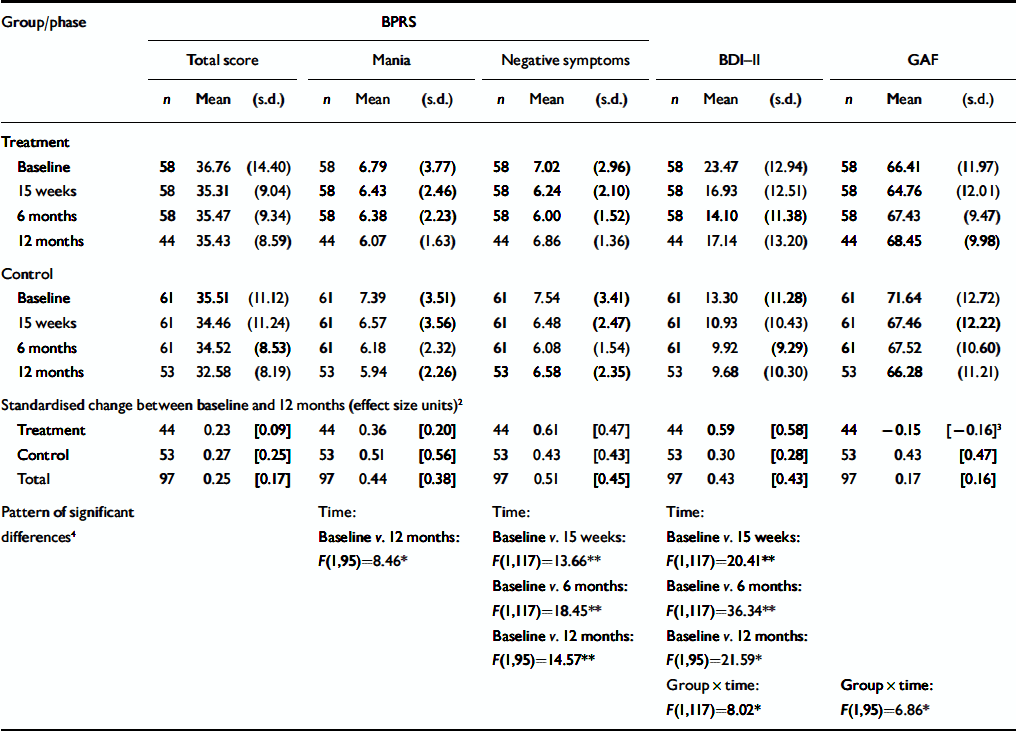
| Group/phase | BPRS | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total score | Mania | Negative symptoms | BDI—II | GAF | |||||||||||
| n | Mean | (s.d.) | n | Mean | (s.d.) | n | Mean | (s.d.) | n | Mean | (s.d.) | n | Mean | (s.d.) | |
| Treatment | |||||||||||||||
| Baseline | 58 | 36.76 | (14.40) | 58 | 6.79 | (3.77) | 58 | 7.02 | (2.96) | 58 | 23.47 | (12.94) | 58 | 66.41 | (11.97) |
| 15 weeks | 58 | 35.31 | (9.04) | 58 | 6.43 | (2.46) | 58 | 6.24 | (2.10) | 58 | 16.93 | (12.51) | 58 | 64.76 | (12.01) |
| 6 months | 58 | 35.47 | (9.34) | 58 | 6.38 | (2.23) | 58 | 6.00 | (1.52) | 58 | 14.10 | (11.38) | 58 | 67.43 | (9.47) |
| 12 months | 44 | 35.43 | (8.59) | 44 | 6.07 | (1.63) | 44 | 6.86 | (1.36) | 44 | 17.14 | (13.20) | 44 | 68.45 | (9.98) |
| Control | |||||||||||||||
| Baseline | 61 | 35.51 | (11.12) | 61 | 7.39 | (3.51) | 61 | 7.54 | (3.41) | 61 | 13.30 | (11.28) | 61 | 71.64 | (12.72) |
| 15 weeks | 61 | 34.46 | (11.24) | 61 | 6.57 | (3.56) | 61 | 6.48 | (2.47) | 61 | 10.93 | (10.43) | 61 | 67.46 | (12.22) |
| 6 months | 61 | 34.52 | (8.53) | 61 | 6.18 | (2.32) | 61 | 6.08 | (1.54) | 61 | 9.92 | (9.29) | 61 | 67.52 | (10.60) |
| 12 months | 53 | 32.58 | (8.19) | 53 | 5.94 | (2.26) | 53 | 6.58 | (2.35) | 53 | 9.68 | (10.30) | 53 | 66.28 | (11.21) |
| Standardised change between baseline and 12 months (effect size units)2 | |||||||||||||||
| Treatment | 44 | 0.23 | [0.09] | 44 | 0.36 | [0.20] | 44 | 0.61 | [0.47] | 44 | 0.59 | [0.58] | 44 | -0.15 | [-0.16]3 |
| Control | 53 | 0.27 | [0.25] | 53 | 0.51 | [0.56] | 53 | 0.43 | [0.43] | 53 | 0.30 | [0.28] | 53 | 0.43 | [0.47] |
| Total | 97 | 0.25 | [0.17] | 97 | 0.44 | [0.38] | 97 | 0.51 | [0.45] | 97 | 0.43 | [0.43] | 97 | 0.17 | [0.16] |
| Pattern of significant differences4 | Time: Baseline v. 12 months: F(1,95)=8.46* | Time: Baseline v. 15 weeks: F(1,117)=13.66** | Time: Baseline v. 15 weeks: F(1,117)=20.41** | ||||||||||||
| Baseline v. 6 months: F(1,117)=18.45** | Baseline v. 6 months: F(1,117)=36.34** | ||||||||||||||
| Baseline v. 12 months: F(1,95)=14.57** | Baseline v. 12 months: F(1,95)=21.59* | ||||||||||||||
| Group × time: F(1,117)=8.02* | Group × time: F(1,95)=6.86* | ||||||||||||||
Intention-to-treat (ITT) analyses
A series of ITT analyses was also performed that paralleled those shown in Tables 2, 3, 4. Reflecting the relatively low rate of attrition in this study (Fig. 1), there were no differences in the patterns of significance compared with those already reported. That is, all of the statistically significant planned comparisons shown in Tables 2, 3, 4 remained significant after imputation of missing data, and there were no additional effects that reached significance. To facilitate comparisons with other RCTs that have utilised ITT analyses, Tables 2, 3, 4 also show standardised differences (in effect size units) between baseline and the 12-month assessment for the ITT data-set. Similarly, Table 3 shows the ITT-based abstinence rates for each of the follow-up assessments.
DISCUSSION
The present study appears to be the first moderately sized RCT of a motivational interviewing/CBT intervention for alcohol and/or other drug use in a sample of people with psychosis. Collectively, there was little evidence of treatment-specific benefits, with no statistically significant differential improvements in substance use at the 12-month assessment (Table 2), and no significant differences in abstinence rates between the treatment and control groups (see Table 3). However, among those individuals who received the motivational interviewing/CBT intervention, there were short-term improvements in depression (differential impact at 6 months=0.50 standardised units), a similar but less marked trend with regard to cannabis use (differential impact at 3 months=0.38 standardised units), effects on general functioning (differential impact at 12 months=0.58 standardised units) and a potentially clinically important effect on amphetamine use (differential impact at 12 months=0.95 standardised units). As described below, although the overall results of this 10-session intervention were modest, they were nevertheless similar to those obtained from a longer and more complex intervention (Reference Barrowclough, Haddock and TarrierBarrowclough et al, 2001; Reference Haddock, Barrowclough and TarrierHaddock et al, 2003) in a sample of 36 patient–caregiver dyads.
Treatment benefits for alcohol and/or other drug use
Both the study by Barrowclough and colleagues (Reference Barrowclough, Haddock and TarrierBarrowclough et al, 2001; Reference Haddock, Barrowclough and TarrierHaddock et al, 2003) and the present study reported short-term benefits of intervention on substance use. At pre-treatment, their motivational interviewing/CBT group had a median of 19.1% of days on which there was abstinence from all substances, which was approximately doubled during the treatment and follow-up phases. Minimal changes in substance use were reported for the control group. In the present study, heavy users of cannabis appeared to benefit from the intervention while it was being administered, but cannabis use returned to the previous high levels once the intervention had been completed. There was also a potentially clinically important treatment benefit with regard to amphetamine use. Although it was not statistically significant, possibly owing to the small numbers of regular amphetamine users, the large effect size associated with the intervention, combined with previous evidence of the effectiveness of CBT among regular amphetamine users (Reference Baker, Lee and ClaireBaker et al, 2005a ), suggests that further studies of CBT for people with psychotic and amphetamine use disorders are needed. However, caution needs to be exercised in relation to the current findings with regard to amphetamine use, as the control group had a relatively low baseline rate of use, and therefore less opportunity to demonstrate change, but conversely they had the highest rate of abstinence at 12 months (see Tables 2 and 3).
Treatment effects for current functioning and depression
Barrowclough and colleagues (Reference Barrowclough, Haddock and TarrierBarrowclough et al, 2001; Reference Haddock, Barrowclough and TarrierHaddock et al, 2003) selected the GAF as their primary outcome measure, specifically to enable the detection of overall changes in symptoms and functioning resulting from the interaction between psychosis and substance use and the multi-component nature of their intervention. Both their study and the present one reported a differential improvement in GAF scores (rated masked) at the final follow-up (12 months in the present study and 18 months in the study by Barrowclough and colleagues, both of which occurred 9 months after treatment). In the present study, this was primarily caused by a deterioration in GAF scores in the control group, with a net change of 0.58 standardised units, whereas the net change of 0.76 units in the Barrowclough study was caused by the sustained superiority in GAF scores for the CBT group. Two RCTs have shown that intervention consisting of motivational interviewing and CBT for substance use problems in people with psychosis can affect general functioning. A modest delayed beneficial effect of CBT on GAF scores at 12 months has also been reported by Kemp et al (Reference Kemp, Kirov and Everitt1998) following a ‘compliance-therapy’ intervention. To help to clarify the relevance of these changes in functioning, future studies of interventions involving motivational interviewing and CBT should include the GAF, together with measures of symptomatology and substance use. Haddock et al (Reference Haddock, Barrowclough and Tarrier2003) also recommend that further trials should seek to identify the active and most important ingredients of successful therapy.
As we have noted previously (Reference Baker, Bucci and LewinBaker et al, 2005b ), the present sample had relatively high levels of functioning. Their average GAF score at baseline was 68.75 (s.d.=12.80, n=130), which was approximately 33% higher than that reported in the Barrowclough study (Reference Barrowclough, Haddock and TarrierBarrowclough et al, 2001; Reference Haddock, Barrowclough and TarrierHaddock et al, 2003), and 85% higher than that reported by Kemp et al (Reference Kemp, Kirov and Everitt1998) for their in-patient study. Perhaps people who present or are referred to community-based treatment studies are generally better functioning than those who are recruited directly from mental health service settings. In any event, it may not be possible to generalise the outcomes of treatment studies that are based on better functioning or more highly motivated samples to other treatment settings. Higher levels of functioning at baseline may influence engagement with treatment and retention, but may also make it more difficult to detect particular treatment benefits. For example, higher-functioning individuals with coexisting psychotic and alcohol use disorders may respond positively to the assessment process and to advice to reduce substance use, within the context of ongoing monitoring.
Barrowclough and colleagues (Reference Barrowclough, Haddock and TarrierBarrowclough et al, 2001; Reference Haddock, Barrowclough and TarrierHaddock et al, 2003) also reported significant benefits of intervention compared with routine care at the 12-month follow-up with regard to positive symptoms and relapse rates, and at the 9-, 12- and 18-month follow-up with regard to negative symptoms. As noted previously, there was a relatively low rate of psychotic symptoms in the sample in the present study (Reference Baker, Bucci and LewinBaker et al, 2005b ). There was a reduction in negative symptoms (and to a lesser extent in mania scores) across the sample as a whole in this study. The observed initial improvement in depression in the treatment group is likely to have been a result of either the generalisation of cognitive and behavioural strategies for substance use to low mood, or the nonspecific support received when attending therapy sessions. The possible non-specific effect of CBT for substance use on depression has previously been noted by us in a study of regular amphetamine users (Reference Baker, Lee and ClaireBaker et al, 2005a ). Thus it appears that people with concurrent depression and substance use disorders (whether or not these are accompanied by psychosis) may derive at least short-term benefits in terms of mood from CBT for substance use disorder.
Possible effects of participation in the study
There were significant improvements over time in the sample as a whole with regard to alcohol consumption, poly-drug use and score on the aggregate substance use index. Similar improvements in alcohol use were reported for the sample as a whole in the study of psychiatric in-patients by Baker et al (Reference Baker, Lewin and Reichler2002). Hulse & Tait (Reference Hulse and Tait2003) also reported that, compared with matched controls, general hospital psychiatric inpatients (10% of whom had psychosis) who received either a motivational interview or an information pack had significantly fewer mental health in-patient episodes and showed other health benefits. The authors of that study suggested that information together with the research process (assessment, etc.) and psychiatric treatment may be sufficient to bring about change.
The need for alternative approaches
Taken together, the findings of the present study, previous RCTs (Reference Barrowclough, Haddock and TarrierBarrowclough et al, 2001; Reference Haddock, Barrowclough and TarrierHaddock et al, 2003) and recent reviews of the literature on this treatment outcome (Reference Kay-Lambkin, Baker and LewinKay-Lambkin et al, 2004; Reference Baker and DaweBaker & Dawe, 2005) suggest that a more complex framework is needed which integrates the available evidence into a coherent treatment and research strategy. A stepped-care approach to treatment is one such framework, within which a series of tiered interventions are applied, with less intensive treatments being offered first, and more intensive targeted treatments being made available contingent on the client's response to the previous tier of treatment (Reference Schippers, Schramade and WalburgSchippers et al, 2002; Reference Baker and DaweBaker & Dawe, 2005). Stepped-care approaches have been tested in a number of different settings, including depression (Reference Scogin, Hanson and WelshScogin et al, 2003), anxiety (Reference Baillie and RapeeBaillie & Rapee, 2004), alcohol problems (Reference Sobell and SobellSobell & Sobell, 2000), smoking (Reference Smith, Infante and AliSmith et al, 2001) and heroin dependence (Reference King, Stoller and HayesKing et al, 2002).
The excellent therapy-attendance figures attest to the beneficial experiences of participants in therapy. Approximately 70% of the present sample attended all 10 therapy sessions, and the median attendance in the study by Barrowclough et al (Reference Barrowclough, Haddock and Tarrier2001) was 22 sessions. Clearly, this challenging client group is able to engage in CBT and appears to derive benefit from it. By examining changes in the percentage of participants who remain above the initial intervention thresholds for substance use (Table 3), we can also gain insight into the intensity of interventions that may be required. For example, in the control group more than two-thirds of those who met the intervention threshold criteria for alcohol or amphetamine use were already below those thresholds at the 15-week follow-up. Such findings reinforce the available research evidence which suggests that even minimal ‘control’ interventions (including assessment alone) can result in significant changes. For some people, giving brief advice within the context of ongoing assessment and monitoring may be sufficient to stimulate the initiation of changes in life circumstances. For others, specific therapy programmes may be required. For example, in both the present study and our previous study of psychiatric in-patients (Reference Baker, Lewin and ReichlerBaker et al, 2002), more than 50% of cannabis users remained above the intervention threshold at the 12-month follow-up.
Limitations
Finally, there are several study limitations that need to be considered.
It is acknowledged that there are several different analytical strategies for assessing change, each with its own particular advantages and disadvantages, ranging from simple change scores (e.g. paired t-tests or repeated-measures ANOVAs) and other more complex linear combinations (e.g. polynomial trend contrasts) to analyses of covariance (ANCOVAs) in which, for example, baseline scores are controlled when assessing differences at follow-up (e.g. Reference Vickers and AltmanVickers & Altman, 2001). On the one hand, analyses that are based on traditional change scores may ignore variance (in change) that is associated with baseline levels, leading to treatment estimates that have higher variability, in essence valuing one unit of change as the same across the full range of scores. On the other hand, when baseline differences are real (e.g. naturally occurring groups), ANCOVAs may introduce directional bias, magnifying post-baseline differences in one direction and masking those in the other (Jamieson, Reference Jamieson1999, Reference Jamieson2004). However, it is clear that decisions about the basic choice of analysis strategy should be made without reference to the data collected (Reference JamiesonJamieson, 1999). In the present study, we opted for a traditional score-change-based approach, in the form of planned comparisons between blocks of assessment time points from repeated-measures ANOVAs, where the primary focus is on group×time interaction comparisons. We also planned and conducted preliminary baseline analyses of key (non-outcome) variables to determine their likely suitability as conventional covariates. In this instance there were no significant differences between the treatment and control groups with regard to key socio-demographic or clinical characteristics (e.g. age, gender, level of education, marital status, illness onset or course, family history), and therefore no covariates were used.
In circumstances such as those of the present study, where there are several possible bases for study entry (e.g. separate thresholds for alcohol, cannabis and amphetamine use) and a range of outcomes of interest (e.g. substance use, symptomatology, general functioning), it becomes increasingly difficult to assume that post-randomisation baseline differences between groups (across all of these outcome measures) are essentially caused by measurement error (i.e. they are not real), and are consequently appropriate for inclusion in an ANCOVA-based strategy for assessing change. One solution might have been to use a complex, stratified randomisation procedure, taking account of baseline levels across all (or most) of the key outcome variables when making initial group allocations, but this was not done in the present study.
The present study was primarily concerned with treatment efficacy – that is, whether or not the actual treatments received were associated with the desired outcomes among the individuals who completed the study, while noting and/or adjusting for any observed or likely recruitment, allocation or participation bias. Arguably, treatment efficacy needs to be demonstrated first, followed by attempts to optimise treatment implementation and effectiveness in real-world settings. However, to facilitate comparison with other RCTs, we also conducted a parallel series of traditional intention-to-treat (ITT) or programme-effectiveness analyses (Reference Wright and SimWright & Sim, 2003). For these analyses, missing follow-up data were imputed by carrying forward the last available observation.
We did not evaluate the psychometric properties of the key self-report or clinician-rated measures within the present study sample (in particular interrater reliability). However, the OTI has features similar to those of other structured interviews, and has been found to have acceptable validity (Reference Darke, Hall and WodakDarke et al, 1992), while the BPRS (Reference Ventura, Lukoff and NuechterleinVentura et al, 1993) and the BDI (Reference Beck, Steer and GarbinBeck et al, 1988) have well-established properties. Likewise, interrater reliability on the GAF was not measured, but it has been documented by Startup et al (Reference Startup, Jackson and Bendix2002) and found to be satisfactory. Similarly, there was no formal assessment of breaks in masking. However, this was unlikely to have been a problem, as the clinicians who conducted the follow-up interviews reported that the participants appreciated the importance of the request not to disclose their group allocation. The absence of a supportive counselling or other non-specific control condition means that we cannot determine the extent to which any of the benefits were primarily a result of contact with a therapist. Furthermore, the therapy sessions were not tape-recorded. However, a therapist checklist was completed at the end of each treatment session. Direct ratings of therapist adherence to the treatment manual should probably be included in future studies. Another area of possible concern is the representativeness of the sample. Relative to the study by Barrowclough et al (Reference Barrowclough, Haddock and Tarrier2001), there were differences in the level of current functioning and in the nature and duration of the interventions. However, despite differences in sampling strategies and in the interventions that were delivered, there were broad similarities in the findings. Recruitment and retention of sufficiently large samples are always a methodological concern. In addition, studies such as the present one and that of Barrowclough et al (Reference Barrowclough, Haddock and Tarrier2001) typically have lower statistical power to detect differences among users of particular substances than overall treatment effects on aggregate indexes of substance use, as is illustrated by the uncertainties associated with the small numbers of regular amphetamine users in the present study. Finally, although we would encourage clinicians to use the treatment manual prepared for this study (Reference Baker, Bucci and Kay-LambkinBaker et al, 2004), further research is needed to develop more effective motivational interviewing/CBT interventions for people with psychosis who are heavy users of substances, especially cannabis, and to extend these interventions to young people with mental health problems who have not yet progressed to substance dependence.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Over two-thirds of the present sample of people with psychotic disorders who were assigned to a motivational interviewing/CBT intervention for substance use attended all 10 treatment sessions.
-
▪ There is a short-term improvement in depression and a similar trend with regard to cannabis use among individuals who receive a motivational interviewing/CBT intervention, together with improved general functioning after 12 months.There is no differential beneficial effect of the intervention on substance use after 12 months, except for a potentially clinically important effect on amphetamine use.
-
▪ Assessment and brief advice in the context of ongoing monitoring appear to have an overall beneficial effect, particularly on alcohol consumption, prompting calls for a consideration of alternative approaches such as stepped care.
LIMITATIONS
-
▪ There was no control for the extra therapy time associated with the motivational interviewing/CBT intervention, the therapy sessions were not recorded, and interrater reliability was not assessed.
-
▪ At recruitment, only a small number of participants were currently using amphetamines.
-
▪ The relatively high levels of functioning in the present sample may have compromised the generalisability of the study findings.
Acknowledgements
We thank the study participants, Hunter Mental Health, the ward and medical staff at James Fletcher Hospital, and the Psychological Assistance Service (PAS) for their enthusiasm and support. We would also like to thank the Neuroscience Institute of Schizophrenia and Allied Disorders (NISAD) Schizophrenia Research Register, Australia, for assisting with the recruitment of the volunteers who participated in this research.
This research was funded by the National Health and Medical Research Council (NHMRC) (grant number 100967).







eLetters
No eLetters have been published for this article.