Diffusion tensor imaging studies of the brain have shown that water diffusion anisotropy in white matter changes throughout life. Reference Bonekamp, Nagae, Degaonkar, Matson, Abdalla and Barker1–Reference Charlton, Barrick, McIntyre, Shen, O'Sullivan and Howe5 During normal brain development, these changes are considered to reflect the degree of neuronal maturation and organisation of white matter tracts. Reference Beaulieu3 The application of diffusion tensor imaging in the study of schizophrenia can therefore inform of developmental changes in white matter associated with this disorder.
Normal cortical development follows a back-to-front pattern starting at the parietal lobes and progressing towards frontal and temporal regions. Reference Thompson, Vidal, Giedd, Gochman, Blumenthal and Nicolson6 In childhood- and adolescent-onset schizophrenia (early-onset schizophrenia) the timing of grey matter volume deficits appears to follow the same pattern being initially evident in the parietal cortex. Reference Thompson, Vidal, Giedd, Gochman, Blumenthal and Nicolson6,Reference Burke, Androutsos, Jogia, Byrne and Frangou7 The prefrontal and temporal deficits seen in individuals with adult onset Reference Shenton, Dickey, Frumin and McCarley8 do not emerge in early-onset schizophrenia until these individuals move into adulthood. Reference Greenstein, Lerch, Shaw, Clasen, Giedd and Gochman9 It remains unclear whether the timing of white matter abnormalities is similarly influenced by age at onset. In keeping with the pattern seen for grey matter, we have reported parietal fractional anisotropy reductions in adolescents with schizophrenia. Reference Kyriakopoulos, Vyas, Barker, Chitnis and Frangou10 However, other studies in early-onset schizophrenia have found reduction in fractional anisotropy in frontal regions, Reference Kumra, Ashtari, McMeniman, Vogel, Augustin and Becker11,Reference Kumra, Ashtari, Cervellione, Henderson, Kester and Roofeh12 in the hippocampal–prefrontal connections Reference White, Kendi, Lehericy, Kendi, Karatekin and Guimaraes13 and in the left inferior temporal and occipital white matter as well as in the left inferior longitudinal fasciculus. Reference Ashtari, Cottone, Ardekani, Cervellione, Szeszko and Wu14 To date, the most widespread white matter fractional anisotropy reductions in early-onset schizophrenia have been reported by Douaud et at Reference Douaud, Smith, Jenkinson, Behrens, Johansen-Berg and Vickers15 in the corticospinal and corticopontine tracts, the left optic radiation, the corpus callosum, the left arcuate fasciculus and white matter tracts in the brainstem. Diffusion tensor imaging studies in people with adult-onset schizophrenia have generally reported reductions in fractional anisotropy in areas corresponding to the major fasciculi connecting the frontal, temporal and parietal cortices, although there is a significant degree of variation between studies in the distribution of these changes. Reference Ardekani, Nierenberg, Hoptman, Javitt and Lim16–Reference Shergill, Kanaan, Chitnis, O'Daly, Jones and Frangou22
Methodological parameters that may contribute to the diversity of findings include differences in acquisition (such as the b-value and number of directions used to estimate the diffusion tensor) and analysis (including the use of regions of interest (ROIs), tractography and group mapping approaches) and (particularly for the latter) in statistical approach, specifically relating to the use of smoothing and of parametric v. non-parametric statistical tests. However, it is possible that the effect of the disorder on white matter may depend on the age (and the stage of brain development) at which it begins. The aim of our study was to use diffusion tensor imaging to assess the effect of the age at onset of schizophrenia on the pattern of white matter abnormalities. We hypothesised that both individuals with adolescent and adult onset would show reduced fractional anisotropy in white matter compared with age-matched healthy controls, but that adolescent-onset schizophrenia would be particularly associated with abnormalities in the parietal white matter, whereas the findings in the adult-onset group would be more widely distributed.
Method
Participants
Participants with first-episode schizophrenia
Adolescents and young adults who had presented to the South London and Maudsley NHS Trust with a first episode of schizophrenia were recruited from child and adolescent psychiatric services and early intervention in psychosis services (Outreach and Support in South London (OASIS) and Lambeth Early Onset (LEO) services). Inclusion required that they met DSM–IV 23 criteria for schizophrenia and had no comorbid Axis I diagnosis or learning disability. The participants with first-episode schizophrenia (n = 34) were divided into two groups: one group (n = 17) comprised individuals with adolescent onset (with onset before the age of 18 years) the other group (n = 17) comprised individuals whose illness began in adult life (Table 1). Data from the adolescent participants have also been presented in a previous publication. Reference Kyriakopoulos, Vyas, Barker, Chitnis and Frangou10
Table 1 Demographic variables and clinical characteristics of study participants
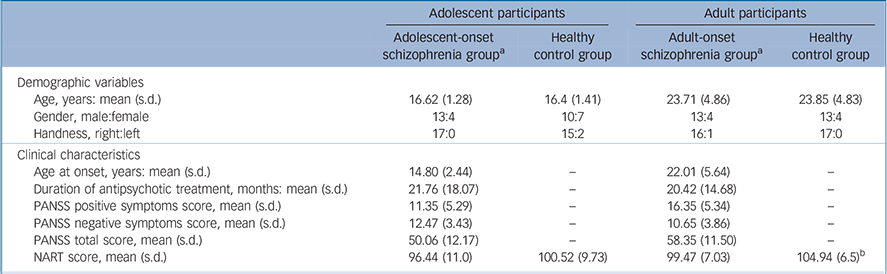
| Adolescent participants | Adult participants | |||||
|---|---|---|---|---|---|---|
| Adolescent-onset schizophrenia group a | Healthy control group | Adult-onset schizophrenia group a | Healthy control group | |||
| Demographic variables | ||||||
| Age, years: mean (s.d.) | 16.62 (1.28) | 16.4 (1.41) | 23.71 (4.86) | 23.85 (4.83) | ||
| Gender, male:female | 13:4 | 10:7 | 13:4 | 13:4 | ||
| Handness, right:left | 17:0 | 15:2 | 16:1 | 17:0 | ||
| Clinical characteristics | ||||||
| Age at onset, years: mean (s.d.) | 14.80 (2.44) | — | 22.01 (5.64) | — | ||
| Duration of antipsychotic treatment, months: mean (s.d.) | 21.76 (18.07) | — | 20.42 (14.68) | — | ||
| PANSS positive symptoms score, mean (s.d.) | 11.35 (5.29) | — | 16.35 (5.34) | — | ||
| PANSS negative symptoms score, mean (s.d.) | 12.47 (3.43) | — | 10.65 (3.86) | — | ||
| PANSS total score, mean (s.d.) | 50.06 (12.17) | — | 58.35 (11.50) | — | ||
| NART score, mean (s.d.) | 96.44 (11.0) | 100.52 (9.73) | 99.47 (7.03) | 104.94 (6.5) b | ||
PANSS, Positive and Negative Syndrome Scale; NART, National Adult Reading Test,
a. All comparisons of the adolescent-onset schizophrenia group with the adult-onset schizophrenia group in demographic variables and clinical characteristics with the exception of age were not statistically significant.
b. P < 0.05. All the other comparisons within age group were not statistically significant.
Healthy control group
Healthy volunteers with no personal history of psychiatric disorder and no family history of psychosis in their first-degree relatives were recruited from the same geographic area as the participants with schizophrenia. Thirty-four individuals were selected to match the total patient sample at group level with respect to age and gender (Table 1).
Exclusion criteria
For all participants, the following exclusion criteria applied: history of head injury; presence of a substance use disorder as defined by DSM–IV; any concomitant neurological condition; and history of any central nervous system disorder.
Assessment of demographic information, IQ and clinical features
Parental socioeconomic status was determined according to the Standard Occupational Classification 24 and handedness was determined by the Annett handedness scale. Reference Annett25 An estimate of general intellectual ability was obtained using the National Adult Reading Test (NART). Reference Nelson and Willison26 The diagnosis of schizophrenia or its absence was based on the Structured Clinical Interview for DSM–IV Axis I Disorders, Reference First, Spitzer, Gibbon and Williams27 supplemented by information from medical records, family members and treating physicians. Age at onset of schizophrenia was defined as the age at which individuals first clearly manifested frank delusions or hallucinations. The current dose and total cumulative exposure to antipsychotic medication was recorded. Cumulative exposure in chlorpromazine equivalents (CPZeq) was calculated according to the comparable daily dose (based on D2 affinity and pharmacokinetics) and to the apparent clinical equivalence in schizophrenia. Reference Bezchlibnyk-Butler and Jeffries28 Psychopathology at the time of scanning was assessed with the Positive and Negative Syndrome Scale (PANSS). Reference Kay, Fiszbein and Opler29 Participants were scanned as close to the onset of the first episode as possible, but only when sufficiently clinically stable to tolerate the procedure.
The study was approved by the ethics committee of the Institute of Psychiatry. All participants gave consent or assent to the study; those over the age of 16 provided written informed consent after detailed description of the study, whereas those under the age of 16 provided their assent and their parents or guardians provided written informed consent.
Image acquisition
Diffusion tensor imaging data were acquired on a GE Signa 1.5 T NVi System running 8.X release software. Head movement was minimised with padding and a foam strap across the forehead. Data were acquired using a multislice, peripherally gated echo planar imaging (EPI) pulse sequence, with each volume acquired from 60 contiguous 2.5 mm thick slices with field of view (FOV) 240×240 mm2 and matrix size 96×96, zero-filled to 128×128 during reconstruction, giving an in-plane voxel size of 1.875×1.875 mm2. Echo time (TE) was 107 ms and effective repetition time (TR) was 15 R–R intervals. At each location, 7 images were acquired without diffusion weighting, together with 64 images with a weighting of 1300 s/mm2 applied along directions uniformly distributed in space.
Image processing and analysis
Following correction of the diffusion-weighted images for eddy-current induced distortion, the diffusion tensor was calculated for each brain voxel, Reference Basser, Mattiello and LeBihan30 and mean diffusivity and fractional anisotropy maps were constructed using in-house software. Images were analysed using a voxel-based approach in standard space. Registration was performed using SPM2 (Wellcome Functional Imaging Laboratory, London, UK) run on Solaris 10 (Sun Microsystems Inc). A two-stage registration process was performed, analogous to the optimised voxel-based morphometry approach first introduced by Good et al. Reference Good, Johnsrude, Ashburner, Henson, Friston and Frackowiak31 The mean T 2-weighted (non-diffusion-weighted, b = 0) images from each participant were first registered to the standard EPI template provided by SPM. The derived mapping parameters for each participant were then applied to the (inherently co-registered) fractional anisotropy images. The normalised fractional anisotropy images of all participants were then averaged and smoothed to create a new, study-specific template to which each individuals' fractional anisotropy images were then re-registered. The registered images were also roughly segmented (using SPMs' default a priori tissue probability information) to give maps of the probability of a tissue being either white or grey matter, and these segmented images were thresholded at a low (10%) probability to provide a binary mask of white matter. (An accurate segmentation was not essential, and a deliberately relatively liberal threshold was used in order to create a slightly ‘over inclusive’ mask.) A 4 mm full width at half maximum (FWHM) smoothing filter was used to improve signal/noise ratio and minimise the effects of residual misregistration. The smoothed images were masked with the mask created above to restrict subsequent analyses to white matter. Finally, after inspection for image quality and gross abnormality, masked images were compared between groups using XBAM (version 3.4, Brain Image Analysis Unit, Institute of Psychiatry, London, UK; www.brainmap.co.uk/xbam.htm), Reference Bullmore, Suckling, Overmeyer, Rabe-Hesketh, Taylor and Brammer32 which uses non-parametric, permutation-based statistics. These maybemore suitablefor this typeof datathan parametric approaches, as the residuals of fit to the general linear model may not show the Gaussian behaviour required by parametric statistics.
Differences between individuals with schizophrenia and controls were examined separately for the adolescent- and adult-onset groups. This included estimation of grey and white matter volume by fitting an analysis of variance (ANOVA) model at each intracerebral voxel in standard space. Initially a liberal statistical threshold (P≤0.05) was set to detect voxels putatively demonstrating differences between groups. Only those voxels to which all participants contributed data were considered; this, along with the masking procedure detailed above, restricted the analysis to core white matter regions, reducing the search volume and thus the number of comparisons. We thus avoided testing at the grey/white interfaces, where the high grey/white contrast of fractional anisotropy images can exacerbate edge effects. We then searched for spatial clusters among the voxels highlighted, and tested the ‘mass’ of each cluster (the sum of suprathreshold voxel statistics it comprises) for significance. XBAM was used to assess statistical significance at both the voxel and cluster levels. Reference Bullmore, Suckling, Overmeyer, Rabe-Hesketh, Taylor and Brammer32 At the cluster level, rather than set a single a priori threshold, we calculated for a range of P-values the number of clusters that would be expected by chance alone. We then set the statistical threshold for cluster significance for this analysis at a P-value such that the expected number of false positive clusters by chance alone would be less than one. In this case it was P≤0.0025.
In investigating cluster level effects, there is an underlying assumption that all regions will be equally ‘smooth’ and from the statistical point of view can be treated equivalently. Although we restricted our analysis to core white matter regions where the signal/noise ratio is relatively uniform, the effects of physiological noise (e.g. motion artefacts) may still vary across the brain. We therefore also inspected the voxel-level maps that treat each voxel independently and inherently allow for such local differences in statistics.
Pearson's chi-squared and Student's t-tests (both two-tailed) were used to compare the distribution of categorical and continuous data respectively, between individuals with schizophrenia and healthy controls in each of the two age groups. We explored the correlation between NART and fractional anisotropy values for all regions where case–control differences were noted; in individuals with schizophrenia we also examined correlations between fractional anisotropy in these regions and age at onset, cumulative antipsychotic exposure, PANSS positive subscale, negative subscale and total scores. To do this, masked images were created for each of the clusters where group differences were identified and applied to the normalised fractional anisotropy images of each participant. The fractional anisotropy values thus extracted were then correlated with clinical variables using Statistical Package for Social Sciences (SPSS) for Windows, version 13.0. Bonferroni correction was applied for multiple comparisons and the level of statistical significance was set at P = 0.005.
The diagnosis×age group interaction was tested by using a 2×2 factorial design implemented in the ANOVA module of XBAM. Reference Bullmore, Suckling, Overmeyer, Rabe-Hesketh, Taylor and Brammer32 Because we had already examined the effect of diagnosis with pair-wise comparisons for participants with adolescent and adult onset separately, this ANOVA model was used to examine the interaction only. Fractional anisotropy values from regions where a significant effect was found were then examined for possible correlations with NART scores in all participants, and with cumulative antipsychotic exposure, PANSS positive subscale, negative subscale and total scores in the whole sample with schizophrenia. The anatomical coordinates of the regions of difference between the groups in all comparisons were identified with the use of the atlases of Talairach & Tournoux Reference Talairach and Tournoux33 and Mori et al. Reference Mori, Wanaka, Nagae-Poetscher and van Zijl34
Results
Participants
There were no significant demographic differences between individuals with schizophrenia and healthy controls; those with schizophrenia had lower NART scores in the adult group (t = −2.36, d.f. = 32, P = 0.025; Table 1) and in the sample as a whole (t = −2.36, d.f. = 66, P = 0.021).
Twenty-eight people with schizophrenia were taking antipsychotic medication (all but one received atypical medication) and six were medication naive (two in the adolescent group and four in the adult group). The mean comparable daily antipsychotic dose at the day of scanning was 205.03 mg (range 0–1200) CPZeq.
Diffusion tensor imaging
Adolescent-onset group
In this group, reductions in fractional anisotropy relative to the age-matched controls were observed in the parietal white matter bilaterally (Fig. 1 and Table 2), extending to regions likely to represent the superior and inferior longitudinal fasciculi, inferior fronto-occipital fasciculus, corticopontine tract and posterior thalamic radiation. Reference Mori, Wanaka, Nagae-Poetscher and van Zijl34 Fractional anisotropy reductions in regions likely to represent the corpus callosum were also noted on the left side. The voxel- and cluster-level maps were very similar in regions showing reduced fractional anisotropy in this group, although the voxel-level map also showed a number of additional regions of increased fractional anisotropy (generally with very small spatial extent), which were not evident in the cluster-level maps. There were no brain regions where white matter fractional anisotropy was greater in the adolescent-onset group than their controls.
Table 2 White matter clusters of reduced fractional anisotropy in individuals with adult-onset schizophrenia compared with adult healthy controls and tracts likely to correspond to these clusters
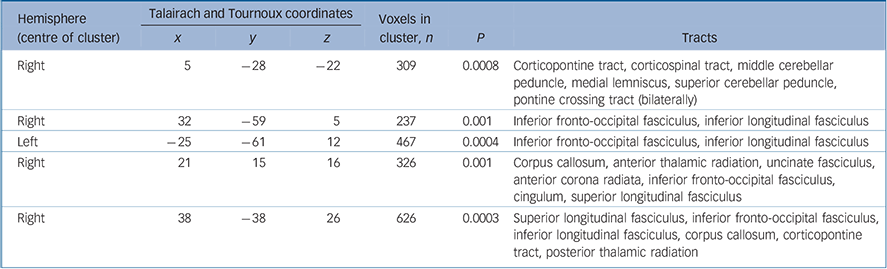
| Hemisphere (centre of cluster) | Talairach and Tournoux coordinates | Voxels in cluster, n | ||||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | P | Tracts | ||||
| Right | 5 | -28 | -22 | 309 | 0.0008 | Corticopontine tract, corticospinal tract, middle cerebellar peduncle, medial lemniscus, superior cerebellar peduncle, pontine crossing tract (bilaterally) | ||
| Right | 32 | -59 | 5 | 237 | 0.001 | Inferior fronto-occipital fasciculus, inferior longitudinal fasciculus | ||
| Left | -25 | -61 | 12 | 467 | 0.0004 | Inferior fronto-occipital fasciculus, inferior longitudinal fasciculus | ||
| Right | 21 | 15 | 16 | 326 | 0.001 | Corpus callosum, anterior thalamic radiation, uncinate fasciculus, anterior corona radiata, inferior fronto-occipital fasciculus, cingulum, superior longitudinal fasciculus | ||
| Right | 38 | -38 | 26 | 626 | 0.0003 | Superior longitudinal fasciculus, inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, corpus callosum, corticopontine tract, posterior thalamic radiation | ||
Adult-onset group
The adult-onset group showed fractional anisotropy reductions in five discrete clusters (Fig. 2 and Table 3). There were bilateral reductions in fractional anisotropy in white matter likely to correspond to the inferior longitudinal and inferior fronto-occipital fasciculi, and in the brain stem and cerebellum. In the right hemisphere, fractional anisotropy was reduced in regions likely to represent the superior and inferior longitudinal fasciculi, inferior fronto-occipital fasciculus, corpus callosum, uncinate fasciculus, cingulum, anterior corona radiata, corticopontine tract and posterior thalamic radiation. Reference Mori, Wanaka, Nagae-Poetscher and van Zijl34 The cluster- and voxel-level maps were very similar. There were no regions within the white matter where fractional anisotropy was greater in the adult-onset group than their controls.
Table 3 White matter clusters of reduced fractional anisotropy in individuals with adolescent-onset schizophrenia compared with adolescent healthy controls and tracts likely to correspond to these clusters
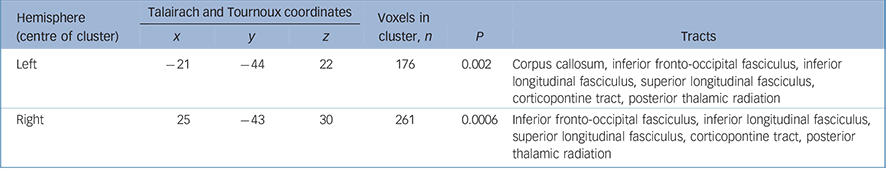
| Hemisphere (centre of cluster) | Talairach and Tournoux coordinates | Voxels in cluster, n | ||||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | P | Tracts | ||||
| Left | -21 | -44 | 22 | 176 | 0.002 | Corpus callosum, inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, superior longitudinal fasciculus, corticopontine tract, posterior thalamic radiation | ||
| Right | 25 | -43 | 30 | 261 | 0.0006 | Inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, superior longitudinal fasciculus, corticopontine tract, posterior thalamic radiation | ||
Effect of age at onset
The combined early- and adult-onset schizophrenia group had reduced white matter fractional anisotropy compared with healthy controls in the brain stem bilaterally (Talairach coordinates of centroid x = 7, y = −29, z = −32), the left temporal lobe (Talairach coordinates x = −39, y = −16, z = −16), the parietal lobe bilaterally (two left clusters with Talairach coordinates x = −22, y = −48, z = 24 and x = −22, y = −37, z = 44 and one right cluster with Talairach coordinates x = 23, y = −48, z = 32) and the white matter adjacent to the left medial frontal gyrus (Talairach coordinates x = −14, y = −2, z = 50). There was one frontal region including the genu of corpus callosum and the anterior cingulum (Talairach coordinates x = −12, y = 31, z = 14) of increased white matter fractional anisotropy in the entire schizophrenia group. With regard to the effect of age, adolescents had higher white matter fractional anisotropy than adults in two bilateral parietal regions (Talairach coordinates x = −21, y = −50, z = 26 and x = 23, y = −52, z = 26). There were no regions of higher white matter fractional anisotropy in the adult group than the adolescent group.

Fig. 1 Fractional anisotropy map indicating the areas where fractional anisotropy in participants in the adolescent-onset schizophrenia group is reduced in comparison with healthy controls (cluster P = 0.0025).
There was a differential effect of age at onset of schizophrenia in two medial regions centred on approximately homotopic parts of the frontal white matter in each hemisphere; this interaction was similar in both hemispheres but more pronounced on the left (Figs 3 and 4, Table 4). Both regions are likely to include parts of the superior fronto-occipital fasciculus, corticopontine tract, anterior thalamic radiation and corpus callosum. The region in the right hemisphere also extended into the right anterior corona radiata, anterior limb of the internal capsule and forceps minor, whereas the left region included the left superior longitudinal fasciculus, superior thalamic radiation and corticospinal tract. Reference Mori, Wanaka, Nagae-Poetscher and van Zijl34
Table 4 White matter clusters emerged in diagnosis×age group interaction analysis in all participants and tracts likely to correspond to these clusters

| Hemisphere (centre of cluster) | Talairach and Tournoux coordinates | Voxels in cluster, n | ||||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | P | Tracts | ||||
| Right | 21 | 17 | 18 | 223 | 0.002 | Anterior corona radiata, anterior limb of internal capsule, superior fronto-occipital fasciculus, corticopontine tract, anterior thalamic radiation, corpus callosum, forceps minor | ||
| Left | -23 | 0 | 26 | 226 | 0.002 | Superior fronto-occipital fasciculus, superior longitudinal fasciculus, corticopontine tract, anterior thalamic radiation, corticospinal tract, superior thalamic radiation, corpus callosum | ||
Correlations
Fractional anisotropy was not significantly correlated with NART score, PANSS scores or the cumulative exposure to antipsychotic medication in any of the regions that showed group differences and group×age interactions, except for one cluster (Talairach coordinates of centroid x = 21, y = 15, z = 16) of reduced fractional anisotropy in the adult-onset schizophrenia group, in which fractional anisotropy correlated with NART score (r = 0.575, P<0.001).
Discussion
In this study, diffusion tensor imaging was used to examine the effect of age at onset of schizophrenia on white matter integrity as inferred by fractional anisotropy. We report different patterns of fractional anisotropy reductions in adolescent-onset compared with adult-onset schizophrenia. In line with our hypothesis, adolescents showed parietal white matter fractional anisotropy reductions, whereas in individuals with adult-onset fractional anisotropy reductions were also noted in the frontal and temporal white matter. Direct comparison of the differences within each age group (adolescents v. adults) revealed two frontal regions where the effect of schizophrenia on fractional anisotropy was modulated by age at onset.

Fig. 2 Fractional anisotropy map indicating the areas where fractional anisotropy in participants in the adult-onset schizophrenia group is reduced in comparison with healthy participants (cluster P = 0.0025).
Data from magnetic resonance imaging studies in adolescents with schizophrenia suggest early parietal grey matter deficits progressing to more anterior regions as individuals move into late adolescence and early adulthood. Reference Thompson, Vidal, Giedd, Gochman, Blumenthal and Nicolson6,Reference Burke, Androutsos, Jogia, Byrne and Frangou7,Reference Greenstein, Lerch, Shaw, Clasen, Giedd and Gochman9 Our findings indicate a similar pattern with respect to white matter abnormalities. In adults with schizophrenia, fractional anisotropy reductions have been reported predominantly in frontal and temporal regions Reference Ardekani, Nierenberg, Hoptman, Javitt and Lim16,Reference Hubl, Koenig, Strik, Federspiel, Kreis and Boesch18–Reference Hao, Liu, Jiang, Gong, Liu and Tan20,Reference Shergill, Kanaan, Chitnis, O'Daly, Jones and Frangou22,Reference Kanaan, Kim, Kaufmann, Pearlson, Barker and McGuire35,Reference Kyriakopoulos, Bargiotas, Barker and Frangou36 and less so in parietal white matter. Reference Ardekani, Nierenberg, Hoptman, Javitt and Lim16,Reference Hubl, Koenig, Strik, Federspiel, Kreis and Boesch18,Reference Buchsbaum, Friedman, Buchsbaum, Chu, Hazlett and Newmark19,Reference Manoach, Ketwaroo, Polli, Thakkar, Barton and Goff21,Reference Kanaan, Kim, Kaufmann, Pearlson, Barker and McGuire35,Reference Kyriakopoulos, Bargiotas, Barker and Frangou36 Consistent with this literature, our adult-onset group had more widespread fractional anisotropy reductions (present in the frontal and temporal lobes in addition to those in parietal regions) compared with their respective healthy controls than the adolescent-onset participants.

Fig. 3 Fractional anisotropy map indicating the areas where the effect of the illness in fractional anisotropy is modulated by age-group membership (adolescent- or adult-onset; cluster P = 0.0025).
A differential effect of age at onset of schizophrenia was noted in two regions in approximately homotopic parts of the left and right frontal white matter (Figs 3 and 4, Table 4). Consistent with the relevant literature on normal development, Reference Lebel, Walker, Leemans, Phillips and Beaulieu37 fractional anisotropy was higher is healthy adults compared with healthy adolescents across the age range of the study sample. Age-related increase in fractional anisotropy during adolescence and early adulthood has been attributed to dendritic changes and increased myelination in prefrontal regions. Reference Jacobs, Driscoll and Schall38,Reference Davis, Stewart, Friedman, Buchsbaum, Harvey and Hof39 In the adult-onset group, there was no correlation between age and frontal fractional anisotropy values that could be indicative of attenuated or absent age-related maturational increase in fractional anisotropy. Within the adolescent-onset group, frontal fractional anisotropy was higher in younger and lower in older participants. Fractional anisotropy is influenced by factors affecting neuronal fibre diameter, density and myelination as well as tract coherence and extracellular diffusion. Although our study raises important questions regarding the interaction between developmental and disease-related changes in white matter in schizophrenia, it is not possible to determine the microstructural nature of white matter abnormalities reflected in our imaging findings. According to the prevalent neurodevelopmental model of schizophrenia, Reference Rapoport, Addington and Frangou40 a disruption in the normal process of cortical layering is assumed to produce ‘dysconnection’ in the connectivity of fibre tracts. Reductions in fractional anisotropy associated with schizophrenia in this and other studies are considered to reflect reduction in tract coherence and direction. It is tempting to postulate that late maturational events occurring from late childhood to late adolescence accentuate this dysconnection and this may explain the lower fractional anisotropy levels in older adolescents compared with younger adolescents with the disorder.
Differences in white matter microstructure changes occurring in individuals with adolescent schizophrenia compared with adult schizophrenia may be under genetic control since relatively higher genetic loading in individuals with early onset has been reported. Reference Addington, Gornick, Duckworth, Sporn, Gogtay and Bobb41 However, although a young age at onset could be considered as a proxy measure of genetic loading, direct testing through analysis of genetic material was not possible in our study and there have not been any studies in schizophrenia literature, to our knowledge, that have examined this to date.
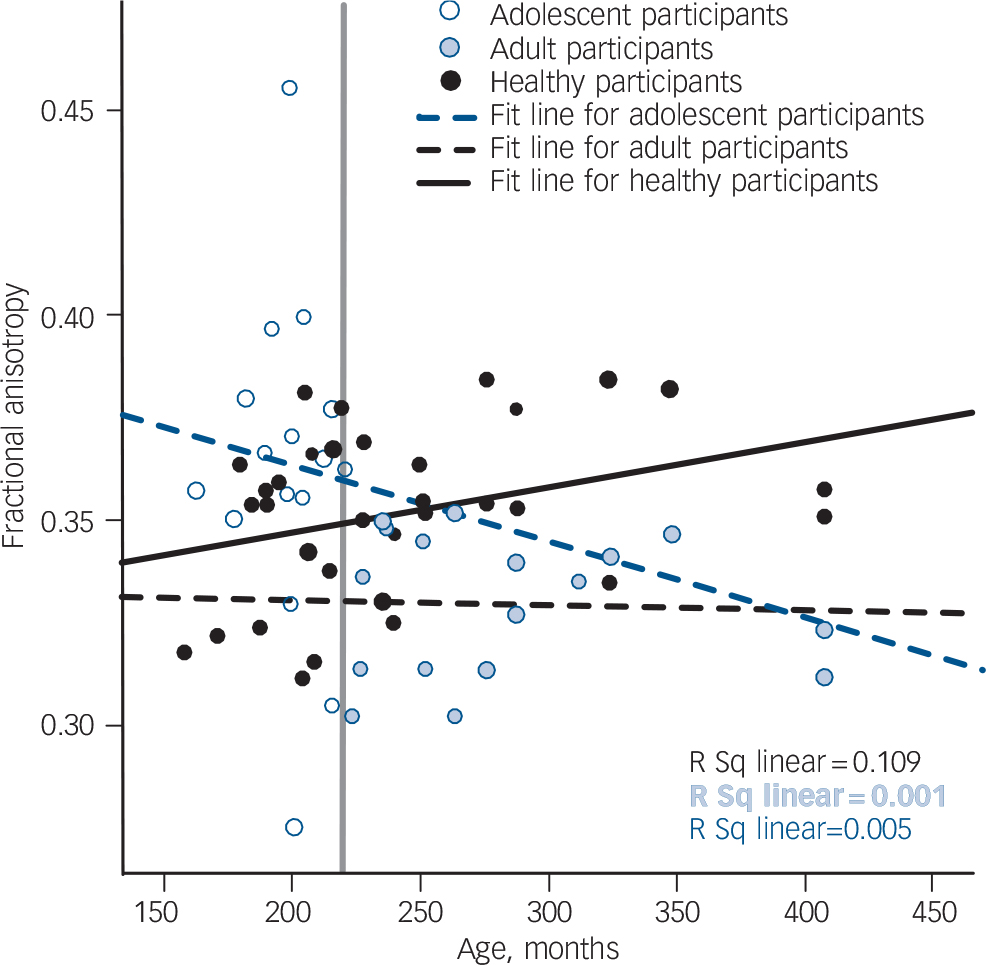
Fig. 4 Scatter plots of mean fractional anisotropy in the left frontal cluster. The vertical grey line indicates the age separating the adult from the adolescent age group.
A strength of the current study is that, despite the differences in ages between the two groups, individuals in the adolescent and adult groups were comparable in terms of illness duration and medication exposure, which could be important sources of variance in fractional anisotropy. At the same time, most participants were on antipsychotic medication at the time of scanning. Although some studies have reported correlations between fractional anisotropy in schizophrenia and measures of antipsychotic medication, most investigators have not found that medication dose or cumulative exposure had a major effect on fractional anisotropy. Reference Kanaan, Kim, Kaufmann, Pearlson, Barker and McGuire35,Reference Kyriakopoulos, Bargiotas, Barker and Frangou36 In addition, white matter fractional anisotropy reductions have been reported in never-medicated individuals with schizophrenia compared with healthy controls. Reference Cheung, Cheung, McAlonan, Deng, Wong and Yip42 Another limitation of the study is the difference in intellectual ability (as indexed by the NART score) between individuals with schizophrenia and healthy controls in the adult-onset group, although this was smaller than one standard deviation (Table 1) and therefore unlikely to be meaningful given the large standard deviation of intellectual ability in the general population. Reference Wechsler43
In conclusion, we found that age at onset of schizophrenia influences the pattern of fractional anisotropy reductions observed; these were more widespread in the adult-onset group compared with their respective age-matched controls. Our study also indicates an interaction between late brain maturational events and frontal white matter changes in schizophrenia, and highlights the importance of longitudinal studies to investigate this finding further.
Funding
This study was supported by grants from Guys & St Thomas' Charitable Foundation (G041001) and GlaxoSmithKline (GSK 084) to P.K.M. and a grant from the National Alliance of Research on Schizophrenia and Depression to S.F. R.A.A.K. is supported by the Wellcome Trust.
Acknowledgements
The authors would like to thank Professor Mick Brammer for his advice.











eLetters
No eLetters have been published for this article.