Bipolar disorder occurs in 1-3% of the population (Reference Angst and SellaroAngst & Sellaro, 2000; Reference Akiskal, Bourgeois and AngstAkiskal et al, 2000). Despite naturalistic treatment in the community or intensive treatment at academic centres, patients remain symptomatic approximately half of the year, and the time depressed exceeds the time manic or hypomanic by a factor of three (Reference Judd, Akiskal and SchettlerJudd et al, 2002; Reference Post, Denicoff and LeverichPost et al, 2003a ; Reference Kupka, Luckenbaugh and PostKupka et al, 2005). Bipolar disorder is potentially lethal, with a 10-20% lifetime risk of dying by suicide (Reference Goodwin and JamisonGoodwin & Jamison, 1990).
Despite these staggering human and public health statistics, there have been relatively few controlled studies of the use of antidepressants in the treatment of acute bipolar depression (Reference Cohn, Collins and AshbrookCohn et al, 1989; Reference Himmelhoch, Thase and MallingerHimmelhoch et al, 1991; Reference Young, Joffe and RobbYoung et al, 2000; Reference Nemeroff, Evans and GyulaiNemeroff et al, 2001) compared with numerous controlled trials in unipolar depression. There has been one moderately sized (n=60) randomised, single-blind study comparing the efficacy of paroxetine and venlafaxine (Reference Vieta, Martinez-Aran and GoikoleaVieta et al, 2002) in bipolar depression. A small comparative trial by Sachs et al (Reference Sachs, Lafer and Stoll1994) suggested that bupropion - which increases brain dopamine levels in the dorsal and ventral striatum upon acute and chronic administration (Nomikos et al, Reference Nomikos, Damsma and Wenkstern1989, Reference Nomikos, Damsma and Wenkstern1992) and also possesses some effects on noradrenaline - showed comparable acute efficacy to the noradrenergic tricyclic antidepressant desipramine, when added to ongoing treatment with a mood stabiliser for acute treatment of bipolar depression. However, the rate of switching into mania or hypomania on desipramine during acute and continuation treatment was considerably higher (37.5%) compared with bupropion (13.3%) (Reference Guille, Shriver and DemopulosGuille et al, 1999). These data suggested the possibility that either the anticholinergic effects associated with the older tricyclic compounds in general or the potent selective effects of desipramine on noradrenaline reuptake could account for these differences. Gijsman et al (Reference Gijsman, Geddes and Rendell2004) in a meta-analysis found comparable efficacy but higher switch rates for tricyclic antidepressants compared with the newer antidepressants in acute trials for bipolar depression.
Based on the early study of Sachs et al (Reference Sachs, Lafer and Stoll1994), we predicted that the three antidepressants bupropion, sertraline and venlafaxine would achieve equal rates of response, but that venlafaxine (like desipramine) would show a higher rate of switching into hypomania or mania because of its additional noradrenergic effects. Consistent with the latter hypothesis, the study of Vieta et al (Reference Vieta, Martinez-Aran and Goikolea2002) found an increased rate of switching on venlafaxine (13.3%) in a single-blind, randomised comparison with paroxetine (3.0%) for 6 weeks in bipolar depression.
METHOD
This study was a 10-week randomised trial of 184 patients comparing bupropion, sertraline and venlafaxine as adjuncts to one or more mood stabilisers. Because of the anticipated prolonged unavailability of one set of masked compounds at study outset, the first 28 patients were randomised by a data-coordinating centre in Bethesda but were treated with open medications at each site; the next 156 patients were studied in a randomised, double-blind fashion. Each of the three drugs had an identically matched placebo and all patients took two sets of compounds throughout this study (one active and one placebo).
The study method has been previously described for a smaller subgroup of these patients in an interim analysis of the overall response and switch rates into hypomania or mania of the antidepressants as a group prior to unmasking the data (Post et al, Reference Post, Altshuler and Frye2001a ,Reference Post, Nolen and Kupka b ). This is the report of the response and switch rates of each of the three separate antidepressants, and it includes the entire cohort of patients randomised to the first acute phase of adjunctive treatment of bipolar depression.
Patients were included when they met criteria for DSM-IV bipolar depression (American Psychiatric Association, 1994) and had an Inventory of Depression Symptomatology (IDS; Rush et al, Reference Rush, Giles and Schlesser1986, Reference Rush, Gullion and Basco1996) scale score of at least 16; a Clinical Global Impression scale for Bipolar Disorder (CGI-BP; Reference Spearing, Post and LeverichSpearing et al, 1997) depression severity score of at least 3; or the decision on the part of the physician of a need to treat the depressive episode because of its functional impact. Most of these patients were also rated for severity of depression and mania on a daily basis on the National Institute of Mental Health - Life Chart Method (NIMH-LCM), as described by Leverich et al (Reference Leverich, Altshuler and Frye2006).
Patients who showed clinically relevant levels of mania - a Young Mania Rating Scale (YMRS; Reference Young, Biggs and ZieglerYoung et al, 1978) score of at least 14 or a CGI-BP mania severity score of at least 3 - at baseline were excluded from the study, leaving a sample of 174 patients. The antidepressants were added to an average of 1.4 other mood stabilisers or antimanic agents. These medications included lithium (64 patients), valproate (93 patients), carbamazepine (16 patients), lamotrigine (8 patients), typical antipsychotics (8 patients) and atypical antipsychotics (30 patients). The maintenance medications were distributed equally among all three antidepressants except for lithium, which was present in 21.6% of those taking bupropion, 36.2% of those taking sertraline and 47.7% of those taking venlafaxine (P=0.01).
All the patients who were randomised had a depressive episode despite ongoing treatment with one or more mood stabilisers within specified dosage and therapeutic blood level guidelines. The minimum blood level guidelines for the mood stabilisers were 0.7 mmol/l for lithium, 50 μg/ml for valproate and 4 μg/ml for carbamazepine. Dosages of these drugs, as well as of typical or atypical antipsychotics, or ongoing benzodiazepines in prophylaxis, were held steady during the course of the protocol except for dose reductions because of side-effects. However, acute augmentation with benzodiazepines or chloral hydrate for a maximum of 7 days was allowed for relief of initial treatment-emergent insomnia or anxiety.
All patients were participants in the Stanley Foundation Bipolar Network at a time when it was funded by the Stanley Medical Research Institute, the National Institute for Mental Health and each local academic site (Reference Leverich, Nolen and RushLeverich et al, 2001; Reference Post, Nolen and KupkaPost et al, 2001b ; Reference Suppes, Leverich and KeckSuppes et al, 2001). All patients provided written informed consent for participation in the Network in general, and additional specific written informed consent for participation in this randomised clinical trial as approved by each local institutional review board. In the randomisation, the patients were stratified on the basis of presence or absence of a prior history of DSM-IV-defined rapid cycling in the year prior to study entry. At the European sites in The Netherlands and Germany, patients were randomised only between sertraline and venlafaxine because bupropion was not approved or available in these countries.
The antidepressants were titrated towards maximum dosages based on side-effects tolerability and clinical discretion during the 10-week acute trial. Starting and maximal dosages respectively for each compound were: bupropion 75-450 mg/day, sertraline 50-200 mg/day and venlafaxine 37.5-375 mg/day.
Patients were seen weekly for 2 weeks and then every 2 weeks for the duration of the 10-week acute-treatment trial. Symptom assessments were conducted using the IDS, the YMRS and the CGI-BP at each visit. The CGI-BP part I, or severity scale, parallels that of the original CGI, but the new format allows separate ratings for severity of depression, mania and overall illness. The scale ranges are 1 not ill, 2 minimally ill, 3 mildly ill, 4 moderately ill, 5 markedly ill, 6 severely ill and 7 very severely ill.
Although those not responding to antidepressant therapy were subsequently offered re-randomisation to another antidepressant (Reference Post, Altshuler and FryePost et al, 2001a ; Reference Leverich, Altshuler and FryeLeverich et al, 2006), the analysis in this report only considered the first parallel-group randomised phase, so that all patients would be represented only once and data would be suitable for independent statistics. All data presented represent the intention-to-treat analysis.
Three outcome variables were assessed: antidepressant response, antidepressant remission and antidepressant-related switch into mania or hypomania. Response was operationalised as either a 50% or greater improvement in IDS score, or a decrease in the CGI-BP depression score of at least 2 points. Response rates were reported at the study end-point and the time to response was calculated for each drug. Remission criteria included an IDS score below 12 and/or a CGI-BP depression severity score of 1 (normal, not ill) at study end-point. A switch into hypomania or mania was operationalised as either a 2-point increase at any point in the trial on the CGI-BP (suggesting a clinically meaningful switch), or a CGI-BP manic severity score of at least 3 (i.e. at least mildly manic) or a YMRS score above 13 at any visit.
Statistical analysis
The Stanley Foundation Bipolar Network ended in 2002 and the analysis of these data was supported by the Stanley Research Medical Institute. The core data repository was transferred from the data coordinating centre in Bethesda to the University of California at Los Angeles where analyses for this study were conducted. The patients were entered into this study at the seven different sites beginning in March 1996 and ending in November 2002.
When demographic data, response rates and switch rates were analysed separately for the 27 open and 147 masked randomisations, they were not found to be statistically significantly different (details available in the data supplement to the online version of this paper). Therefore, the data are presented for the combined analysis of the 174 patients for simplicity of presentation and in order to have maximum power for detecting differences in switch rates among the three antidepressant drugs. This was felt to be justified because our initial hypothesis was that there would be no significant difference in initial degrees of acute responsiveness among these three antidepressants, but as in the Sachs et al (Reference Sachs, Lafer and Stoll1994) data for desipramine, venlafaxine would have a higher switch rate than the other two drugs.
Kaplan-Meier curves were used to construct survival curves among the treatment arms in time-to-event data. Differences in strata were assessed using the log-rank test. Chi-squared tests were used to assess significance for categorical data.
RESULTS
Patient characteristics are summarised in Table 1 and the flow of patients is show in Fig. 1. Participants showed balanced gender distribution, were an average of 41.7 years of age and had 19.1 years of illness, averaging 14.3 prior episodes of depression and 12.2 prior episodes of mania. The majority (73%) of diagnoses were bipolar I disorder, 26% were bipolar II disorder and 1% bipolar disorder not otherwise specified. More than a quarter (27%) had a prior history of rapid cycling. Fifty-one patients were randomised to receive bupropion, 58 to sertraline and 65 to venlafaxine. Patients were treated with an average maximum daily dosage of 286 (s.d.=132) mg for bupropion, 192 (s.d.=104) mg for sertraline or 195 (s.d.=112) mg for venlafaxine.

Fig. 1 Study profile.
Table 1 Demographic factors and course of illness data
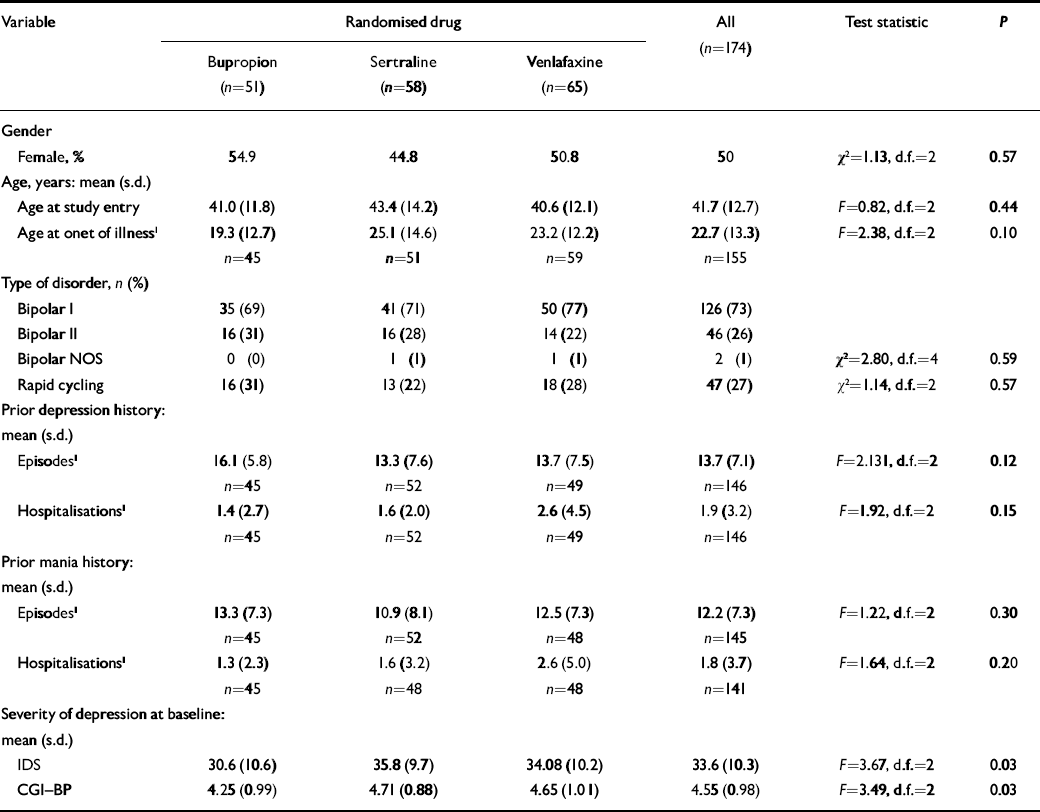
| Variable | Randomised drug | All (n=174) | Test statistic | P | ||||
|---|---|---|---|---|---|---|---|---|
| Bupropion (n=51) | Sertraline (n=58) | Venlafaxine (n=65) | ||||||
| Gender | ||||||||
| Female, % | 54.9 | 44.8 | 50.8 | 50 | χ2=1.13, d.f.=2 | 0.57 | ||
| Age, years: mean (s.d.) | ||||||||
| Age at study entry | 41.0 (11.8) | 43.4 (14.2) | 40.6 (12.1) | 41.7 (12.7) | F=0.82, d.f.=2 | 0.44 | ||
| Age at onet of illness 1 | 19.3 (12.7) | 25.1 (14.6) | 23.2 (12.2) | 22.7 (13.3) | F=2.38, d.f.=2 | 0.10 | ||
| n=45 | n=51 | n=59 | n=155 | |||||
| Type of disorder, n (%) | ||||||||
| Bipolar I | 35 (69) | 41 (71) | 50 (77) | 126 (73) | ||||
| Bipolar II | 16 (31) | 16 (28) | 14 (22) | 46 (26) | ||||
| Bipolar NOS | 0 (0) | 1 (1) | 1 (1) | 2 (1) | χ2=2.80, d.f.=4 | 0.59 | ||
| Rapid cycling | 16 (31) | 13 (22) | 18 (28) | 47 (27) | χ2=1.14, d.f.=2 | 0.57 | ||
| Prior depression history: | ||||||||
| mean (s.d.) | ||||||||
| Episodes 1 | 16.1 (5.8) | 13.3 (7.6) | 13.7 (7.5) | 13.7 (7.1) | F=2.131, d.f.=2 | 0.12 | ||
| n=45 | n=52 | n=49 | n=146 | |||||
| Hospitalisations 1 | 1.4 (2.7) | 1.6 (2.0) | 2.6 (4.5) | 1.9 (3.2) | F=1.92, d.f.=2 | 0.15 | ||
| n=45 | n=52 | n=49 | n=146 | |||||
| Prior mania history: | ||||||||
| mean (s.d.) | ||||||||
| Episodes 1 | 13.3 (7.3) | 10.9 (8.1) | 12.5 (7.3) | 12.2 (7.3) | F=1.22, d.f.=2 | 0.30 | ||
| n=45 | n=52 | n=48 | n=145 | |||||
| Hospitalisations 1 | 1.3 (2.3) | 1.6 (3.2) | 2.6 (5.0) | 1.8 (3.7) | F=1.64, d.f.=2 | 0.20 | ||
| n=45 | n=48 | n=48 | n=141 | |||||
| Severity of depression at baseline: | ||||||||
| mean (s.d.) | ||||||||
| IDS | 30.6 (10.6) | 35.8 (9.7) | 34.08 (10.2) | 33.6 (10.3) | F=3.67, d.f.=2 | 0.03 | ||
| CGI–BP | 4.25 (0.99) | 4.71 (0.88) | 4.65 (1.01) | 4.55 (0.98) | F=3.49, d.f.=2 | 0.03 | ||
CGI–BP, Clinical Global Impression – Bipolar Disorder; IDS, Inventory of Depression Symptomatology; NOS, not otherwise specified
1. Not reported by several patients in each group
About a third of the patients withdrew from the trial prematurely, for lack of improvement or worsening mood, including 29% of those taking bupropion (n=15), 28% taking sertraline (n=16) and 38% taking venlafaxine (n=25) (Table 2). Other withdrawals for side-effects or administrative reasons did not differ among the three drugs.
Table 2 Early discontinuation from 10-week adjunctive antidepressant trial
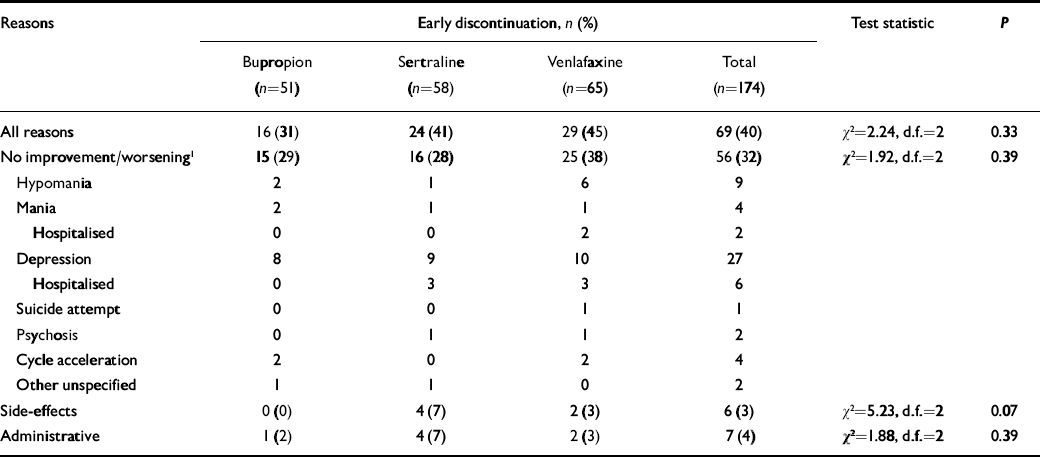
| Reasons | Early discontinuation, n (%) | Test statistic | P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Bupropion (n=51) | Sertraline (n=58) | Venlafaxine (n=65) | Total (n=174) | ||||||
| All reasons | 16 (31) | 24 (41) | 29 (45) | 69 (40) | χ 2=2.24, d.f.=2 | 0.33 | |||
| No improvement/worsening 1 | 15 (29) | 16 (28) | 25 (38) | 56 (32) | χ 2=1.92, d.f.=2 | 0.39 | |||
| Hypomania | 2 | 1 | 6 | 9 | |||||
| Mania | 2 | 1 | 1 | 4 | |||||
| Hospitalised | 0 | 0 | 2 | 2 | |||||
| Depression | 8 | 9 | 10 | 27 | |||||
| Hospitalised | 0 | 3 | 3 | 6 | |||||
| Suicide attempt | 0 | 0 | 1 | 1 | |||||
| Psychosis | 0 | 1 | 1 | 2 | |||||
| Cycle acceleration | 2 | 0 | 2 | 4 | |||||
| Other unspecified | 1 | 1 | 0 | 2 | |||||
| Side-effects | 0 (0) | 4 (7) | 2 (3) | 6 (3) | χ 2=5.23, d.f.=2 | 0.07 | |||
| Administrative | 1 (2) | 4 (7) | 2 (3) | 7 (4) | χ 2=1.88, d.f.=2 | 0.39 | |||
1. Multiple symptoms were possible
Overall, the percentage of patients who left the study prior to 10 weeks for any reason was 31% of those taking bupropion, 41% of those taking sertraline and 45% of those taking venlafaxine.
Response and remission rates
At week 10, using the IDS or CGI-BP criterion, response rates were 49% for bupropion, 53% for sertraline and 51% for venlafaxine; remission rates (either IDS score ⩽12 or CGI-BP score=1) were 41%, 36% and 34% respectively (Table 3). There was no significant difference between the groups. To evaluate if cotreatment with lithium influenced these results a log linear model was fitted to the data. The inclusion of a relationship between lithium and response or remission did not improve the fit of the log linear model (response: Δχ2=0.196, d.f.=1, P>0.5; remission: Δχ2=0.112, d.f.=1, P>0.5).
Table 3 Rates of antidepressant response, remission and switching into hypomania or mania
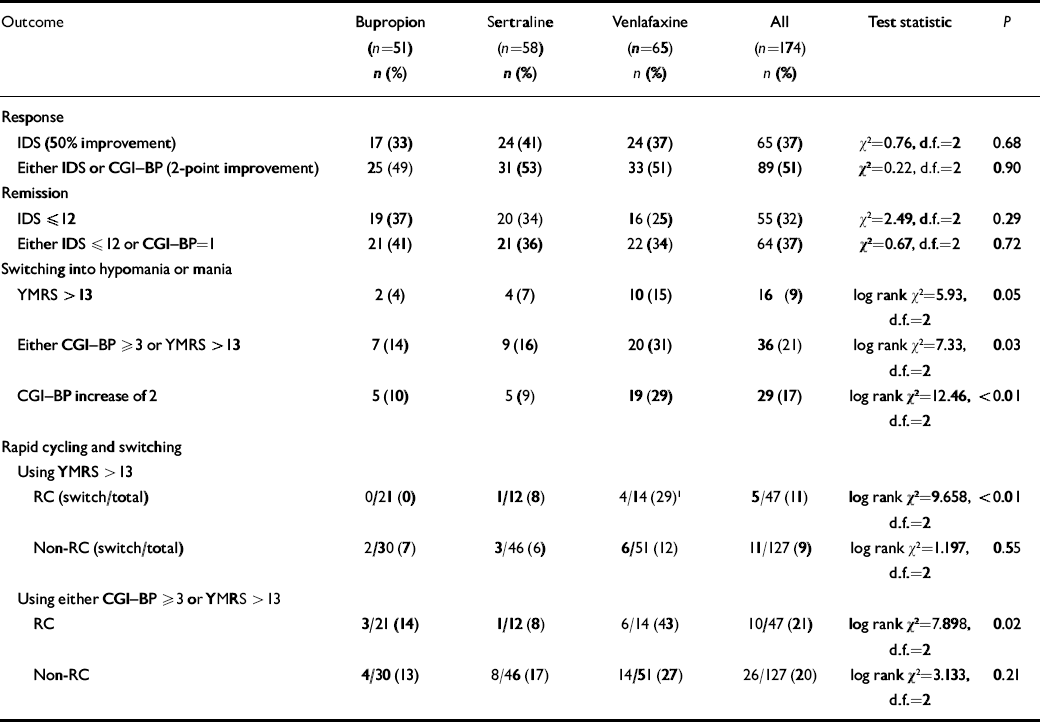
| Outcome | Bupropion (n=51) n (%) | Sertraline (n=58) n (%) | Venlafaxine (n=65) n (%) | All (n=174) n (%) | Test statistic | P |
|---|---|---|---|---|---|---|
| Response | ||||||
| IDS (50% improvement) | 17 (33) | 24 (41) | 24 (37) | 65 (37) | χ2=0.76, d.f.=2 | 0.68 |
| Either IDS or CGI–BP (2-point improvement) | 25 (49) | 31 (53) | 33 (51) | 89 (51) | χ2=0.22, d.f.=2 | 0.90 |
| Remission | ||||||
| IDS ⩽ 12 | 19 (37) | 20 (34) | 16 (25) | 55 (32) | χ2=2.49, d.f.=2 | 0.29 |
| Either IDS ⩽ 12 or CGI–BP=1 | 21 (41) | 21 (36) | 22 (34) | 64 (37) | χ2=0.67, d.f.=2 | 0.72 |
| Switching into hypomania or mania | ||||||
| YMRS > 13 | 2 (4) | 4 (7) | 10 (15) | 16 (9) | log rank χ2=5.93, d.f.=2 | 0.05 |
| Either CGI–BP ⩾ 3 or YMRS > 13 | 7 (14) | 9 (16) | 20 (31) | 36 (21) | log rank χ2=7.33, d.f.=2 | 0.03 |
| CGI–BP increase of 2 | 5 (10) | 5 (9) | 19 (29) | 29 (17) | log rank χ2=12.46, d.f.=2 | <0.01 |
| Rapid cycling and switching | ||||||
| Using YMRS > 13 | ||||||
| RC (switch/total) | 0/21 (0) | 1/12 (8) | 4/14 (29)1 | 5/47 (11) | log rank χ2=9.658, d.f.=2 | < 0.01 |
| Non-RC (switch/total) | 2/30 (7) | 3/46 (6) | 6/51 (12) | 11/127 (9) | log rank χ2=1.197, d.f.=2 | 0.55 |
| Using either CGI–BP ⩾ 3 or YMRS > 13 | ||||||
| RC | 3/21 (14) | 1/12 (8) | 6/14 (43) | 10/47 (21) | log rank χ2=7.898, d.f.=2 | 0.02 |
| Non-RC | 4/30 (13) | 8/46 (17) | 14/51 (27) | 26/127 (20) | log rank χ2=3.133, d.f.=2 | 0.21 |
CGI–BP, Clinical Global Impression – Bipolar Disorder; IDS, Inventory of Depression Symptomatology; RC, rapid cycling; YMRS, Young Mania Rating Scale
Switch rates
Most patients did not switch into hypomania or mania when prescribed acute adjunctive antidepressant therapy, regardless of the antidepressant prescribed. However, on most measures and analyses venlafaxine showed a higher risk of patients switching into hypomania or mania than bupropion or sertraline. Using the requirement of a 2-point or greater increase on the CGI-BP mania severity rating, switching occurred in 10% of patients taking bupropion, 9% taking sertraline, and 29% taking venlafaxine. To control for the effect of withdrawals on the relative risk of switching, these data were analysed using survival analysis (Fig. 2). Results revealed a significant overall difference between the three groups (log rank χ2=12.462, d.f.=2, P=0.002). Controlling for lithium yielded the same result (log rank χ2=11.99, d.f.=2, P<0.01). Post hoc analysis of this result demonstrated that the effect was mainly driven by a significant difference in the risk of switching time between venlafaxine and both sertraline and bupropion (venlafaxine v. sertraline, adjusted for lithium: log rank χ2=6.70, d.f.=1, P=0.01; venlafaxine v. bupropion, adjusted for lithium: log rank χ2=8.16, d.f.=1, P<0.01), whereas there was no significant difference between sertraline and bupropion (adjusted for lithium: log rank χ2=0.02, d.f.=1, P=0.90).
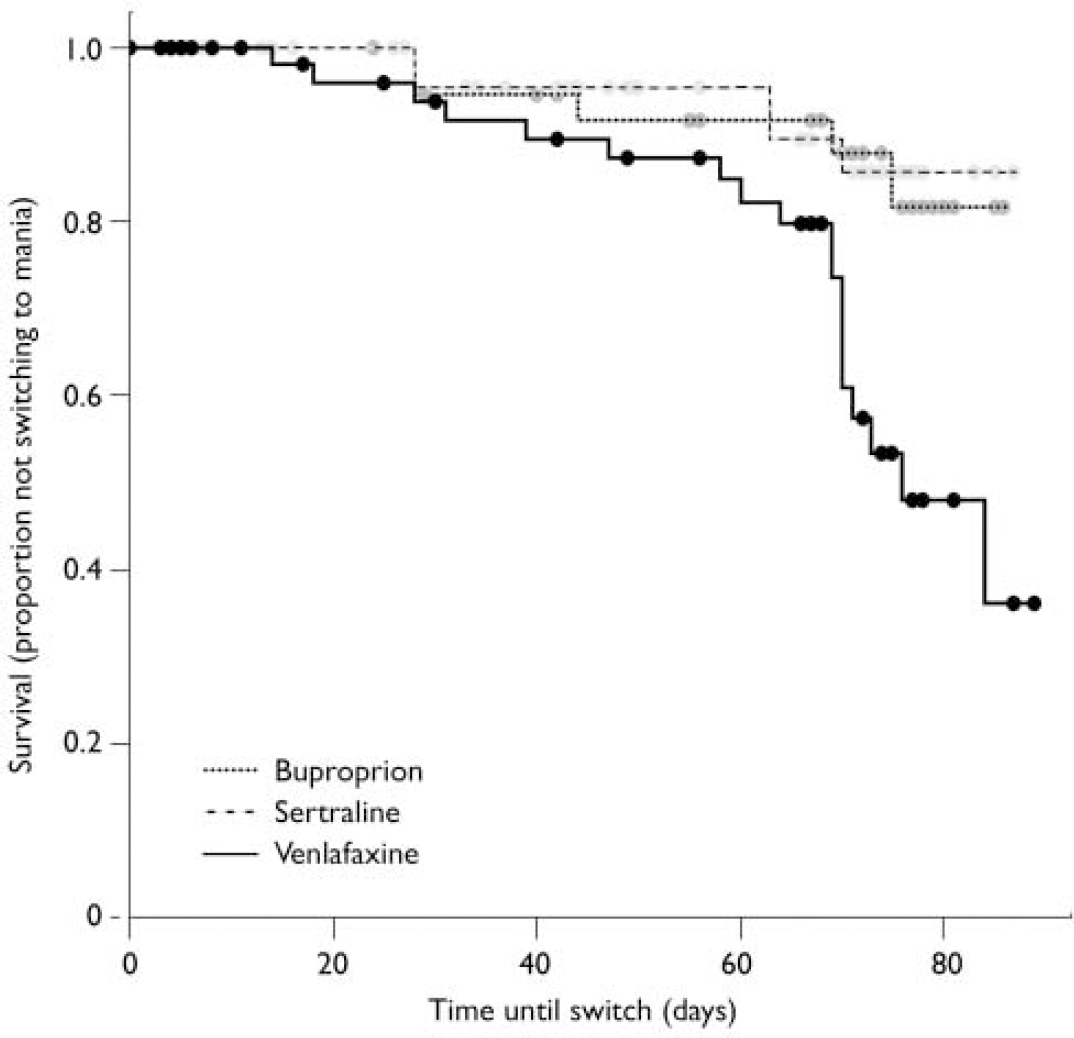
Fig. 2 Increased switch rate (defined as a 2-point increase in manic severity score on the Clinical Global Impression - Bipolar Disorder scale) for venlafaxine compared with bupropion and sertraline.
Using the more conservative YMRS threshold score of 413, only 4% of patients on bupropion and 7% of patients on sertraline switched into hypomania or mania by study end-point, but 15% of patients on venlafaxine had switched by study end-point (log rank χ2=5.91, d.f.=2, P=0.052; Fig. 3). The effect of medication on the survival rates was not significant. In addition, controlling for possible effects of lithium did not influence the results (log rank χ2=5.80, d.f.=2, P=0.055).
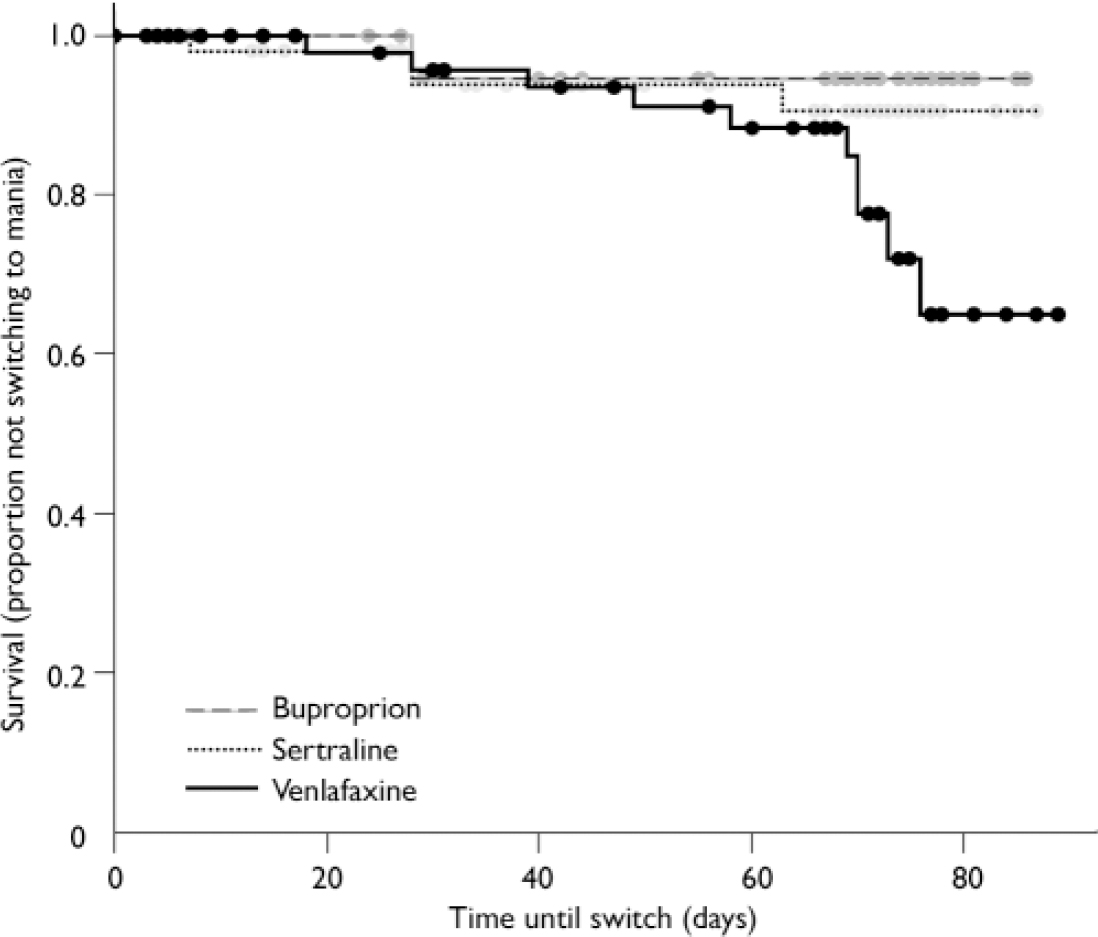
Fig. 3 Increased switch rate, defined more conservatively by Young Mania Rating Scale scores >13, on venlafaxine compared with bupropion and sertraline.
Using the combined switch criterion of CGI-BP severity of mania ⩾3 or YMRS>13, the switch rate of the bupropion group was 14%, the switch rate of the sertraline group was 16% and the switch rate for venlafaxine was 31%. This difference was significant both when lithium was not included (log rank χ2=7.33, d.f.=2, P=0.03) and when lithium was included (log rank χ2=7.55, d.f.=2, P=0.02). The results of a post hoc analysis again showed that the difference was driven by venlafaxine. The hazard for switching was not significantly different between bupropion and sertraline after adjustment for lithium (log rank χ2=0.38, d.f.=1, P=0.54), whereas the patients treated with venlafaxine experienced significantly higher switch rates than the patients treated with bupropion (adjusted for lithium, log rank χ2=6.35, d.f.=1, P=0.01). The difference in the risk for switching between venlafaxine and sertraline was not significant at α=0.05, but the data suggest a trend towards a difference (adjusted for lithium, log rank χ2=3.18, d.f.=1, P=0.07).
Rapid cycling
There was a strong interaction between the rapid-cycling status of patients and the relative risk of switching for the three medication groups. In those without rapid-cycling disorder the risk of switching was identical for all three medication groups (log rank χ2=1.197, d.f.=2, P=0.55), but the difference between the three medications was highly significant among rapid-cycling patients (log rank χ2=9.66, d.f.=2, P<0.01). The pattern of this difference for the rapid-cycling group was the familiar result that bupropion had a significantly lower risk for switching than venlafaxine (log rank χ2=9.07, d.f.=1, P<0.01), whereas there was no significant difference between bupropion and sertraline (log rank χ2=1.9, d.f.=1, P=0.17) or between sertraline and venlafaxine (log rank χ2=2.1, d.f.=1, P=0.15).
DISCUSSION
Overall response and switch rates on antidepressant augmentation
To our knowledge, this is the largest randomised comparative study of the response and switch rates of modern (i.e. non-tricyclic) antidepressants in the adjunctive treatment of acute bipolar depression. All of these unimodal antidepressants are approved by the US Food and Drug Administration (FDA) for the treatment of major depression, but are not FDA-approved for use in bipolar depression and are not widely studied by European authorities.
The three agents with different mechanisms of action were assessed for their relative magnitude of acute antidepressant response when added to ongoing treatment with mood stabilisers in this 10-week trial. Overall response (49-53%) and remission rates (34-41%) were similar to those often seen in antidepressant monotherapy trials in unipolar illness, using the traditional 50% improvement and absolute criteria respectively. Substantial numbers of patients (31-45%) withdrew prematurely from the trial because of lack of improvement or worsening of either depressed or manic mood, indicating a continuing need to find more effective agents for even the acute treatment of bipolar depression.
Most patients in this acute treatment trial did not switch into hypomania or mania with the addition of an antidepressant to their ongoing mood stabiliser regimen. Overall, across all medication groups, 9% switched by the more stringent criteria of YMRS score > 13 (Table 3), whereas more than double that (21%) switched using the CGI-BP severity score (⩾3) of at least mild mania.
The CGI-BP cut-off of mild mania is a more permissive measure than the YMRS score, and this two-fold difference in what is categorised as a switch depending on which scale or cut-off score is used needs to be considered by investigators in the future when designing trials and specifying outcome measures. Use of different threshold criteria may account for some of the large discrepancies in the field regarding reported switch rates. Similarly, the inclusion or exclusion of patients with rapid-cycling disorder in a study also contributes to these differences in switch rates.
Switch rates among the three antidepressants
Venlafaxine had a greater risk for inducing switching than the other two agents, i.e. bupropion as a dopamine-active agent and sertraline as a representative serotonin selective reuptake inhibitor (SSRI) (Nomikos et al, Reference Nomikos, Damsma and Wenkstern1989, Reference Nomikos, Damsma and Wenkstern1992; Reference Ascher, Cole and ColinAscher et al, 1995).
Venlafaxine's dual actions on serotonin and noradrenaline reuptake (Reference Montgomery, Feighner and FrazerMontgomery et al, 1993), which may account for its greater efficacy in patients with unipolar depression compared with SSRIs in recent meta-analyses (Reference Thase, Entsuah and RudolphThase et al, 2001; Reference Stahl, Entsuah and RudolphStahl et al, 2002), could have contributed to the higher rate of switching with this agent compared with the other two agents.
These data are consistent with those from the single-blind randomised study of Vieta et al (Reference Vieta, Martinez-Aran and Goikolea2002), who found a greater switch risk for venlafaxine (mean dosage 180 mg) compared with the SSRI paroxetine (mean dosage 30 mg), although patients were only assessed for 6 weeks and the YMRS criterion for a switch was a score of 11 as opposed to the 14 used here. Nonetheless, the switch rates for venlafaxine (13.3%) v. paroxetine (3.0%) in that study were of a similar magnitude to the switch rates in this study for venlafaxine (15.4%) v. sertraline (6.9%).
These findings could also be consistent with the higher switch rates for the tricyclic antidepressants (Reference Gijsman, Geddes and RendellGijsman et al, 2004) which represent largely combined serotonin and noradrenaline reuptake inhibitors, or noradrenaline selective ones (e.g. nortriptyline and desipramine). Sachs et al (Reference Sachs, Lafer and Stoll1994) and Guille et al (Reference Guille, Shriver and Demopulos1999) also found a higher switch rate for desipramine than for bupropion.
Interestingly, in our study the greater switch rates on venlafaxine compared with the other drugs were largely accounted for by the increased switch risk in the rapid-cycling group, and those without rapid-cycling disorder did not show this differential risk. It is especially noteworthy that those with rapid-cycling disorder did not appear more switch-prone when exposed to bupropion or sertraline than those with the non-rapid-cycling form on these drugs, as many might have predicted (log rank χ2=0.321, d.f.=1, P=0.571).
Methodological limitations
This study has several methodological limitations, including the combination of data for the first 27 open randomised patients with the next 147 studied in a double-blind fashion. However, this did not have a major impact on the observed rates of response, remission or switching. Generally similar findings were observed when only the 147 masked patients were included (further information available in the data supplement to the online version of this paper).
A problem not addressed by our study is the rate of response or switching related to antidepressant agents over that which might occur naturally through course of illness variation. It was decided not to include a placebo arm in this study to make it most similar to naturalistic treatment in the community and to focus on comparison of switch rates among the three agents. This was also intended to enhance participant recruitment in an out-patient setting in which a high percentage of patients were working full-time or part-time and generally wished to be treated with active agents as rapidly as possible. Thus, we do not know with any degree of certainty whether any of the antidepressants was efficacious, i.e. significantly more effective than placebo. This concern is also heightened by the finding of Nemeroff et al (Reference Nemeroff, Evans and Gyulai2001) that the effectiveness of paroxetine did not exceed that of placebo when used as an adjunct to lithium (unless lithium levels were low). However, given that venlafaxine was more likely to be associated with hypomania or mania than two other active drugs (bupropion or sertraline), it would appear that venlafaxine carries an increased risk of switching compared with two other widely used antidepressants, especially in the treatment of patients with rapid-cycling bipolar illness. This differential liability of venlafaxine compared with two other active comparators to some extent obviates the need for a placebo comparison group, at least in relation to the rate of switching on venlafaxine.
The study also did not address the optimal duration of antidepressant treatment, even though participants whose condition responded to therapy were offered continuation treatment on a masked basis (Post et al, Reference Post, Altshuler and Frye2001a , Reference Post, Leverich and Nolen2003b ). However, based on data in three recent naturalistic studies (Altshuler et al, Reference Altshuler, Kiriakos and Calcagno2001, Reference Altshuler, Suppes and Black2003; Reference Joffe, Macqueen and MarriottJoffe et al, 2005), it has been suggested that for the small minority of people with bipolar disorder who both respond to acute antidepressant treatment and remain well for at least 6 weeks, continuation of the antidepressant medication over the following year may be superior to its discontinuation because it is associated with a reduction in the occurrence of new depressive episodes without any increase in switch rates into mania. However, interim results of a 5-year study of 33 patients (Reference Ghaemi, El-Mallakh and BaldassanoGhaemi et al, 2005) indicate that discontinuation of antidepressant may be either non-inferior or perhaps slightly superior to antidepressant continuation, which appeared to increase affective morbidity in non-rapid-cycling bipolar disorder.
Other limitations of this study include the flexible dosage titration, such that the rate of dosage increase and final levels achieved could have affected either response rates or switch vulnerability. However, the relatively low maximum dosage of venlafaxine compared with the other two drugs suggests that the high switch rate on venlafaxine was not related to an overly aggressive dose titration of this drug compared with the others.
Strengths of this study include its double-blind evaluation of 147 patients; that the study was relatively large compared with many previous studies of bipolar depression; that the 174 patients randomised to one of the three second-generation antidepressants with different mechanisms of action were sufficient to discern a significant difference in switching into hypomania or mania on venlafaxine compared with bupropion and sertraline; and that the sample was representative of people receiving out-patient treatment, including those with a history of rapid-cycling disorder (Reference Kupka, Luckenbaugh and PostKupka et al, 2005).
Clinical and research implications
Other study designs, such as that used by Young et al (Reference Young, Joffe and Robb2000) in patients with non-rapid-cycling disorder, are now necessary to put these results in perspective, by examining the use of antidepressants with a low risk of switch (i.e. bupropion, sertraline or a related SSRI) compared with a second mood stabiliser (especially lamotrigine) or an atypical antipsychotic, in order to begin to develop an evidence-based algorithm for the best approach to the treatment of breakthrough bipolar depression in both rapid-cycling and non-rapid-cycling disorder. Since time depressed exceeds that of time manic by a factor of three in naturalistically treated out-patients (Reference Judd, Akiskal and SchettlerJudd et al, 2002; Post et al, Reference Post, Denicoff and Leverich2003a ,Reference Post, Leverich and Nolen b ,Reference Post, Speer and Leverich c ; Reference Nolen, Luckenbaugh and AltshulerNolen et al, 2004; Reference Kupka, Luckenbaugh and PostKupka et al, 2005), such direct comparisons for effectiveness, tolerability and switch risk would be timely and potentially highly informative for clinical practice.
The results of this study reveal non-significantly different acute antidepressant response and remission rates among the three mechanistically different second-generation antidepressants used adjunctively in the acute treatment of bipolar depression. However, there was a significantly increased risk of switching into hypomania or mania on several measures during acute treatment with venlafaxine compared with bupropion or sertraline. This was largely accounted for by the increased switch rate in the rapid-cycling group taking venlafaxine. When daily NIMH-LCM ratings were used, venlafaxine also had a three times higher ratio than bupropion of full duration/severity switches compared with brief hypomanias in the 1-year continuation phase of the study, further suggesting that the increased risk of a full switch on venlafaxine does not dissipate after the end of the 10-week acute trial, as it tended to do for both bupropion and sertraline (Reference Leverich, Altshuler and FryeLeverich et al, 2006). Clinicians should be aware of the risk of hypomania or mania for those prescribed venlafaxine (especially those with a history of four or more episodes in the prior year) when considering the choice of antidepressant for the common problem of bipolar depression breaking through ongoing treatment with one or more mood stabilisers.
BJP, 189,
Acknowledgements
We gratefully acknowledge the support of the Stanley Medical Research Institute and the National Institute of Mental Health in the conduct of this research. Drug and matching placebo were generously supplied by GlaxoSmithKline (bupropion), Pfizer US Pharmaceuticals (sertraline) and Wyeth Pharmaceuticals (venlafaxine). Additional superb statistical support was provided by Sun Hwang, Gerhard Hellemann and Chad Polio. Jeffrey Hatef and Sean O'Neill provided technical support and randomisation tables. We thank Stephanie Reardon for administrative support and Harriet Brightman, Maria Martinez and Chris Gavin for manuscript preparation.









eLetters
No eLetters have been published for this article.