Schizophrenia is associated with diverse cognitive and behavioural impairments which may have an impact on independent living. One classification of schizophrenia into three sub-syndromes includes the category ‘psychomotor poverty’, encompassing symptoms of alogia, flatness of affect and avolition (Reference Liddle, Friston and FrithLiddle et al, 1992). This last symptom involves an inability to initiate and persist in goal-directed behaviour, which can seriously constrain patients’ abilities to maintain social interaction. Methods of measuring and influencing the magnitude of spontaneous motor activity in people with schizophrenia are needed to lessen the negative impact of this debilitating symptom (Reference Farrow, Hunter and WilkinsonFarrow et al, 2005).
In this study we recorded the spontaneous, unconstrained motor behaviour of people with schizophrenia in order to determine whether such behaviour might be influenced by modafinil, a novel wakefulness-promoting agent currently licensed in the USA and the UK for the treatment of narcolepsy (Cephalon, 1999). Congruent with its wakefulness-promoting properties, in animal models modafinil increases activity in the anterior hypothalamus and anterior cingulate – structures implicated in arousal (Reference Lin, Hou and JouvetLin et al, 1996). Of particular relevance to modafinil's application in schizophrenia is its activity with respect to the human dopaminergic system. Animal models have differentiated modafinil's pharmacological properties from those of amphetamines (Reference Lin, Hou and JouvetLin et al, 1996), and there is evidence that its wakefulness-promoting effects are not antagonised by haloperidol (Reference Mignot, Nishino and GuilleminaultMignot et al, 1994). In humans, peak modafinil plasma levels occur 2–4 h following oral dosing (Cephalon, 1999). As well as its use in the treatment of narcolepsy, modafinil has been investigated in a range of psychiatric disorders that have fatigue or anergia as a central feature, such as depression (Reference DeBattista, Lembke and SolvasonDeBattista et al, 2004). There have been preliminary reports of its general use in schizophrenia to ameliorate negative symptoms (e.g. Reference Rosenthal and BryantRosenthal & Bryant, 2003; given at a dosage of 100–200 mg per day).
In order to investigate whether the lower dosage of modafinil might be of use in the remediation of avolition in schizophrenia we conducted a trial of its acute effects, including an objective measure of volume of movement.
METHOD
In a randomised double-blind study with a placebo-controlled crossover design, 18 right-handed patients with schizophrenia (DSM–IV; American Psychiatric Association, 1994) received a single dose of either 100 mg modafinil or placebo. A nineteenth participant who relapsed midway through the study was unable to provide two actigraphic data-sets and was excluded from all analyses (a CONSORT diagram is presented in a data supplement to the online version of this report). All participants were community-based out-patients but for monitoring purposes were admitted on two separate occasions (during ‘drug’ and ‘placebo’ conditions, a week apart) to a psychiatric ward for 24 h. Modafinil or placebo was administered at 08.00 h and movement recording began at 12.00 h. Participants had a mean age of 38 years (s.d.=9), an illness duration of 15 years (s.d.=10), a Scale for the Assessment of Negative Symptoms (SANS; Reference AndreasenAndreasen, 1985) score of 11.0 (s.d.=2.3), a Scale for the Assessment of Positive Symptoms (Reference AndreasenAndreasen, 1985) score of 3.7 (s.d.=2.1), and a Mini-Mental State Examination (Reference Folstein, Folstein and McHughFolstein et al, 1975) score of 29.4 (s.d.=0.6). Twelve patients were taking oral atypical antipsychotic medication, two were taking oral typical medication and four were receiving typical depot medication. All patients were stable at the time of the study, having received no change in oral medication for a minimum of 4 weeks or in depot medication for 12 weeks prior to enrolment.
Patients wore an Actiwatch (Cambridge Neurotechnology Ltd, Cambridge, UK) to measure their cumulative activity over a 20 h period. The Actiwatch is a wrist-worn device containing a miniature uniaxial accelerometer, which produces a digital integration of the amount and duration of all movement over 0.05 g (equivalent to an acceleration of approximately 0.5 m/s2). As an indicator of normal daytime activity, a study of 107 young people aged 16–19 years recorded mean Actiwatch readings of 162 565 (s.d.=68 620), a dimensionless measure, over a 24 h period (Nancy Butte, personal communication, 2005). All participants gave written informed consent, and the study was approved by the North Sheffield Research Ethics Committee.
RESULTS
Over a 20 h period, during the modafinil condition participants exhibited significantly greater motor activity (mean 135 090, s.d.=59 341) than when receiving placebo (mean 120 778, s.d.=56783): mean increase 12%; paired t=2.46, P=0.012 (Fig. 1). There was a significant negative correlation between SANS avolition score and Actiwatch-measured motor activity (r=–0.41; P=0.044, one-tailed t-test) during the placebo condition, but no such correlation during the modafinil condition (r=–0.31; P=0.104). There was no correlation between age, duration of illness or chlorpromazine equivalent drug dosage and motor activity.
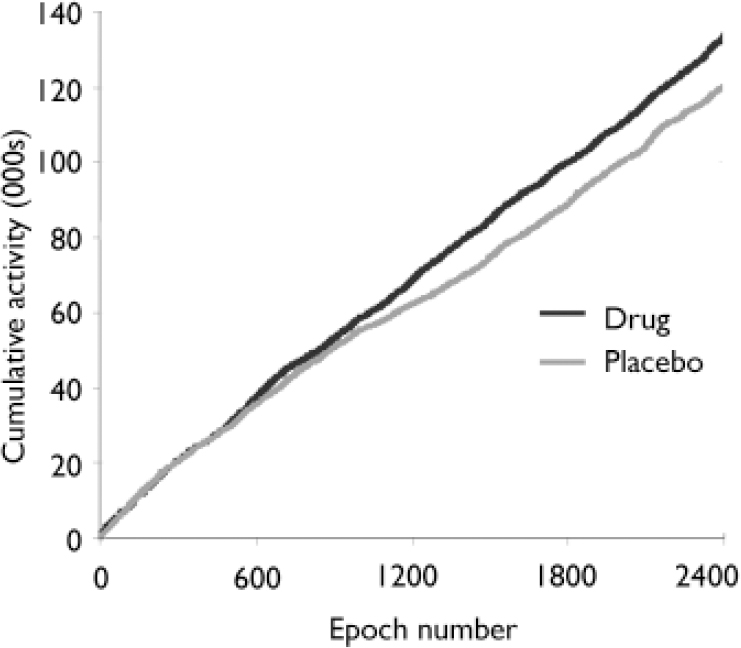
Fig. 1 Average cumulative activity over 20 h (2400 epochs of 30 s) for 18 patients with schizophrenia during drug (modafinil) and placebo conditions. Patients were significantly more active during the drug condition (P=0.012). Cumulative activity measure (y axis) isdimensionless (see text for details).
DISCUSSION
In a group of community-based patients with chronic but stable schizophrenia, administration of a single dose of 100 mg of modafinil was associated with significantly increased motor activity over a 20 h period. This increase in activity was detected on average approximately 9 h after drug administration. Modafinil's positive effect on activity may reflect a generalised increase in motor behaviour, or an effect that is more specific to those most ‘avolitional’ at the outset. In support of the latter proposal, post hoc examination of our data revealed that there was a significant negative correlation between motor activity on placebo and percentage increased activity on modafinil (r=–0.43; P=0.037).
Using the Actiwatch to augment ratings obtained using the SANS facilitates a more objective, scalar measure of bodily movement and severity of avolition. This additional quantification is simple to acquire, unobtrusive to patients and may be particularly useful where change is anticipated (as with putative behavioural or pharmacological interventions).
Our study findings are potentially limited, however, in two respects. First, we studied patients within the confines of a psychiatric ward and therefore must remain circumspect in drawing inferences about ambulatory, community-based activity. Second, although we have a sum of the total activity undertaken by our participants, we are limited in the extent to which we may infer purpose (i.e. goal direction) in such activity. Nevertheless, our data suggest that administration of modafinil is associated with an increase in unconstrained motor activity in chronic schizophrenia and this may have implications for future strategies aimed at increasing volitional behaviour in those affected by negative symptoms. In the light of recent reports of modafinil's cognitive-enhancing effects in chronic schizophrenia (Reference Turner, Clark and Pomarol-ClotetTurner et al, 2004), and with further understanding of the brain systems through which it acts (Reference Spence, Green and WilkinsonSpence et al, 2005; Reference Hunter, Ganesan and WilkinsonHunter et al, 2006), the clinical potential for its use remains promising, although further work is required.
Acknowledgements
M.D.H. is supported by the Wellcome Trust. We thank Professor Nancy Butte, Baylor College of Medicine, Texas, USA, for normative Actiwatch data. We also thank Dr Russell Green and nursing staff from Rowan Ward, The Longley Centre, Northern General Hospital, Sheffield, UK.




eLetters
No eLetters have been published for this article.