The incidence, prevalence and predictors of depression after stroke, together with its associated outcomes, have been investigated in the past few decades. Previous reviews on this topic summarised the evidence available until 2000 Reference Turner-Stokes and Hassan1 and then again until 2004. Reference Hackett and Anderson2,Reference Hackett, Yapa, Parag and Anderson3 More recently, Kouwenhoven et al reviewed prevalence, predictors and outcomes of depression within a month of stroke. Reference Kouwenhoven, Kirkevold, Engedal and Kim4 However, the studies included in these reviews had important limitations such as small sample size, short follow-up and weak analyses. There are also no updated reviews of the long-term incidence, prevalence, predictors and outcomes of depression after stroke.
This systematic review and meta-analysis summarises the available evidence on incidence, prevalence, predictors and associated outcomes of depression after stroke, both in the short and long term.
Method
The Meta-analysis Of Observational Studies in Epidemiology (MOOSE) criteria Reference Stroup, Berlin, Morton, Olkin, Williamson and Rennie5 were used to undertake this review and meta-analysis.
Observational studies reporting prevalence, incidence, cumulative incidence, duration, predictors or outcomes of depression after stroke were searched in the following databases: EMBASE (1947 – August 2011), MEDLINE (1948 – August 2011), PsycINFO (1806 – August 2011) and ISI Web of Science (1900 – August 2011). The search strategy is presented in the online supplement. Reference lists of all systematic reviews identified were hand-searched for relevant studies. Reference Turner-Stokes and Hassan1–Reference Kouwenhoven, Kirkevold, Engedal and Kim4
Only studies defining depression as a diagnosis made using DSM-IV 6 criteria, a score above a cut-off point in a validated scale, or another validated method of diagnosis were included. There were no restrictions on the basis of language, sample size or duration of follow-up. Studies were excluded if they had any of the following: studies limited to specific clinical characteristics (e.g. strokes in specific locations, strokes of a specific subtype); they were limited to specific patient characteristics (e.g. patients of a specific age group); studies of mixed populations (e.g. stroke and head injury) unless separate results for stroke patients were identified; convenience sampling; unstructured assessment of mood; mood reported only as a continuous variable (not categorising patients as depressed or not depressed); studies with retrospective recruitment; and studies using only univariate analyses.
In some cases, similarities between studies indicated the possibility of multiple publications from the same cohort. In the absence of explicit cross-referencing, we considered articles to be from the same cohort if there was evidence of overlapping recruitment sites, study dates and grant funding numbers, or there were similar reported patient characteristics in the studies. Where several articles reported results from the same population, data were taken from the publication with the longest follow-up. When more than one method of assessment for depression was used, the result of the assessment that was discussed more in-depth by the authors was included in the meta-analysis. When the prevalences of major and minor depression were reported separately, they were grouped as depression.
Studies of predictors of depression that were included used depression as a dependent variable in a statistical model where potential predictors were explanatory variables. Studies of outcomes of depression that were included used outcomes as a dependent variable in a model where depression was an explanatory variable. Studies using only univariate analysis were not included as their results could be highly confounded. Reference Counsell and Dennis7 For studies of predictors or outcomes, information was collected on all of the variables analysed as potential predictors, outcomes and confounders. Only studies reporting outcomes measured at a later time point than depression were included. Information was collected on all of the variables analysed as potential predictors, outcomes and confounders. The quality of studies was assessed according to accepted criteria presented in a previous systematic review. Reference Hackett and Anderson2 Authors of studies were contacted when there were questions about whether papers met the inclusion criteria and also to verify methods and results that may not have been reported.
Statistical methods
A meta-analysis was undertaken to obtain pooled estimates of the prevalence of depression. Studies were classified into four categories: acute phase (within 1 month of stroke); medium-term phase (1–6 months); long-term phase (6 months to 1 year); very long-term phase (more than 1 year after stroke). A second meta-analysis was conducted in which studies were classified as population, hospital or rehabilitation studies. For studies with follow-up assessments at more than one time point, only results from the last follow-up were included in the meta-analysis. This was done to obtain pooled estimates of prevalence in the long term after stroke avoiding the bias that would have been introduced by entering repeated estimates of the same study in the meta-analysis. However, data from measurements at all time points were also recorded and are presented in the following tables. Studies with time of follow-up reported as an interval (e.g. 1–24 months) were included in the category of the earliest time point as it was considered to be the least affected by drop out due to mortality. Categorisation of these studies according to their mid time point of follow-up was also attempted but the differences of the estimates using earliest time point and mid time point was found to be negligible. A funnel plot was used to investigate possible publication bias.
The number of studies reporting estimates of natural history of depression after stroke other than prevalence (e.g. incidence) was small. The assessments for depression had been conducted at different time points in each of these studies. Therefore, a meta-analysis to obtain pooled estimates of other measures of natural history was not conducted. Results presented by individual studies were reported separately.
Results
Fifty studies, published between 1983 and 2011, reporting incidence, prevalence, cumulative incidence, duration, predictors or associated outcomes of depression after stroke were included in this review (Fig. 1). In all of them the analyses were based on the result of assessments for depression conducted after stroke, not accounting for whether the onset of depression occurred before or after the stroke (Table 1, Table 2 and online Table DS1).

Fig. 1 Results of the literature search.a
a. Many studies, together with prevalence of depression after stroke, reported either predictors or outcomes of depression after stroke.
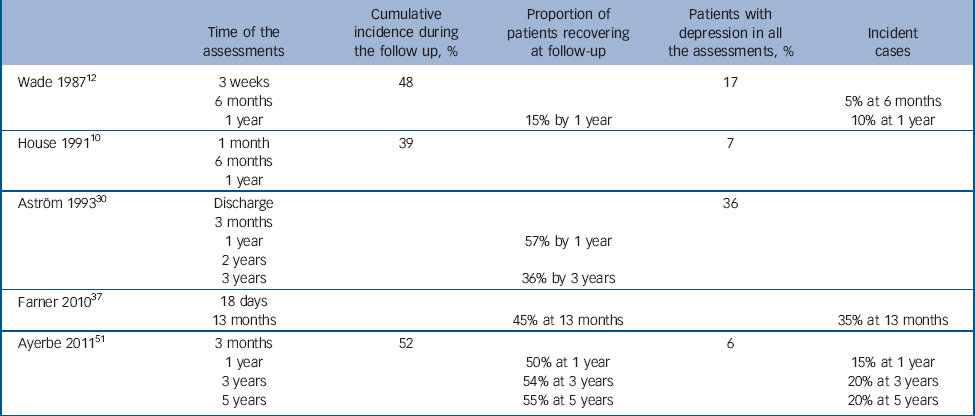
Table 1 The natural history of depression after stroke
| Time of the assessments |
Cumulative incidence during the follow up, % |
Proportion of patients recovering at follow-up |
Patients with depression in all the assessments, % |
Incident cases |
|
|---|---|---|---|---|---|
| Wade 1987 Reference Wade, Legh-Smith and Hewer12 | 3 weeks | 48 | 17 | ||
| 6 months | 5% at 6 months | ||||
| 1 year | 15% by 1 year | 10% at 1 year | |||
| House 1991 Reference House, Dennis, Mogridge, Warlow, Hawton and Jones10 | 1 month | 39 | 7 | ||
| 6 months | |||||
| 1 year | |||||
| Aström 1993 Reference Astrom, Adolfsson and Asplund30 | Discharge | 36 | |||
| 3 months | |||||
| 1 year | 57% by 1 year | ||||
| 2 years | |||||
| 3 years | 36% by 3 years | ||||
| Farner 2010 Reference Farner, Wagle, Engedal, Flekkoy, Wyller and Fure37 | 18 days | ||||
| 13 months | 45% at 13 months | 35% at 13 months | |||
| Ayerbe 2011 Reference Ayerbe, Ayis, Rudd, Heuschmann and Wolfe51 | 3 months | 52 | 6 | ||
| 1 year | 50% at 1 year | 15% at 1 year | |||
| 3 years | 54% at 3 years | 20% at 3 years | |||
| 5 years | 55% at 5 years | 20% at 5 years |
Natural history of depression after stroke
In total, 43 studies, including 20 293 patients, reported depression after stroke (Table DS1). Overall, 6 were population-based studies, Reference Burvill, Johnson, Jamrozik, Anderson, Stewart-Wynne and Chakera8–Reference Wolfe, Crichton, Heuschmann, McKevitt, Toschke and Grieve13 15 were hospital studies Reference Bayer14–Reference Storor and Byrne28 and 22 were rehabilitation studies. Reference Angeleri, Angeleri, Foschi, Giaquinto, Nolfe and Saginario29–Reference Van de Weg, Kuik and Lankhorst50 The number of patients assessed for depression in each study ranged from 14 to 13 999. Only nine studies assessed more than 200 patients Reference Burvill, Johnson, Jamrozik, Anderson, Stewart-Wynne and Chakera8,Reference Chausson, Olindo, Cabre, Saint-Vil and Smadja9,Reference Paul, Dewey, Sturm, Macdonell and Thrift11–Reference Wolfe, Crichton, Heuschmann, McKevitt, Toschke and Grieve13,Reference Eriksson, Asplund, Glader, Norrving, Stegmayr and Terent18,Reference Sienkiewicz-Jarosz, Milewska, Bochynska, Chelmniak, Dworek and Kasprzyk27,Reference Gillen, Tennen, McKee, Dott and Affleck38,Reference Langhorne, Stott, Robertson, MacDonald, Jones and McAlpine43 and only one study assessed more than 1000 patients. Reference Eriksson, Asplund, Glader, Norrving, Stegmayr and Terent18

Fig. 2 Pooled prevalence of depression stratified by length of follow-up.
Across the 43 studies, 29 studies used validated scales, 12 studies used DSM criteria, and 2 studies used a validated question. Overall, 11 different methods were used to assess depression. The cut-off points for the same scale used to assess depression across different studies were not consistent.
Only 8 studies reported the prevalence of depression more than 1 year after stroke, and only 13 studies assessed patients at more than one time point.
The pooled prevalence of depression observed at any time point was 29% (95% CI 25–32), with a prevalence of 28% (95% CI 23–34) within a month of stroke, 31% (95% CI 24–39) at 1–6 months, 33% (95% CI 23–43) at 6 months to 1 year, and 25% (95% CI 19–32) at more than 1 year (Fig. 2). The pooled prevalence of depression at any time point in population studies was 22% (95% CI 17–28), in hospital studies 30% (95% CI 24– 36), and 30% (95% CI 25–36) in rehabilitation studies (Fig. 3). The prevalence rates did not differ significantly over time or in studies of different settings. Heterogeneity was significant for all investigated categories. Studies with small sample sizes tended to report larger estimates of prevalence.
Five studies reported other measures of natural history of depression after stroke, including incidence, cumulative incidence and duration of depression (Table 1). Reference House, Dennis, Mogridge, Warlow, Hawton and Jones10,Reference Wade, Legh-Smith and Hewer12,Reference Astrom, Adolfsson and Asplund30,Reference Farner, Wagle, Engedal, Flekkoy, Wyller and Fure37,Reference Ayerbe, Ayis, Rudd, Heuschmann and Wolfe51 Incidence in year 1 ranged from 10 to 15% in the two studies reporting it. Cumulative incidence ranged from 39 to 52% in three studies with follow-up periods between 1 and 5 years. Three studies reported that 15–50% of patients with depression within 3 months of stroke had recovered 1 year later. The proportion of patients with depression in all the assessments ranged from 6% to 36% in four studies, with follow-up periods between 1 and 5 years. All the longitudinal studies presented a dynamic natural history, with new cases and recovery of depression occurring over time. Reference House, Dennis, Mogridge, Warlow, Hawton and Jones10,Reference Wade, Legh-Smith and Hewer12,Reference Astrom, Adolfsson and Asplund30,Reference Farner, Wagle, Engedal, Flekkoy, Wyller and Fure37,Reference Ayerbe, Ayis, Rudd, Heuschmann and Wolfe51

Fig. 3 Prevalence of depression stratified by study setting.
Predictors of depression after stroke
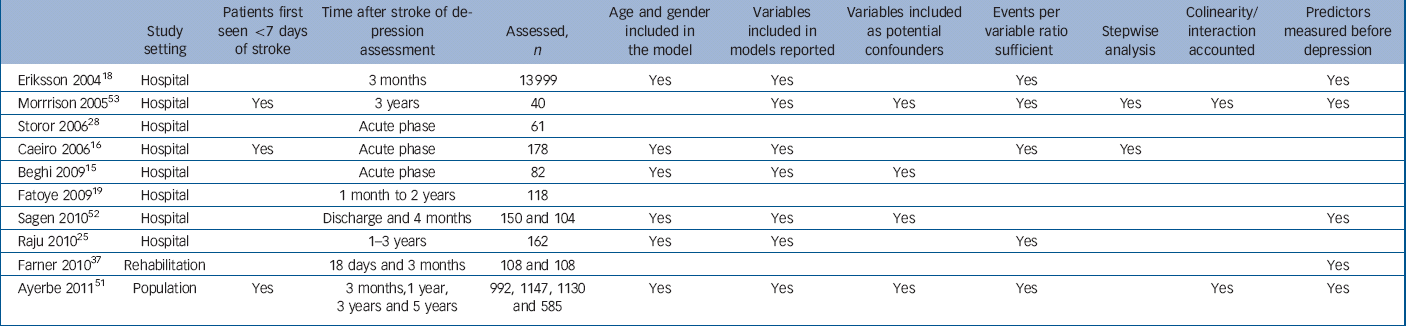
A total of 16 045 patients were assessed in 10 studies reporting predictors of depression. The number of patients assessed for depression in each study ranged from 40 to 13 999. Seven studies assessed more than 100 patients, Reference Caeiro, Ferro, Santos and Figueira16,Reference Eriksson, Asplund, Glader, Norrving, Stegmayr and Terent18,Reference Fatoye, Mosaku, Komolafe, Eegunranti, Adebayo and Komolafe19,Reference Raju, Sarma and Pandian25,Reference Farner, Wagle, Engedal, Flekkoy, Wyller and Fure37,Reference Ayerbe, Ayis, Rudd, Heuschmann and Wolfe51,Reference Sagen, Finset, Moum, Morland, Vik and Nagy52 of which only two studies assessed more than 1000 patients. Reference Eriksson, Asplund, Glader, Norrving, Stegmayr and Terent18,Reference Ayerbe, Ayis, Rudd, Heuschmann and Wolfe51 The quality assessment of these studies is presented in Table 2. Eight studies were hospital based, Reference Beghi, Cornaggia, Di Giacomo, Primati and Clerici15,Reference Caeiro, Ferro, Santos and Figueira16,Reference Eriksson, Asplund, Glader, Norrving, Stegmayr and Terent18,Reference Fatoye, Mosaku, Komolafe, Eegunranti, Adebayo and Komolafe19,Reference Raju, Sarma and Pandian25,Reference Storor and Byrne28,Reference Sagen, Finset, Moum, Morland, Vik and Nagy52,Reference Morrison, Pollard, Johnston and MacWalter53 one was a population-based study Reference Ayerbe, Ayis, Rudd, Heuschmann and Wolfe51 and one was a rehabilitation-based study. Reference Farner, Wagle, Engedal, Flekkoy, Wyller and Fure37 Only four studies assessed the patients more than 1 year after stroke. Reference Raju, Sarma and Pandian25,Reference Farner, Wagle, Engedal, Flekkoy, Wyller and Fure37,Reference Ayerbe, Ayis, Rudd, Heuschmann and Wolfe51,Reference Morrison, Pollard, Johnston and MacWalter53
The assessments for depression were carried out using scales in seven studies, DSM criteria in two studies and a validated question in another study. The time of these assessments ranged from the acute phase to 5 years after stroke. Seven studies stated all the variables included in the models. Six studies did not report that potential confounders had been included in the models. In five studies, depression and its predictors had been measured at the same time, making the model less predictive. The odds ratio and 95% confidence intervals of the associations were not always presented.
Many different predictors were investigated across the ten studies (online Table DS2). Disability was investigated in five studies. Two studies reported disability at baseline as a predictor of depression. Reference Ayerbe, Ayis, Rudd, Heuschmann and Wolfe51,Reference Morrison, Pollard, Johnston and MacWalter53 Another two studies reported disability to be associated with depression at follow-up. Reference Eriksson, Asplund, Glader, Norrving, Stegmayr and Terent18,Reference Raju, Sarma and Pandian25 Finally, another study found that disability after stroke was not associated with depression. Reference Sagen, Finset, Moum, Morland, Vik and Nagy52 Medical history of psychiatric disorders was investigated in different ways in five studies: pre-stroke depression was reported as a predictor of depression after stroke in one study; Reference Caeiro, Ferro, Santos and Figueira16 another study reported pre-stroke treatment for depression as a predictor of depression post-stroke; Reference Ayerbe, Ayis, Rudd, Heuschmann and Wolfe51 and three studies investigated medical history of psychiatric disorders, Reference Beghi, Cornaggia, Di Giacomo, Primati and Clerici15,Reference Fatoye, Mosaku, Komolafe, Eegunranti, Adebayo and Komolafe19,Reference Storor and Byrne28 two of which found a significant association with depression after stroke. Reference Fatoye, Mosaku, Komolafe, Eegunranti, Adebayo and Komolafe19,Reference Storor and Byrne28 Cognitive impairment after stroke predicted depression in two studies that investigated this association. Reference Fatoye, Mosaku, Komolafe, Eegunranti, Adebayo and Komolafe19,Reference Ayerbe, Ayis, Rudd, Heuschmann and Wolfe51 In both of these, cognitive impairment had been defined with a score above a cut-off point in a scale, rather than with clinical assessment, so no details were given on whether the association was between depression and the executive domain or with other domains of cognitive function. Three studies reported stroke severity not to be associated with depression after stroke. Reference Beghi, Cornaggia, Di Giacomo, Primati and Clerici15,Reference Raju, Sarma and Pandian25,Reference Morrison, Pollard, Johnston and MacWalter53 However, a large population-based study reported independent measures of stroke severity such as the Glasgow Coma Scale, dysphagia and incontinence to be associated with depression. Reference Ayerbe, Ayis, Rudd, Heuschmann and Wolfe51 Another study reported hemiparesis to be associated with depression. Reference Fatoye, Mosaku, Komolafe, Eegunranti, Adebayo and Komolafe19 Anxiety predicted depression in two studies Reference Sagen, Finset, Moum, Morland, Vik and Nagy52,Reference Morrison, Pollard, Johnston and MacWalter53 and was associated with depression at follow-up in a third study. Reference Raju, Sarma and Pandian25 Social isolation at follow-up was associated with depression in one study Reference Ayerbe, Ayis, Rudd, Heuschmann and Wolfe51 and another reported an association between living alone after stroke and depression. Reference Eriksson, Asplund, Glader, Norrving, Stegmayr and Terent18 Age and gender did not predict depression in six out of the seven studies that investigated the associations. Other potential predictors that were investigated, including comorbidities, history of stroke, education, family type or neuroticism, are presented in Table DS2.
Table 2 Studies of predictors of depression after stroke
| Study setting |
Patients first seen <7 days of stroke |
Time after stroke of de- pression assessment |
Assessed, n |
Age and gender included in the model |
Variables included in models reported |
Variables included as potential confounders |
Events per variable ratio sufficient |
Stepwise analysis |
Colinearity/ interaction accounted |
Predictors measured before depression |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eriksson 2004 Reference Eriksson, Asplund, Glader, Norrving, Stegmayr and Terent18 | Hospital | 3 months | 13 999 | Yes | Yes | Yes | Yes | ||||
| Morrrison 2005 Reference Morrison, Pollard, Johnston and MacWalter53 | Hospital | Yes | 3 years | 40 | Yes | Yes | Yes | Yes | Yes | Yes | |
| Storor 2006 Reference Storor and Byrne28 | Hospital | Acute phase | 61 | ||||||||
| Caeiro 2006 Reference Caeiro, Ferro, Santos and Figueira16 | Hospital | Yes | Acute phase | 178 | Yes | Yes | Yes | Yes | |||
| Beghi 2009 Reference Beghi, Cornaggia, Di Giacomo, Primati and Clerici15 | Hospital | Acute phase | 82 | Yes | Yes | Yes | |||||
| Fatoye 2009 Reference Fatoye, Mosaku, Komolafe, Eegunranti, Adebayo and Komolafe19 | Hospital | 1 month to 2 years | 118 | ||||||||
| Sagen 2010 Reference Sagen, Finset, Moum, Morland, Vik and Nagy52 | Hospital | Discharge and 4 months | 150 and 104 | Yes | Yes | Yes | Yes | ||||
| Raju 2010 Reference Raju, Sarma and Pandian25 | Hospital | 1–3 years | 162 | Yes | Yes | Yes | |||||
| Farner 2010 Reference Farner, Wagle, Engedal, Flekkoy, Wyller and Fure37 | Rehabilitation | 18 days and 3 months | 108 and 108 | Yes | |||||||
| Ayerbe 2011 Reference Ayerbe, Ayis, Rudd, Heuschmann and Wolfe51 | Population | Yes | 3 months,1 year, 3 years and 5 years | 992, 1147, 1130 and 585 | Yes | Yes | Yes | Yes | Yes | Yes |
Outcomes of depression after stroke
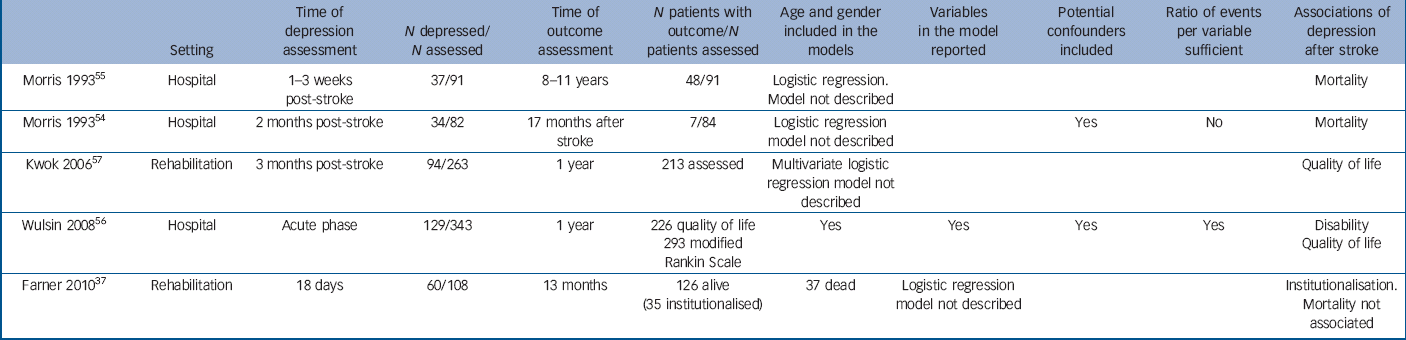
Five studies reported health outcomes associated with depression after stroke (Table 3): three were hospital studies Reference Morris, Robinson and Samuels54–Reference Wulsin, Alwell, Moomaw, Lindsell, Kleindorfer and Flaherty56 and two were rehabilitation studies. Reference Farner, Wagle, Engedal, Flekkoy, Wyller and Fure37,Reference Kwok, Lo, Wong, Wai-Kwong, Mok and Kai-Sing57 The number of patients assessed for outcomes ranged from 84 to 293. Depression was assessed between the acute phase and 3 months after stroke. Three studies reported outcomes observed more than a year after stroke. Reference Farner, Wagle, Engedal, Flekkoy, Wyller and Fure37,Reference Morris, Robinson and Samuels54,Reference Morris, Robinson, Andrzejewski, Samuels and Price55 Only one study described the statistical model used in the analysis. Reference Wulsin, Alwell, Moomaw, Lindsell, Kleindorfer and Flaherty56 Disability was found to be an outcome of depression in one study with an odds ratio of 2.68 (95% CI 1.50 to 4.78). Reference Wulsin, Alwell, Moomaw, Lindsell, Kleindorfer and Flaherty56 Lower quality of life was found to be an outcome of depression in two studies that investigated this association. Both of them used linear regression. One of them reported a coefficient for quality of life of −0.52 (95% CI −0.70 to −0.33) Reference Wulsin, Alwell, Moomaw, Lindsell, Kleindorfer and Flaherty56 and the other presented separate coefficients for the physical domain (–1.8, 95% CI −1.4 to −2.2), pshychological domain (–26, 95% CI −2.4 to −2.8), social domain (–1.2, 95% CI −0.8 to −1.6) and environmental domain (–2.0, 95% CI −1.6 to −2.4) of quality of life. Reference Kwok, Lo, Wong, Wai-Kwong, Mok and Kai-Sing57 Higher mortality was found to be an outcome of depression in two of the three studies that investigated this association. Reference Morris, Robinson and Samuels54,Reference Morris, Robinson, Andrzejewski, Samuels and Price55
Discussion
Natural history of depression after stroke
Depression had a cumulative incidence of up to 52% within 5 years of stroke, with a pooled prevalence of 29% that remained stable in the first 10 years after stroke across different study settings. Studies assessing patients more than once suggested that most patients who have depression after stroke became depressed shortly after the acute event, a significant proportion of them recovered from depression in subsequent assessments, and new cases made the overall prevalence of depression stable. The natural history of depression more than 5 years after stroke remains unknown. Factors affecting the variation of the prevalence of depression reported by individual studies included the different methods used to diagnose depression, source of patient recruitment and the timing of assessment, together with the different study settings. Without greater methodological uniformity, it will remain difficult to determine whether heterogeneity in study findings is showing real differences in population characteristics or is simply an artefact caused by measurement bias and other errors. These estimates may still be inaccurate because of potential underreporting of abnormal mood, especially in patients with communication impairment Reference Hackett, Yapa, Parag and Anderson3 and the possibility of overreporting depression by using screening questionnaires.
Table 3 Outcomes of depression after stroke
| Setting | Time of depression assessment |
N depressed/ N assessed |
Time of outcome assessment |
N patients with outcome/N patients assessed |
Age and gender included in the models |
Variables in the model reported |
Potential confounders included |
Ratio of events per variable sufficient |
Associations of depression after stroke |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Morris 1993 Reference Morris, Robinson, Andrzejewski, Samuels and Price55 | Hospital | 1–3 weeks post-stroke | 37/91 | 8–11 years | 48/91 | Logistic regression. Model not described | Mortality | |||
| Morris 1993 Reference Morris, Robinson and Samuels54 | Hospital | 2 months post-stroke | 34/82 | 17 months after stroke | 7/84 | Logistic regression model not described | Yes | No | Mortality | |
| Kwok 2006 Reference Kwok, Lo, Wong, Wai-Kwong, Mok and Kai-Sing57 | Rehabilitation | 3 months post-stroke | 94/263 | 1 year | 213 assessed | Multivariate logistic regression model not described | Quality of life | |||
| Wulsin 2008 Reference Wulsin, Alwell, Moomaw, Lindsell, Kleindorfer and Flaherty56 | Hospital | Acute phase | 129/343 | 1 year | 226 quality of life 293 modified Rankin Scale | Yes | Yes | Yes | Yes | Disability Quality of life |
| Farner 2010 Reference Farner, Wagle, Engedal, Flekkoy, Wyller and Fure37 | Rehabilitation | 18 days | 60/108 | 13 months | 126 alive (35 institutionalised) | 37 dead | Logistic regression model not described | Institutionalisation. Mortality not associated |
A previous systematic review published in 2005 reported that the prevalence of depression after stroke was stable across studies conducted at different time points and in different settings, with an overall prevalence of 33% (95% CI 29–36). Reference Hackett, Yapa, Parag and Anderson3 This systematic review includes 15 new studies, with 7 studies conducted in Europe, 3 studies conducted in Oceania, 3 in Asia, 1 in the Caribbean and 1 in Africa. However, the prevalence observed in our study and the one previously reported Reference Hackett, Yapa, Parag and Anderson3 are very similar, showing overlapping confidence intervals. Our results show the great stability of the prevalence of depression across studies assessing patients at different time points in different areas of the world.
Only one population-based study recruited controls to allow estimates of the relative risks of depression after stroke. Reference House, Dennis, Mogridge, Warlow, Hawton and Jones10 The authors reported that the prevalence of depression in stroke survivors was twice that found in controls, although this difference was only significant at the 6-month follow-up assessment. Another robust examination of the relative risk of depression in stroke survivors was undertaken in the Framingham Study, which reported that significantly more stroke survivors had depression compared with controls who were matched for age and gender. Reference Kase, Wolf, Kelly-Hayes, Kannel, Beiser and D'Agostino58
Predictors of depression after stroke
Disability after stroke and history of depression pre-stroke are predictors of depression after stroke that are most consistently reported, with four studies presenting a significant association. Other predictors were cognitive impairment, stroke severity, lack of social or family support, and anxiety. Depression pre-stroke and anxiety were not reported as predictors in a previous review. Reference Hackett and Anderson2 Risk factors for depression not connected to stroke (e.g. genetic factors) may explain the strong association between depression before and after stroke. The associations between stroke severity and depression were not completely consistent. The association between stroke severity and disability may be a possible explanation for the inconsistent association between severity and depression observed in our study. Whether the association between stroke severity and depression is independent or partly or completely explained by the association between severity and disability remains unknown. The association observed in our review between depression and impaired cognition is complex as both can be cause or effect of each other and they also have common risk factors. Patients with cognitive impairment deserve special attention in any case, as their risk of depression may be increased and they may be unable to report their symptoms. No association was found between depression and other variables representing neurological damage, such as stroke subtype, lesion location or laterality of stroke. A previous systematic review of depression and stroke lesion location concluded that there was no evidence suggesting that the risk of depression after stroke is affected by the location of the brain lesion. Reference Carson, MacHale, Allen, Lawrie, Dennis and House59 The importance of neurological damage on depression after stroke appears to be limited to cognitive impairment and stroke severity. Other medical conditions did not predict depression after stroke. The results of this review suggest that depression after stroke is mostly associated with the experience and consequences of the stroke itself.
Predictors of depression after stroke observed in this review can be considered in clinical practice. Clinicians should pay particular attention to patients with disability and a history of depression, as the risk of depression after stroke seems to be higher in these groups. Patients with cognitive impairment, severe strokes, anxiety and living in isolation also deserve close monitoring and consideration for preventive interventions to reduce the risk of depression and improve stroke outcomes.
Outcomes of depression after stroke
The evidence on the outcomes of depression after stroke is still limited, with only five studies investigating this area. The very brief description of the statistical models reported in most studies makes it difficult to assess the validity of the results. Without information on all the variables included in the models, it is not possible to differentiate between outcomes of depression, and outcomes of stroke or all the other comorbidities that may come with this combination of disorders. Low quality of life Reference Morris, Robinson and Samuels54,Reference Wulsin, Alwell, Moomaw, Lindsell, Kleindorfer and Flaherty56 and mortality Reference Morris, Robinson and Samuels54,Reference Wulsin, Alwell, Moomaw, Lindsell, Kleindorfer and Flaherty56 were outcomes of depression identified most often. In an attempt to investigate the causal associations between depression and its outcomes, only studies where the outcomes had been assessed after depression have been included in this review. A previous systematic review reported many possible outcomes of depression after stroke, including higher disability rates, higher mortality, poor involvement in rehabilitation, longer hospital stay and poor cognitive function. Reference Turner-Stokes and Hassan1 However, in that review, the authors included studies where depression and its potential outcomes had been assessed at the same time. This makes it difficult to know whether depression is actually a cause or a consequence of the variable investigated as a potential outcome. Reference Turner-Stokes and Hassan1 We found weak evidence or none at all that other variables apart from disability, lower quality of life and mortality may be outcomes of depression in stroke patients.
Strengths and limitations
The comprehensive search and critical assessment of studies of unselected stroke patients conducted in this review allows estimation of the natural history of predictors and outcomes of depression after stroke obtained with a large number of patients. The funnel plot showed that some studies with smaller samples reported prevalence estimates that were larger than average, while no studies reported prevalence under 10% (online Fig. DS1). Although this could be interpreted as publication bias, it could nonetheless be interpreted as a genuine prevalence of depression which is not less than 10%. The diversity of the methods used across studies may have an effect on the external validity of each individual one. In this review, this effect was minimised by conducting a comprehensive search, and the categorisation of studies by setting and length of follow-up. The summary of results of individual studies provides estimates that can be used in clinical practice and in the development of further research.
Although the guidelines for reporting meta-analyses of observational studies were used as a reference, Reference Stroup, Berlin, Morton, Olkin, Williamson and Rennie5 this review does have several limitations. Only one person extracted most of the data (L.A.). Even so, all data were checked for accuracy on multiple occasions and all analyses were conducted several times and checked by a senior statistician (S.A.). Finally, it is possible that some ‘multiple publications’ have been miscoded or missed altogether. Particular attention was paid to addressing this source of publication bias, because the lack of cross-referencing of data from some cohorts has served to mislead the research community, specifically in the area of depression after stroke.
Clinical and research implications
Depression after stroke requires periodic clinical attention in the long term that should focus on patients at highest risk. The natural history, predictors and outcomes of depression after stroke require further research. This should ideally be conducted in population-based studies of large sample sizes and long follow-up. Studies investigating, in the long term, incidence and prevalence of depression at different time points, the time of depression onset and recovery, and recurrence patterns, are needed. Adherence of future studies of predictors to standard methods accepted for prognostic models Reference Counsell and Dennis7 in stroke cohorts is required to make results easy to interpret and applicable in clinical practice. The identification of predictors of depression after stroke would help clinicians to identify patients at higher risk of this problem, a much needed focus for clinical trials of preventive interventions for post-stroke depression. Finally, in order to understand the impact of depression specifically in stroke patients, the association between depression after stroke and other health outcomes should be investigated further.









eLetters
No eLetters have been published for this article.