Treatment-resistant psychosis (TRP) is broadly defined as a suboptimal response to at least two non-clozapine antipsychotics prescribed at an adequate dose and duration.Reference Howes, McCutcheon, Agid, de Bartolomeis, van Beveren and Birnbaum1 TRP affects approximately one-third of individuals diagnosed with psychosis and is one of the leading causes of disability worldwide. TRP is associated with severe long-term consequences on social and occupational functioning, with total treatment costs up to 11 times that of schizophrenia that is responsive to standard treatment.Reference Kennedy, Altar, Taylor, Degtiar and Hornberger2
Since the seminal work by Kane and colleagues in the 1980s, clozapine has been regarded as the most effective antipsychotic in TRP, supported by evidence from randomised controlled trials and observational studies.Reference Siskind, Siskind and Kisely3,Reference Leucht, Cipriani, Spineli, Mavridis, Orey and Richter4 Despite this, clozapine is vastly underutilised,Reference Bachmann, Aagaard, Bernardo, Brandt, Cartabia and Clavenna5 and its initiation is often substantially delayed, partly owing to the stringent requirement of indefinite blood monitoring to detect the potentially fatal but rare risk of agranulocytosis.Reference Farooq, Choudry, Cohen, Naeem and Ayub6 This adverse effect was first identified in the 1970s during clozapine's pre-marketing development where fatalities among 18 Finnish individuals were ultimately linked to CIA.Reference Crilly7 The risk of CIA, assessed at a prevalence of approximately 0.4–0.8%Reference Li, Zhong, Lu, Zheng, Wang and Rao8 has for a long time distorted the perception among clinicians and patients of the risk versus benefit of this uniquely effective treatment.Reference Parkes, Mantell, Oloyede and Blackman9 For instance, after the Finnish epidemic, the notion of so-called ‘clozapine-induced neutropenia’ became widely accepted. However, evidence to date demonstrates that clozapine's association is strictly with agranulocytosis (i.e. severe neutropenia), and an association with milder forms of neutropenia has been largely assumed and indeed perpetuated by surveillance bias.
Clozapine monitoring schemes were originally established for the early detection of moderate neutropenia as a means of preventing the development of agranulocytosis and ultimately death.Reference Oloyede, Blackman, Whiskey, Bachmann, Dzahini and Shergill10 However, several lines of evidence indicate that the threshold model for discontinuation substantially overestimates rates of potential CIA. Over-diagnosis of CIA or potential CIA in turn leads to unnecessary discontinuation of clozapine.Reference Myles, Myles, Xia, Large, Bird and Galletly11,Reference Myles, Myles, Xia, Large, Kisely and Galletly12 For example, evidence shows that 80% of people suspected of having clozapine-related dyscrasia can be safely restarted on treatment.Reference Oloyede, Whiskey, Casetta, Dzahini, Dunnett and Gandhi13 One explanation for this mismatch between assumed clozapine toxicity and success on re-exposure is that haematological phenotypes such as benign ethnic neutropenia (BEN) are often the cause of isolated neutropenic readings.Reference Manu, Sarvaiya, Rogozea, Kane and Correll14 Unsurprisingly, clinicians have highlighted the need for revisions to the definition and criteria for identifying CIA.Reference Oloyede, Taylor and MacCabe15
The basis of the current threshold monitoring system for clozapine is that agranulocytosis is defined by a single neutrophil count below 0.5 × 109 and the assumption that all such cases in people taking clozapine are caused by clozapine. However, an isolated below-threshold count may be neither pathological nor related to clozapine.Reference van Staa, Boulton, Cooper, Hagenbeek, Inskip and Leufkens16 Examining the pattern of neutrophil count changesReference Taylor, Vallianatou, Whiskey, Dzahini and MacCabe17 is very probably a more accurate measure of clozapine-associated agranulocytosis. It is thus unsurprising that efforts to delineate risk factors for CIA (derived from a threshold monitoring system) have yielded contradictory findings. For instance, Alvir and colleagues identified an increased risk of agranulocytosis in females receiving clozapine.Reference Alvir, Lieberman, Safferman, Schwimmer and Schaaf18 However, since this study in 1993, no report has replicated these findings.Reference Deliliers19 Munro et al suggested an increased risk in Asian individuals compared with White individuals,Reference Munro, O'Sullivan, Andrews, Arana, Mortimer and Kerwin20 but this too has not been replicated. Since then, other studies have generated diverging conclusions.
In this study, we aimed to determine the epidemiology and timing of CIA using pattern-based criteria, to investigate demographic differences of CIA in a large retrospective cohort study design.
Method
Data sources
This retrospective observational study was based on data from the largest clozapine manufacturer in the UK – Viatris, monitored by the Clozaril® Patient Monitoring Service (CPMS) database from the period between May 2000 and February 2021 inclusive.
Definition of haematological monitoring
In accordance with the Medicines and Healthcare Products Regulatory Agency (MHRA), UK regulations for clozapine prescription include regular monitoring of haematological parameters for all individuals receiving Clozaril®, notably the white blood cell (WBC) count, and the absolute neutrophil count (ANC). In the UK, weekly monitoring is mandated for the first 18 weeks of clozapine treatment, and in weeks 19–52 monitoring is mandatory biweekly.Reference Oloyede, Casetta, Dzahini, Segev, Gaughran and Shergill21 Thereafter, 4-weekly haematological monitoring is required indefinitely. Blood test results are categorised into one of the following three categories: green (WBC >3.5 × 109/L, ANC >2.0 × 109/L), amber (WBC 3.0–3.5 × 109/L, ANC 1.5–2.0 × 109/L) and red (WBC <3.0 × 109/L, ANC <1.5 × 109/L). Since 2002, ANC and/or WBC discontinuation thresholds were reduced by 0.5 × 109/L for those with confirmed BEN status.Reference Oloyede, Casetta, Dzahini, Segev, Gaughran and Shergill21
In the event of an amber blood test result, blood tests must be performed two times per week, until the WBC and/or ANC stabilises or increases. In the event of a red result, individuals are assessed for signs of infection, clozapine is stopped immediately and daily haematological monitoring is initiated until ANC and/or WBC is above threshold. In exceptional cases, clozapine manufacturers allow for re-exposure of clozapine (i.e. ‘clozapine rechallenge’) following discontinuation due to suspected CIA and thus registration on the Central Non-Rechallenge Database (CNRD), which has been fully described elsewhere.Reference Oloyede, Whiskey, Casetta, Dzahini, Dunnett and Gandhi13,Reference Oloyede, Casetta, Dzahini, Segev, Gaughran and Shergill21
Study population
For individuals registered on the CNRD with at least one ANC <1.5 × 109/L and/or WBC <3.0 × 109/L (i.e. neutropenia), CPMS provided the WBC and absolute neutrophil count (ANC) data and individuals’ sociodemographic information, i.e. gender, age, self-reported ethnicity, clozapine indication (i.e. TRP, off-label, other), date of blood test(s) and clozapine rechallenge status information. Self-reported ethnicity was stratified as follows: White, Black, Asian and Other. For the exploratory analysis between demographic/clinical factors and prevalence of CIA, age was stratified into quartiles (≤29, 30–40, 41–48 and >48 years). For people who were rechallenged on clozapine after experiencing agranulocytosis, only the first event was included in the primary analysis.
Measures
In this study, we calculated the prevalence of agranulocytosis, stratified by gender, age and ethnicity.
Individuals were diagnosed with CIA using two different ascertainment methods:
(a) At least one ANC <0.5 × 109/L – threshold-based agranulocytosis
(b) Two consecutive ANC (over 2 or more days) <0.5 × 109/L – pattern-based agranulocytosis as suggested by Taylor et alReference Taylor, Vallianatou, Whiskey, Dzahini and MacCabe22
The number of people who were determined to have transient agranulocytosis were described. Transient agranulocytosis was defined as one ANC <0.5 × 109/L followed by subsequent ANC ≥0.5 × 109/L. The number of individuals who transitioned from a normal ANC count (≥1.5 × 109/L) to threshold-based and pattern-based agranulocytosis were recorded. The number of people who recorded pattern-based agranulocytosis within 14 days of a mild to moderate neutropenia (ANC 0.5–1.5 × 109/L) were recorded and stratified by ethnicity. The rationale for 14 days was to assess current monitoring requirements. Heat maps were produced to visually represent the impact of demographic and clinical factors on the proportion of individuals recording neutropenia, and threshold- and pattern-based agranulocytosis.
Statistical analysis
The statistical analyses were performed using SciPy software (version 1.12.0 for Windows).Reference Virtanen, Gommers, Oliphant, Haberland, Reddy and Cournapeau23 The 20-year prevalence of agranulocytosis was calculated for the entire cohort and the CNRD sample using the threshold- and pattern-based criteria. The mean, s.d., median and interquartile range (IQR) were calculated for continuous data. Additional subgroup analyses were performed according to gender, age and ethnicity. Frequencies and percentages were calculated for categorical data.
Ethics approval statement
Consent was not obtained from all participants, as we used non-identifiable data provided by Clozaril® (Viatris) monitored by CPMS. Ethical approval was not required according to the UK HRA.
Results
Baseline characteristics
Between May 2002 and February 2021, 75 553 people were registered on the CPMS database for clozapine treatment – data on ethnicity were not available. In the same study period, 3029 were registered on the CPMS CNRD and included in this study. The mean age of the CNRD cohort was 39 years (s.d. 13) at clozapine initiation, and 62% were male. Overall, 85% identified as White, 9% as Black and 4% as Asian. The median number of blood samples per person was 93.7 (s.d. 83.7, range 1–900), and the mean observation period was 1909 days (~5.2 years) (s.d. 2043, range 0–7602 days). Of the people registered on the CNRD, 2436 (80%) had mild to moderate neutropenia, and 245 (8%) were determined to have transient agranulocytosis (see Supplementary Appendix available at https://doi.org/10.1192/bjp.2024.104). Clinical and demographic characteristics of the study population are shown in Table 1.
Table 1 Sociodemographic and clinical characteristics of people registered on the Central Non-Rechallenge Database (CNRD)
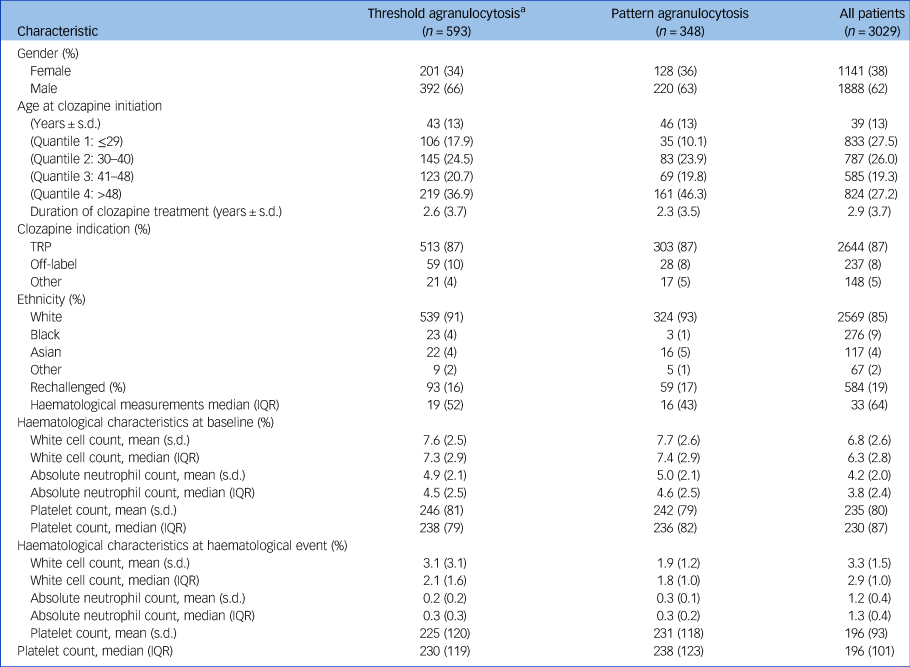
a. Threshold-based agranulocytosis cohort includes individuals who registered pattern-based agranulocytosis. See Supplementary Appendix for distinct group characteristics. TRP, treatment-resistant psychosis; IQR, interquartile range.
Agranulocytosis and prevalence
Table 2 outlines the prevalence of agranulocytosis in the CNRD cohort using threshold- and pattern-based criteria. In the total sample (75 533), prevalence of threshold-based agranulocytosis and pattern agranulocytosis was (0.8%) and (0.5%), respectively. The period prevalence of threshold-based agranulocytosis in the CNRD was 19.5%, compared with 11.4% using the pattern-based criterion. Overall, 43.3% of threshold-based agranulocytosis cases emerged during the first 18 weeks of clozapine treatment, and 53.3% during the first year, while these rates were 53.2 and 58.9%, respectively, for the pattern-based criterion (Supplementary Appendix).
Table 2 Prevalence (ever) of agranulocytosis (threshold-based v. pattern-based) in the Central Non-Rechallenge Database (CNRD) sample
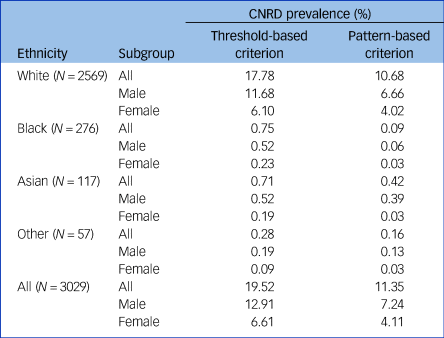
Among ethnic groups, the prevalence was highest among White individuals (10.7%) and lowest in Black individuals (0.1%). In general, the prevalence rates were highest in the >48 age group. Prevalence of pattern-based agranulocytosis was slightly higher among males (7.3) compared with females (4.1).
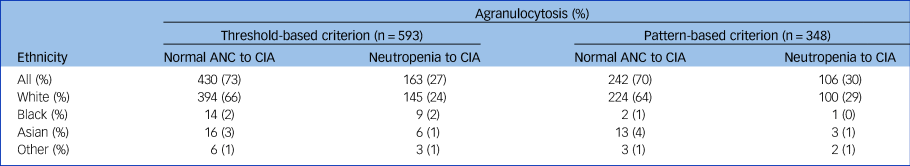
As shown in Table 3, most people transitioned from a normal ANC count to agranulocytosis with threshold-based (73%) and pattern-based (70%) criteria.
Table 3 Proportion of individuals that transitioned from neutropenia or a normal absolute neutrophil count (ANC) to clozapine-induced agranulocytosis (CIA) (threshold-based v. pattern-based) in the Central Non-Rechallenge Database (CNRD) sample
Agranulocytosis timing
The median time to pattern-based agranulocytosis was 0.28 (IQR 3.25) years and 0.62 (IQR 3.52) years for threshold-based agranulocytosis. Overall, 43% of people recorded threshold-based agranulocytosis in the first 18 weeks of treatment. The corresponding figure for pattern-based agranulocytosis was 53.16% (see Supplementary Appendix). For threshold-based criteria, 55% of individuals recorded agranulocytosis at 1 year and 76% by 4 years. For pattern-based criteria, 58% of individuals recorded agranulocytosis at 1 year and 80% by 4 years (see Supplementary Appendix). Figure 1 displays the cumulative frequency of neutropenia and agranulocytosis stratified by threshold and pattern-based criteria for the entire cohort.
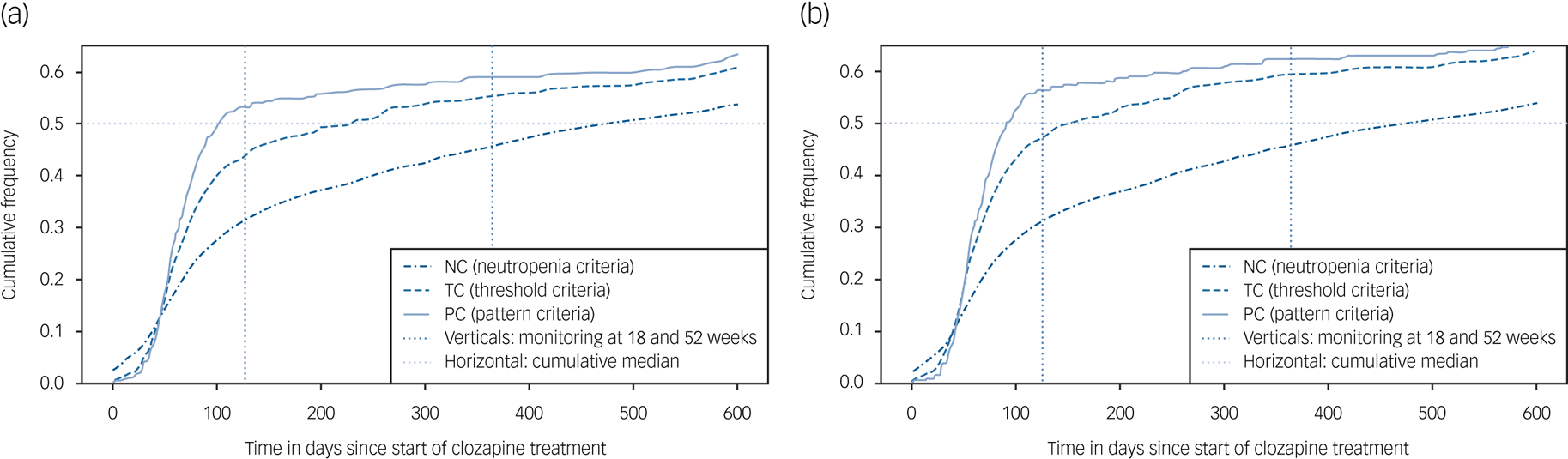
Fig. 1 Cumulative frequency of haematological events (neutropenia, threshold-based and pattern-based agranulocytosis) in people registered on the Central Non-Rechallenge Database (CNRD): (a) includes all recorded clozapine indications and (b) restricted to only people with treatment-resistant psychosis.
Agranulocytosis demographic differences
The heatmaps in Fig. 2 present the (a) prevalence of haematological events and (b) median time in days to the haematological event, stratified by ethnicity, gender and age quantiles. Haematological events including neutropenia, threshold-based agranulocytosis and pattern-based agranulocytosis.
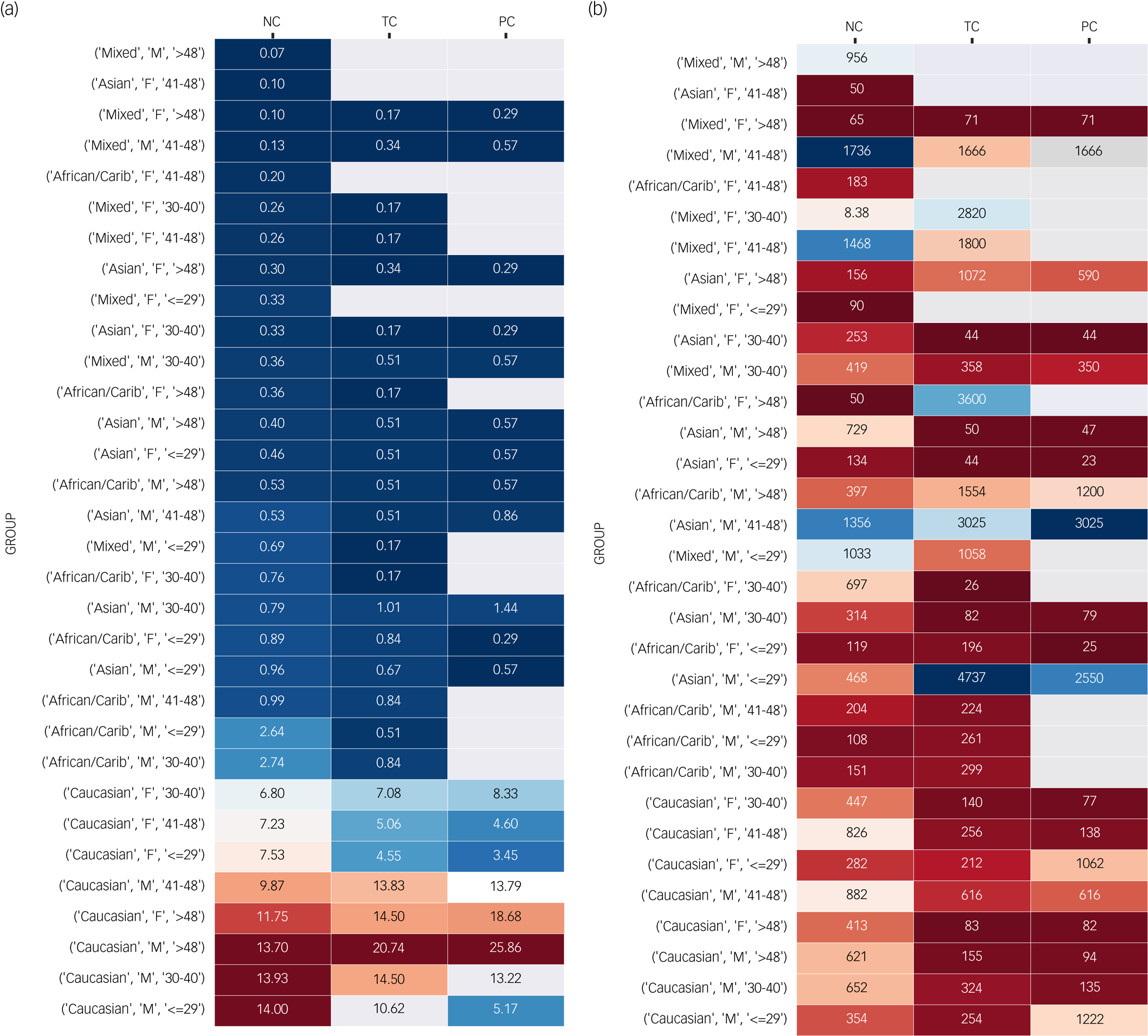
Fig. 2 Heatmaps represent (a) the prevalence and (b) the median time of different haematological events (neutropenia, threshold-based and pattern-based agranulocytosis) for different demographic groups (ethnicity, gender, age). See the Supplementary Appendix for a more detailed version which includes clozapine indication and clozapine rechallenge status. For figure (a), blue represents low prevalence and burgundy represents high prevalence. For figure (b), blue represents longer medians and burgundy represents short medians. Areas in white represent no event in the respective demographic group.
Discussion
Summary of findings
To our knowledge, our study is the first to examine how diagnostic criteria for agranulocytosis affect estimated prevalence rates in people prohibited from receiving clozapine treatment. Overall, we found a substantial difference in the prevalence when a pattern-based criterion for CIA was applied. Review of a large and diverse cohort enabled us, for the first time, to demonstrate the relevance of diagnostic criteria when examining demographic differences in the epidemiology of CIA. In marked contrast to previous studies, CIA (as pattern-based criterion) was least likely to occur in Black individuals, warranting further exploration. In recent years, there has been considerable discussion as to the appropriate classification of agranulocytosis in people receiving clozapine. Our data provide added evidence that the current haematological threshold model overestimates the incidence of CIA.
Comparison with other studies
To date, no published study has specifically examined the impact of diagnostic criteria on prevalence rates of CIA in individuals deemed at high risk. Nonetheless it can be said that CIA epidemiology remains incompletely understood, likely because of broad diagnostic criteria (ANC < 0.5 × 109/L) and heterogeneity of the patient populations studied, and the varied duration of patient follow-up. In a meta-analysis comprising over 260 000 people across 12 countries, the pooled prevalence of agranulocytosis was 0.4% (95% CI 0.3–0.6%). By comparison, recent literature by Northwood et al reported a CIA prevalence of 1.2 and 0.8% deemed unrelated to clozapine.Reference Alvir, Lieberman, Safferman, Schwimmer and Schaaf18,Reference Munro, O'Sullivan, Andrews, Arana, Mortimer and Kerwin20 Personal communications with CPMS revealed that 75 533 people were registered for clozapine use within our study period, providing an estimated prevalence of 0.8% with threshold-based criterion and 0.5% with pattern-based criterion. While direct comparisons with our cohort are only speculative, our somewhat higher threshold-based prevalence compared with the meta-analysis may be attributed to differences in follow-up periods. For instance, the follow-up periods of most studies included in the meta-analysis by Li et alReference Li, Zhong, Lu, Zheng, Wang and Rao8 were considerably shorter than the 20-year time span in our study. Notably, in their subgroup analysis, the authors noted a slightly higher pooled prevalence (0.5% v. 0.4%) of moderate neutropenia (<1.0 × 109/L) compared with agranulocytosis (<0.5 × 109/L). Still, it is likely that application of pattern-based diagnostic criteria in existing studies would have yielded comparable results to the present study and improve the quality of epidemiological and clinical studies concerning CIA in the future.
Earlier evidence indicates that the risk of CIA is greatest in the first 18 weeks of clozapine treatment and is significantly reduced after 1 year, with its incidence falling to 0.07% in the second year.Reference Atkin, Kendall, Gould, Freeman, Liberman and O'Sullivan24 More recently, it has been similarly shown that the weekly incidence rate for CIA peaked at 9 weeks (0.128%), falling to a rolling average weekly incidence of 0⋅001% by 2 years. Consistent with those observations, we observed that a large proportion of agranulocytosis cases occurred within the first year (55% with threshold-based criterion). This was notably more pronounced when using a pattern-based criterion (59% with pattern-based criterion), again underlying how stringent diagnostic criteria may reduce false positive rates. Despite existing reports, a somewhat unexpected finding in our study was the large proportion of people who recorded agranulocytosis beyond two years (37%). Overall, these findings should be interpreted with caution as we were limited in our ability to determine non-clozapine causes for these apparent haematological aberrations, such as chemotherapy.
Risk factors for clozapine-induced agranulocytosis (CIA)
Previous studies on risk factors and prevalence for CIA and its outcome have found inconsistent and diverging conclusions. For example, in their study on CIA incidence and risk factors in the US, Alvir et alReference Alvir, Lieberman, Safferman, Schwimmer and Schaaf18 reported a slightly higher risk in females (RR 1.60, 95% CI 0.99–2.58, after adjustment for age), whereas these findings were not seen in Denmark,Reference Johannsen, Petersen, Nielsen, Jørgensen, Jimenez-Solem and Fink-Jensen25 GermanyReference Stübner, Grohmann, Engel, Bandelow, Ludwig and Wagner26 or Italy.Reference Deliliers19 By contrast – at least when using the threshold-based criterion – prevalence was higher in males than females (19.5% compared with 12.9%) for CIA in our study. The reason for this is unclear; however, epidemiological data from a US cohort of children and adolescents treated with clozapine described an increased risk of moderate neutropenia in males.Reference Maher, Tan, Tossell, Weisinger, Gochman and Miller27 Moreover, it is plausible that there was a greater proportion of non-clozapine causes of agranulocytosis in males, such as infection or concomitant medication such as valproate in our sample.
With respect to ethnicity, Munro et al found an increased risk in Asian individuals compared with White individuals.Reference Munro, O'Sullivan, Andrews, Arana, Mortimer and Kerwin20 However, an earlier study in the same setting reported no difference between ethnic groups regarding agranulocytosis.Reference Atkin, Kendall, Gould, Freeman, Liberman and O'Sullivan24 The authors suggested an increased risk of neutropenia in Black individuals; however, this is likely related to undetected BEN. Interestingly, in the absence of ethnicity data, Northwood et al found that a low baseline ANC (<2.5 × 109/L) predicted an increased risk of minor neutropenia and serious neutropenia unrelated to clozapine, but not increased risk of serious neutropenia related to clozapine. Considering the predominant prevalence of BEN in Black populations, it is likely that these data corroborate our hypothesis of a reduced risk of CIA in this population. Overall, our findings warrant further investigations to characterise clinical and genetic risk factors for CIA. Of note, our study found an increased prevalence of agranulocytosis in White individuals, which is probably because of a genuinely lower risk of CIA in Black individuals. An alternative influence could be earlier treatment discontinuation (as described by Atkins et al)Reference Atkin, Kendall, Gould, Freeman, Liberman and O'Sullivan24 and thus reduced exposure time in Black individuals; nevertheless, further studies are required to elucidate this. Of note, the increased prevalence in older people from our study is consistent with existing literature. Remarkably, the increased prevalence of CIA in White individuals is striking in its similarity to the findings of the risk of agranulocytosis with phenothiazines in the late 1950s.Reference Lambo28
Clinical implications
Agranulocytosis, also known as severe neutropenia, is an acute condition involving severe and dangerously low neutrophil counts that place people at a high risk of severe infection. When assessing whether a below-threshold haematological reading is indicative of CIA, current literature emphasises consideration of factors such as the length of clozapine exposure, concomitant medication and possible infections. However, little attention is paid to the actual diagnostic criteria for agranulocytosis. By most standards, agranulocytosis is defined as a neutrophil count of less than 0.5 × 109/L. A subthreshold ANC always provokes clozapine cessation, as mandated by official monitoring programmes. However, this is often done prematurely – serious infections are most likely to occur at counts of <0.2 × 109/L. Emerging evidence has demonstrated a distinct pattern of continuous and rapid neutrophil count decline to zero or near zero in those with clinically relevant CIA.Reference Taylor, Vallianatou, Whiskey, Dzahini and MacCabe22 Consistently, in our cohort, 70% of people transitioned from a normal ANC to agranulocytosis without passing through neutropenia, casting further doubt on the utility of fortnightly and certainly monthly monitoring beyond the highest period. While not explored in this study, it is certainly plausible that the remaining 30% of people were identified by weekly monitoring.
Taking this into consideration, our study showed a clinically important difference in prevalent CIA using pattern-based compared with threshold-based diagnostic criteria, which could have important implications for epidemiologic surveillance and patient prognosis. Current monitoring schemes require clozapine to be discontinued in the event of an ANC <1.5 × 109/L, with the aim of preventing agranulocytosis by detecting early a fall in ANC. As shown in the Supplementary Appendix, in 1 in 10 people registered on the CNRD, there was a single event of an ANC count <0.5 × 109/L, which is then followed by a spontaneous and prompt normalisation of the ANC. Nevertheless, in most cases such a single event which will inevitably lead to clozapine discontinuation, which in turn puts people at a high risk of severe psychotic relapse from avoidable clozapine discontinuation, reduced responsiveness and discontinuation symptoms.Reference Blackman, Oloyede, Horowitz, Harland, Taylor and MacCabe29
Based on the current findings and to avoid the above-mentioned adverse consequences, it is important that monitoring schemes and regulatory bodies take consideration of neutrophil patterns to correctly discriminate between clozapine and non-CIA. It is plausible that use of pattern-based criteria may improve understanding of risk factors for true CIA and reduce ethnic disparities in clozapine prescription by emphasising the misconceptions about the increased risk of CIA among Black individuals. Overall, a pattern-based classification for CIA may represent a fundamentally important step towards reduced treatment discontinuation and improved care for those with TRP.
Strengths and limitations
This study has several strengths and limitations that require acknowledgement. A major strength of our study is the large sample size that spans an observation period of more than 20 years. Moreover, our cohort is ethnically diverse, with nearly 10% of individuals being of Black ethnicity.
This study has some considerable limitations that need to be accounted for. The main weakness is the need to estimate total number of people receiving clozapine over the observation period. Only the CNRD group had the exact number of people known. In addition, the prevalence estimates, which are based on people from the UK and Ireland, may not generalise to other populations with different ethnicity mixes. A further limitation is that no data on the outcomes of individuals with agranulocytosis (i.e. death) were available, and that information on somatic comorbidities or concomitant medication, which might influence/increase the risk of agranulocytosis (e.g. valproateReference Lally, Malik, Krivoy, Whiskey, Taylor and Gaughran30), was lacking. In addition, people who recorded threshold- and pattern-based agranulocytosis ceased clozapine at the initial instance of neutropenia. Thus, we are unable to determine if clozapine cessation prevented some people from transitioning from threshold- to pattern-based agranulocytosis. Despite our efforts to improve the diagnostic accuracy of CIA through pattern-based criteria, we could not confirm that all cases of severe neutropenia in our sample are necessarily associated with clozapine treatment;Reference Johannsen, Petersen, Nielsen, Jørgensen, Jimenez-Solem and Fink-Jensen25 for example, it is plausible that individuals with BEN may occasionally be misdiagnosed as having CIA by pattern-based agranulocytosis. Finally, we were not able to confirm that all individuals studied were first-starters of clozapine; that is, some may have previous been prescribed clozapine by another clozapine supplier. However, the key finding from our study was that pattern-based criteria give a substantially lower estimate of CIA risk than threshold-based criteria.
Current threshold-based definitions of agranulocytosis over-diagnose cases of potentially CIA. This study demonstrates that a pattern-based definition of agranulocytosis yields markedly lower incidence and prevalence rates of clozapine-associated agranulocytosis. The use of pattern-based criteria for CIA may lead to better patient outcomes through lower clozapine discontinuation rates. Our findings of White ethnicity as having greatest agranulocytosis prevalence warrant further exploration.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2024.104
Data availability
The data that support the findings of this study are available from Clozaril® Patient Monitoring Services. Restrictions apply to the availability of these data, which were used under licence for this study.
Author contributions
E.O.: conceptualisation, methodology, validation, writing – original draft; C.J.B.: conceptualisation, methodology, writing – original draft; O.D.: methodology, writing – review & editing; J.M.L.A.: data curation, formal analysis, writing – review & editing; S.D.S.: methodology, data curation, software, formal analysis, writing – review & editing; K.V.: resources, writing – review & editing; B.F.: methodology, software, formal analysis, writing – review & editing; E.W.: resources, writing – review & editing; D.T.: conceptualisation, methodology, validation, writing – review & editing. All authors have reviewed the manuscript draft critically for important intellectual content and approved the final version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Declaration of interest
None.
Transparency declaration
This manuscript is an honest, accurate and transparent account of the study being reported; no important aspects of the study have been omitted; and any discrepancies from the study as planned have been explained.









eLetters
No eLetters have been published for this article.