The mechanism of action of electroconvulsive therapy (ECT) remains unclear despite its status as a highly effective treatment for major depressive episodes. Studies of ECT have focused on the serotonergic and noradrenergic systems, Reference Fochtmann1 but without conclusive findings. Two probes explored in studying mechanisms of antidepressant drug response are indoleamine depletion Reference Delgado, Charney, Price, Aghajanian, Landis and Heninger2 and catecholamine depletion. Reference Miller, Delgado, Salomon, Berman, Krystal and Heninger3 Individuals with depression treated successfully with noradrenergic antidepressants tended to transiently relapse with catecholamine depletion, whereas those treated successfully with serotonergic antidepressants tended to transiently relapse with indoleamine depletion. We have shown that neither indoleamine depletion nor catecholamine depletion, given alone, led to signs of relapse in individuals soon after successful treatment with ECT. Reference Cassidy, Weiner, Cooper and Carroll4,Reference Cassidy, Murry, Weiner and Carroll5
It is possible that ECT operates through multiple neurotransmitter systems. Moreover, these neurotransmitter systems are known to interact with each other. For example, a compensatory increase in serotonergic function may have attenuated or blocked a response to catecholamine depletion, Reference Gobert, Rivet, Audinot, Newman-Tancredi, Cistarelli and Millan6 particularly if the mechanism of ECT were to relate to both systems. We therefore conducted a double catecholamine and indoleamine depletion study in individuals with depression.
Method
Seven male and two female in-patients (mean age 50.4 years (s.d. = 11.3), range 30–70) initially meeting DSM–IV criteria for major depressive episode, 7 four with psychotic features, and who had responded to courses of bilateral ECT were included. Each gave written informed consent for participation in the study, which was approved by both the institutional review board at Duke University Medical Center and the research committee at John Umstead Hospital. The mean Montgomery–Åsberg Depression Rating Scale (MADRS) Reference Montgomery and Åsberg8 score prior to ECT was 35.0 (s.d. = 5.4, range 26–43); ECT was administered as described previously Reference Cassidy, Murry, Weiner and Carroll5 (mean number of treatments received 10.4, s.d. = 3.9, range 7–18).
The study used a double-blind, counterbalanced, placebo-controlled design over two 4-day periods commencing after the next to last and the last ECT treatments. In each 4-day period, Day 1 was designated pre-baseline. Day 2 was designated baseline: the catecholamine depletion protocol was commenced. On Day 3, the catecholamine depletion protocol was continued, and indoleamine depletion was added to achieve combined depletion. The definitive behavioural and biochemical assessments were performed at 15.00 h on Day 3. Day 4 was designated a follow-up day. Consistent with clinical practice when the study was performed, participants remained off psychotropic medication throughout the ECT treatments, and throughout this study.
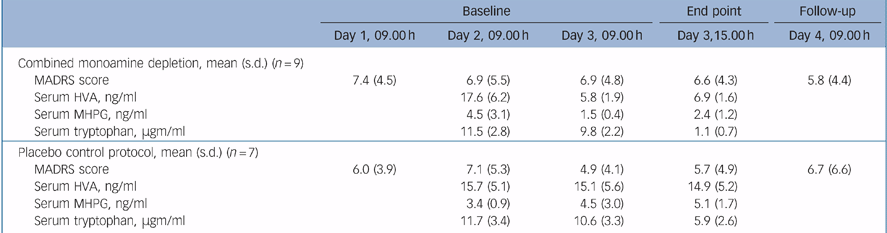
Participants were evaluated at 09.00 h each day with the MADRS. Stability of MADRS scores from Day 1 to Day 2 was required. Serum was collected on Day 2 (baseline) at 09.00 h for measurement of homovanillic acid (HVA), 3-methoxy-4-hydroxyenylethyleneglycol (MHPG) and tryptophan levels. Catecholamine depletion was effected with alpha-methyl-p-tyrosine (AMPT) 1 g orally at 09.00 h, 12.00 h and 15.00 h on Day 2, and at 09.00 h and 12.00 h on Day 3. For the placebo condition, we gave 50 mg diphenhydramine as an active placebo at the same times to mask the sedation associated with AMPT. On Day 3, serum was obtained again at 09.00 h for measurement of HVA, MHPG and tryptophan levels. To achieve indoleamine depletion, individuals who had received active AMPT were given a full-strength tryptophan-free amino acid mixture Reference Cassidy, Murry and Carroll9 at 09.00 h on Day 3. Participants who had received the active placebo were given a quarter-strength tryptophan-free amino acid mixture to preserve masking. To further limit tryptophan availability, individuals fasted on Day 3 until after the completion of the afternoon ratings. At 03.00 h on Day 3, MADRS ratings were completed to document the primary end-point, and serum was again obtained. Within the next 3 days, participants received their final ECT treatment, and the study was repeated with the alternate active or placebo protocol. They were given a low monoamine diet during the study. Tryptophan was assayed by high-performance liquid chromatography, and HVA and MHPG were assayed as described elsewhere. Reference Fri, Wiesel and Sedvall10 Insufficient serum was available for three of the MHPG samples.
Changes in MADRS scores at depletion end-point (15.00 h on Day 3) compared with post-ECT baseline (09.00 h on Day 2) were analysed to compare the effect of combined catecholamine and indoleamine depletion with the effect of the placebo protocol on mood state using t-tests. Each individual served as their own control. Two participants who completed only the active arms of the study were excluded from this analysis. Their MADRS scores at baseline and at the depletion end-point were 1 and 1 respectively for the one person, and 12 and 13 for the other.
Results
Mean post-ECT baseline MADRS scores (7.0, s.d. = 4.4) were reduced in comparison to pre-ECT scores (mean = 35.0, s.d. = 5.4, t = 10.7, d.f. = 8, P<0.001). Following the administration of AMPT and the full-strength amino acid load, mean serum HVA levels decreased 60.8% and MHPG level decreased 46.6% from baseline to the combined depletion end-point, while the mean serum tryptophan level drawn immediately prior to the active 100% amino acid load decreased 88.8% at the combined depletion end-point (Table 1). A substantial 67% decrease in both mean serum HVA levels and mean serum MHPG were observed following 24 hours of AMPT alone (Day 3, 09.00 h), compared with mean levels at baseline (Day 2, 9.00 h; Table 1). No clinical changes were noted during the combined catecholamine/indoleamine depletion in any individual. Pairs of MADRS scores at baseline and at the depletion end-point during active depletion were: 5, 6; 3, 4; 14, 13; 0, 2; 8, 5; 5, 6; 11, 9; 1, 1; and 12, 13. Change in MADRS scores during depletion were not significant (t = 0.755 d.f. = 12, 95% CI 3.477 to –0.905). Analysis of MADRS scores also indicated neither period (t = 2.012, d.f. = 12, 95% CI 3.915 to –0.201) nor carry-over (t = 1.779, d.f. = 12, 95% CI 2.862 to –3.528) effects. Secondary analyses of change in MADRS scores from baseline (Day 2, 9.00 h) following 1 day of AMPT or active placebo (Day 3, 9.00 h) revealed no significant differences between experimental interventions (t = 0.988, d.f. = 12, 95% CI 5.9826 to –1.4106). Neither period (t = 1.329, d.f. = 12, 95% CI 6.208 to –1.064) nor carry-over (t = 1.003, d.f. = 12, 95% CI 6.406 to –7.906) effects were observed.
Table 1 Mean Montgomery–Åsperg Depression Rating Scale (MADRS) scores and biochemical assessments during depletion and active placebo
| Baseline | End point | Follow-up | |||
|---|---|---|---|---|---|
| Day 1, 09.00 h | Day 2, 09.00 h | Day 3, 09.00 h | Day 3, 15.00 h | Day 4, 09.00 h | |
| Combined monoamine depletion, mean (s.d.) (n = 9) | |||||
| MADRS score | 7.4 (4.5) | 6.9 (5.5) | 6.9 (4.8) | 6.6 (4.3) | 5.8 (4.4) |
| Serum HVA, ng/ml | 17.6 (6.2) | 5.8 (1.9) | 6.9 (1.6) | ||
| Serum MHPG, ng/ml | 4.5 (3.1) | 1.5 (0.4) | 2.4 (1.2) | ||
| Serum tryptophan, μgm/ml | 11.5 (2.8) | 9.8 (2.2) | 1.1 (0.7) | ||
| Placebo control protocol, mean (s.d.) (n = 7) | |||||
| MADRS score | 6.0 (3.9) | 7.1 (5.3) | 4.9 (4.1) | 5.7 (4.9) | 6.7 (6.6) |
| Serum HVA, ng/ml | 15.7 (5.1) | 15.1 (5.6) | 14.9 (5.2) | ||
| Serum MHPG, ng/ml | 3.4 (0.9) | 4.5 (3.0) | 5.1 (1.7) | ||
| Serum tryptophan, μgm/ml | 11.7 (3.4) | 10.6 (3.3) | 5.9 (2.6) | ||
Discussion
Combined functional blockade of catecholaminergic and serotonergic systems had no effect on maintenance of an acute response to ECT. This result augments our findings from single blockade of these systems. Viewed collectively, these studies do not support a major role of catecholamine or serotonin, either alone or through a synergistic mechanism, in maintaining the early response to ECT in major depression. The combined depletion strategy makes it unlikely that homeostatic compensatory effects between noradrenergic and serotonergic systems prevented relapses in our earlier studies of single amine depletion. Although sample size was limited in this study, the mean rating scores (Table 1) suggest that if a statistically significant difference were noted between combined amine depletion and the placebo protocol, the magnitude of the effect would be clinically trivial.
Our findings bear on the issue of relapse after successful ECT. Despite the efficacy of ECT in major depression, relapse rates remain unacceptably high, even with the use of maintenance ECT. Relapse rates about 24–28 weeks following ECT for a depressive episode have been reported at 80–84% on no prophylactic medication, Reference Sackeim, Haskett, Mulsant, Thase, Mann and Pettinati11 60% on lithium alone, 37–39% on lithium and nortriptyline, Reference Sackeim, Haskett, Mulsant, Thase, Mann and Pettinati11,Reference Kellner, Knapp, Petrides, Rummans, Husain and Rasmussen12 and 37% with maintenance ECT. Reference Kellner, Knapp, Petrides, Rummans, Husain and Rasmussen12 Our results suggest that the classical catecholamine and indoleamine neurotransmitter systems are not of primary importance for maintaining an acute response to ECT. New approaches to maintenance therapy are, therefore, needed.




eLetters
No eLetters have been published for this article.